3.1. Capillary Water Absorption and Water Absorption with Karsten Tubes
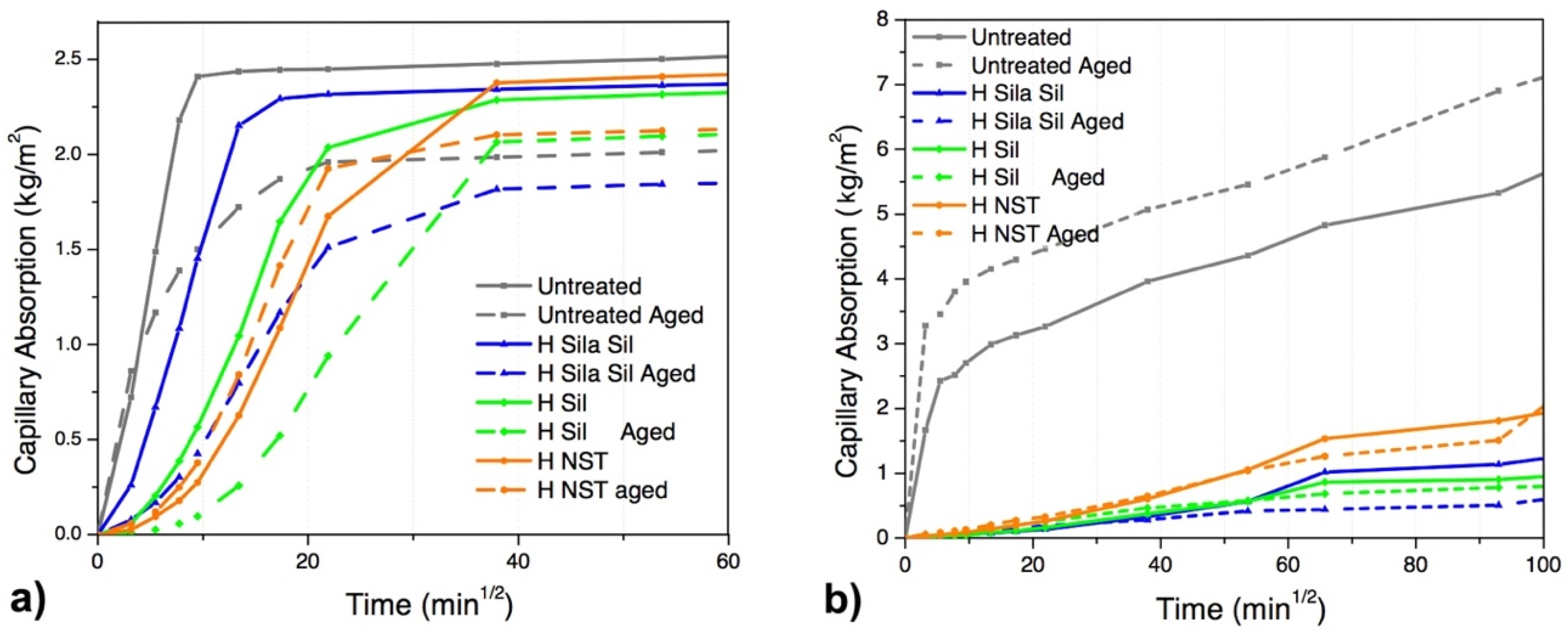
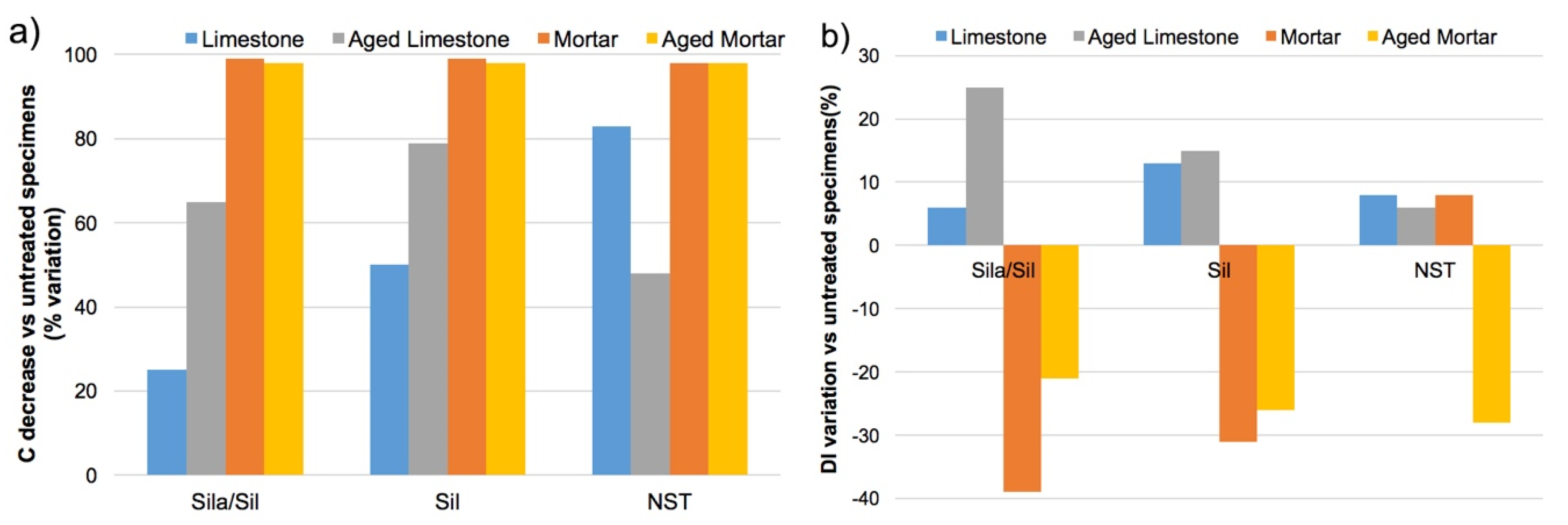
The results show that, in the case of limestone specimens, the capillary water absorption coefficient (C) of treated specimens considerably reduced after the application of the hydrophobic products (33% in the case of H
Sila-Sil, 51% with H
sil, 88% with H
NST) (
Table 4,
Figure 2).
All hydrophobic products penetrated within the porous network of the substrate, reducing the wettability and providing a hydrophobic coating [
9]. The higher reduction of the capillary water absorption coefficient of the specimens treated with H
NST, which can penetrate deeper within the treated substrate due to its nanosize [
1], can be attributed to the chain arrangement (creation of Si-O-Ti bonds) of the TiO
2-SiO
2 nanoparticles. The copolymerization of the TiO
2 and silane within the silica network can give rise to the formation of a homogeneous organic–inorganic hybrid xerogel, with improved hydrophobic properties [
23,
24].
After accelerated aging tests, untreated specimens show a significant reduction of the capillary water absorption (around 44%), which can be attributed to the modification (destruction) of capillary pores (typically observed in altered and decayed materials) [
36]. In fact, a significant increase of porosity is observed mostly between 5 to 10 freeze-thaw cycles [
37]. Thus, artificial aging induces the generation of new pores and the expansion of existing pores in the specimens.
Additionally, the specimens with HNST treatment, which show the highest reduction of capillary water absorption before artificial aging, show an opposite trend after artificial aging, with a decrease of up to 60% when compared to the unaged specimens. On the other hand, aged specimens treated with HSila/Sil and HSil show a significant reduction of the capillarity water absorption (80% and 50%, respectively), when compared to the untreated aged specimens.
This can be justified by the lower durability of H
NST treatment to hygrothermal aging cycles. As a matter of fact, it is reported [
38] that weathering can induce a weakening of the film adhesion and reduce the durability of TiO
2-SiO
2 protective coatings.
After aging, all specimens still maintain a significant reduction (40%, 21% and 52% for HSila/Sil, HSil and HNST, respectively) of the capillary water absorption when compared to the untreated specimens.
Concerning mortar specimens, all treatments show a higher reduction of the capillarity water absorption when compared to treatments on limestone, although the total amount of absorbed water is higher (total saturation of the specimens is not completely achieved at the end of the test). A remarkable reduction of the capillarity water absorption was observed in all treated mortar specimens (>95%), when compared to untreated specimens. This behavior is attributed to the higher open porosity of rendering mortars (
Table 1), allowing the easier penetration of hydrophobic products into the pores of the substrate coating [
19]. The deeper penetration of the hydrophobic products leads to a reduction of the wettability of the substrate, resulting in an almost hydrophobic surface. All treatments show a similar behavior, almost waterproofing the mortar specimens, and the treatments show a good durability after artificial aging, with a minimal increase of the capillarity water absorption coefficient when compared to unaged specimens.
Additionally, all aged treated mortar specimens significantly reduce their capillary water absorption coefficient when compared to aged untreated specimens. However, it is worth noting that a slight decrease of the capillarity water absorption coefficient was observed if comparing unaged treated specimens to aged treated ones. This modification can be attributed to the possible alteration of the pore size distribution of the substrates.
A possible cause for the reduction of capillary water absorption after freeze-thaw cycles can be the reduction of capillary suction, resulting from an increase in the amount of bigger pores (above the capillary range) as a consequence of micro-cracking. Additionally, in the case of the cement-based mortar, the exposure to water with these cycles can induce a self-healing effect that promotes hydration reactions, further explaining the reduction of the capillary absorption rate with ageing.
When considering the results of water absorption by Karsten tube in the limestone specimens, a trend similar to that seen in the capillarity water absorption test was observed (75% in the case of H
Sila-Sil, 93% with H
sil, 92% with H
NST). In the case of the mortar specimens, the reduction was similar to that observed in capillary water absorption tests (96% in the case of H
Sila-Sil, 98% with H
sil, 99% with H
NST) (
Table 5).
If comparing the results of capillary water absorption and water absorption under low pressure of the untreated specimens, the opposite trend is seen. In fact, there is a reduction of capillary water absorption after aging; however, an increase of water absorption under low pressure is observed in equivalent conditions [
39]. It is generally assumed that pores ranging from 1 to 10 μm act as capillary pores, whereas pores >10 μm contribute to the water permeability through gravity (e.g., percolation) or wind driven water ingress [
40,
41]. Thus, this confirms that artificial aging cycles possibly contribute to an increase in the amount of pores with dimensions greater than 10–20 μm.
Furthermore, HSila/Sil e HSil treatments decrease the water absorption coefficient under pressure after artificial aging, both when applied on mortar or limestone. On the other hand, a decrease of 25% in the C60 was observed in the aged limestone specimens treated with HNST, if compared to the unaged specimens, whereas an opposite trend is observed when considering treated mortar specimens.
These results point out that HSila/Sil e HSil treatments have lower variation of the water absorption after artificial aging, compared to HNST treatments. In fact, the latter shows both an increase of the capillary water absorption and water absorption under low pressure, possibly due to its physical-chemical alteration.
3.2. Drying Rate
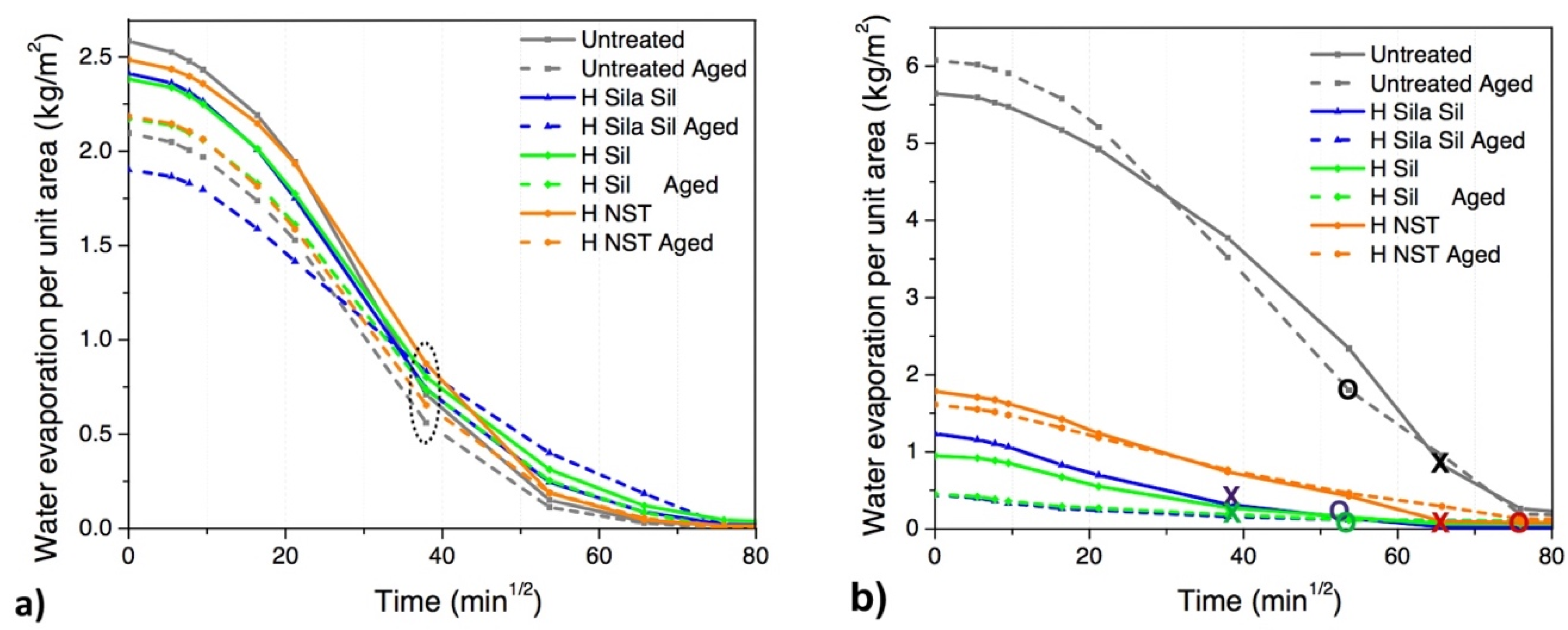
When observing the drying curves, two stages can be observed (
Figure 3). In the first stage of drying, called the constant drying period or initial drying rate, the drying front is at the surface and the drying rate is constant and controlled by the external conditions [
42]. This first phase (initial drying rate) ends after 24 h in the case of all treated and untreated limestone and treated mortar specimens, whereas for untreated mortar specimens it ends after ≥72 h (
Figure 3). When compared to the sound untreated specimens, all treated specimens decrease the initial drying rate products (first stage of drying), both in the limestone (3–12%) and mortar specimens (6–36%). More specifically, H
Sila/Sil has an almost negligible influence on the initial drying rate of the treated specimens (3% reduction, when compared to untreated specimens), whereas H
Sil and H
NST induce a slightly higher reduction (up to 12%).
In the second stage of drying, identified by the change in the slope of the drying curve, the moisture content can no longer support the demands of the evaporation flux, and, thus, the drying process occurs in the vapour phase. The transition between the first and second step of drying (i.e., the critical moisture content) occurs when the superficial moisture has evaporated. In this phase, the drying front progressively recedes into the material and the properties of the liquid and of the substrate control the rate of drying [
42].
When considering the second stage of drying in limestone specimens, although the critical moisture content is identified at 24 h in all cases, it can be observed that (aged and unaged) untreated specimens almost achieve complete drying at 72 h, and a similar trend is observed in the case of the specimens treated with H
NST (
Figure 3a). On the other hand, H
Sil and H
Sila/Sil slightly delay the drying process (the second step of drying ends at 96 h), when compared to H
NST treatment.
It can be concluded that the hydrophobic treatments induce only a slight retarding effect on the drying behavior of the limestone.
When considering the mortar specimens, all the hydrophobic treatments remarkably reduce the total amount of water absorbed; however, the H
NST treatment takes a longer time to dry completely when compared to the H
Sil and H
Sila/Sil treatments. Conversely, the H
NST treatment only slightly influences the initial drying rate (6% reduction) when compared to the H
Sila/Sil treatment (13%) and, especially, the H
Sil treatment (36%). Additionally, in accordance with the results observed in the previous section, H
NST treatment also increases the drying time (8%) in the mortar specimens, whereas H
Sil and H
Sila/Sil show a significant decrease (30–39%). The difference in the behavior of the hydrophobic products is also observed in the second step of drying, which starts at 24 h in the case of H
Sila/Sil and H
Sil treatment, whereas the critical moisture content is identified at 72 h in the case of the H
NST treatment (as in the case of untreated specimens). Results obtained with contact angle measurements in a previous work confirm this trend and the drying index (
Table 6), that is, a higher reduction of the wettability on both substrates in the case of H
Sila/Sil treatment [
19].
After hygrothermal aging, H
Sila/Sil treatment show an improvement of the initial drying rate (20% and 27%, for limestone and mortar specimens, respectively), with worse results when compared to H
NST treatment (reduction of 5% and 24%, for limestone and mortar specimens, respectively) and H
Sil treatment (reduction of 9% and 12%, for limestone and mortar specimens, respectively). It can be seen that artificial aging slightly speeds up the drying process of the mortar specimens (the critical moisture content is identified at 48 h in the case of untreated aged specimens, and at 72 h with untreated unaged specimens). Additionally, in accordance with previous observations, the second step of drying of aged specimens treated with HSil and HSila/Sil starts at 48 h, and at 96 h in the case of the H
NST treatment (
Figure 3b).
After artificial aging, HSila/Sil treatment shows the highest variation of drying behavior, with an increase (26%) of the DI for limestone and decrease of 21% for mortar specimens (the slower the drying, the higher the DI). In accordance with previous observations, HSil treatment also induces an increase (15%) of the DI for limestone, and, conversely, a significant decrease (26%) for mortar specimens. On the other hand, HNST treatment shows the best performance on limestone specimens, with only a slight DI increase (6%), and, however, a significant decrease for mortar specimen (28%).
3.3. Water Vapor Permeability
The results of the water vapor permeability test, as expressed by the water vapor diffusion resistance coefficient (µ), show a µ decrease for all hydrophobic treatments on limestone and mortar specimens, reducing the breathability of the substrates (
Table 7). The reduction in water vapor permeability is an inevitable consequence of the water repellence properties of polymer film; however, the lowest possible decrease is pursued [
43].
This reduction is more significant in limestone specimens, whereas it is almost negligible in mortar specimens. In fact, an increase of µ of 227% (HSila/Sil), 129% *(HSil) and 74% (HNST) is observed on treated limestone specimens, when compared to untreated ones, whereas a minimal µ increase (<4%) is observed on the treated mortar specimens.
After artificial aging, only the HNST treatment applied on limestone maintains reasonably higher µ values (increase of 7%) when compared to untreated specimens, whereas HSil and HSila/Sil treatments still induce a drastic µ increase (46% and 188%, respectively). In the case of mortar specimens, the higher increase of µ was observed with HSila/Sil treatment (9%), and only a slight µ decrease in the case of HSil and HNST treatments (<2%). In general, the hydrophobic treatment that illustrates the most suitable behavior to water vapor permeability was HNST, with a moderate reduction of the µ on both substrates, even after artificial aging.