1. Introduction
A number of efforts are currently made to eliminate volatile organic compounds (VOC) from common use, meaning that water-based paints are coming to the fore [
1,
2,
3]. The majority of water-based paints contain up to 80% water plus some organic solvents such as glycol ethers [
4]. These paints are beneficial in that they are environmentally friendly, meeting both U.S. (EPA (U.S. Environmental Protection Agency)) and European (BPD (Biocidal Products Directive)) regulations requiring the VOC content to be below the limit of 350 g per liter of water [
5,
6]. As a drawback, however, water-based paints (during storing) and their coatings films are subject to microbial colonization, which is an effect that needs to be prevented not just because of their possible bio-deterioration associated with economic impact [
7], but also from health and safety reasons, as pathogenic microorganisms can be transferred easily from coatings to humans [
8,
9].
Water-based acrylate dispersions (latexes) constitute a universal, high-quality and environmentally friendly option [
10]. Their synthesis has been brought to a high level [
11]. The drawbacks of common latex coatings such as low resistance to solvents, adhesive properties at elevated temperatures and brittleness at low temperatures are overcome by using various crosslinking strategies [
12,
13]. Among them, the interfacial crosslinking, using the keto-hydrazide self-crosslinking pathway, is based on a reaction of the carbonyl group (diacetone acrylamide) at the polymeric chain and a diamine (adipic acid dihydrazide (ADH)) dissolved in the aqueous phase. The advantages of this keto-hydrazide self-crosslinking include a fast reaction at normal temperatures and one-component composition [
12,
13,
14].
Microbial infectious diseases are a serious health, social and economic problem that has drawn public attention worldwide as a human health threat [
15]. The prevention of biofilm formation and bacterial adhesion on various materials is subject to research [
16,
17]. A wide selection of additives (often toxic), providing permanent protection against the adverse effects of microorganisms, exists for paints and coatings [
18,
19,
20]. Antibiotics are among the most frequently used agents for this purpose. Bacteria are becoming increasingly resistant to antibiotics, however, due to the wide application of the latter; therefore, finding new alternatives to antibiotics is urgently required [
21,
22,
23]. Various inorganic and organic compounds have been applied in the past, but many of them are now banned because of their environmental harmfulness and failure to meet applicable European legislation. Specifically, the European Union adopted its Directive 98/8/EC establishing a framework of rules governing the marketing of biocidal products with a view to ensuring a high degree of protection of humans and the environment. New findings stimulate additional limitations to be placed on the quantities and numbers of substances exhibiting antimicrobial effects; therefore, new alternatives to antimicrobial protection need to be sought out [
17,
24,
25].
Nanoparticles provide new approaches to the development of antibacterial materials because metal oxide nanoparticles demonstrate the potential for reducing bacterial contamination [
26,
27,
28] and, at the same time, can exert a favorable effect on the structural properties of the paint film [
29,
30]. MgO is an important inorganic oxide that is widely used in many industrial areas. MgO powder microparticles find a range of applications, e.g., as rubber fillers, in adhesives, cosmetic products, artificial silk and paper. This material is also added to paints where enhanced chemical resistance is desirable; it is an ingredient in anticorrosion paints that resist the effects of sea water and in waterproof paints for wood [
31,
32,
33,
34]. It has been demonstrated that MgO is harmless to human health and the environment [
35,
36,
37], yet it exhibits a destructive antibacterial activity with respect to both Gram-positive and Gram-negative bacteria and even to viruses and spores [
38,
39,
40].
One of the drawbacks of latex-based coatings is flash corrosion, which is a rapid, widespread corrosion occurring solely upon the application of water-based paints to metals [
41]. The occurrence and development of flash corrosion are influenced by a number of factors, particularly ambient humidity and temperature during the film drying process, polymeric dispersion type, metallic surface pre-treatment, pH of the drying paint and the presence of air pollutants. The film formation of water-based paints, occurring while the water evaporates, includes the coalescence of polymeric particles, their deformation and, ultimately, diffusion of the polymeric chains of these particles [
42]. If the process of water evaporation from the paint films is slowed down, e.g., by high air humidity, the coalescence is preceded by soluble iron salt transfer into the paint film. Application of the aqueous polymeric dispersion alone on a steel substrate is usually accompanied by flash corrosion observed over the entire metal area, which acquires a red-brown color. If a pigmented paint containing the same binder is used, then local, sharply bound defects develop whose size and number may depend on the paint film thickness and porosity. Flash corrosion can be efficiently counteracted by the use of inhibitors which are frequently toxic, such as sodium benzoate and sodium nitrite [
43,
44].
The goal of this work was to formulate a new latex binder exhibiting chemical resistance and biocidal efficiency in the paint films in which it is used. To this end, a one-component water-based polymeric dispersion was synthesized so that MgO nanoparticles were included in the polymerization system at a concentration of 1.5% with respect to the monomers used (based on solids). A reference system possessing the same composition but with no nanoparticles (blank) was also formulated. The paint film efficiency was assessed with respect to the biocidal effect due to the presence of magnesium nanoparticles. The antimicrobial efficiency of the pigmented vs. transparent polymeric films was assessed, potential commercial biocidal product savings were estimated, and a number of additional benefits of the binder were highlighted.
2. Materials and Methods
2.1. Incoming Materials
The latexes were prepared from monomeric methyl methacrylate (MMA), n-butyl acrylate (BA), methacrylic acid (MAA) and diacetone acrylamide (DAAM) supplied by Sigma-Aldrich, Prague, Czech Republic. Adipic acid dihydrazide (ADH, active substance content > 98%; Sigma-Aldrich, Prague, Czech Republic) served as the crosslinking agent; Disponil FES 993 (anion-active surfactant based on sodium polyglycol ether sulfate; BASF, Chrudim, Czech Republic), as the emulsifier; ammonium persulfate (active substance content > 99.9%; Lach-Ner, Neratovice, Czech Republic), as the initiator; and nanostructural MgO with no surface treatment, particle size < 200 nm (commercial name JR-NMg30, Xuancheng Jingrui New Materials Co., Xuancheng, China), as the antimicrobial and antifungal ingredient of the latex.
2.2. Synthesis
The semi-continuous emulsion polymerization process was used to prepare two latex types: one with MgO nanoparticles added as a component of the polymerization system (“LM”) and the other with an identical composition, but with no nanoparticles added (“L0”). Details of the composition are listed in
Table 1. To provide well-coalesced coatings on the one hand and non-tackiness of coatings films on the other hand, the monomer proportions were selected so that the calculated glass transition temperature (
Tg) of the latex polymer was around 10 °C. (Calculation was performed according to the Fox equation [
45].) ADH was added to the latex after synthesis to facilitate copolymer crosslinking via DAAM carbonyl groups.
The latexes were prepared in a glass reaction vessel at a polymerization temperature of 85 °C under nitrogen by following the procedure shown in
Table 2. Distilled water, emulsifier and initiator were added to the reaction vessel prior to starting the dropwise addition of the emulsion of monomers. The emulsion addition was divided into two phases. (In the course of emulsion addition, a temperature of 85 °C was maintained in the reaction vessel.) In the first phase, the emulsion was added dropwise for 60 min, followed by a 15 min period for completion of the polymerization process while maintaining a temperature of 85 °C in the polymerization system. In the second phase, the monomer emulsion was added dropwise and combined with the addition of MgO nanoparticles for the LM latex: the MgO nanoparticles were dispersed in the acrylate monomers on a T18 digital ULTRA TURRAX (IKA Works, Staufen, Germany) dispersing machine at 20,000 rpm for 30 min, followed by exposure to the action of ultrasound in a KRAINTEK K-12.F (Kraintek s.r.o., Podhájská, Slovak Republic) bath for another 30 min. All of the ingredients were then mixed together and dispersed for another 3 min. This made up the emulsion of the monomers containing MgO nanoparticles for dropwise addition to the reaction vessel. When all of the emulsion had been added, the system was allowed to stand for 120 min for completion of the polymerization process. The pH of the cold latex containing no nanoparticles was adjusted to 8.5 using 10% aqueous ammonia. This alkalinization step could be omitted for the latex with the nanoparticles because its pH was 10.34 due to hydration of the nanostructural MgO. The self-crosslinking latex binders were obtained by mixing with a 10% aqueous solution of ADH at the molar ratio of DAAM:ADH = 2:1.
2.3. Description of the Self-Crosslinking Latexes
The coagulate and coarse impurity contents of the latexes were determined by sieve analysis according to ČSN 64 9008; the zeta potential and particle size were determined by the dynamic light scattering (DLS) method on a Zetasizer Nano ZS (Malvern Panalytical, Malvern, UK) [
46]; pH was measured with a Mettler Toledo FiveEasy FE20 pH-meter (Merck KGaA, Darmstadt, Germany) [
47]; non-Newtonian (apparent) viscosity was measured on a RotoVisco (RT10/94 viscometer (HAAKE, Vreden, Germany)) in the cone-plate arrangement of the Searle type where the bottom plate was steady and the rotor was movable, at a speed of 0–250 min
−1, and with a slot width of 0.05 mm, duration of 180 s, and temperature of 21 °C (held constant with a Thermo Scientific Haake A10, Thermo Fisher Scientific, Waltham, MA, State, USA); the minimum film-forming temperature (MFFT) was determined by using a MFFT-60 instrument (Rhopoint Instruments, East Sussex, UK) according to ISO 2115; the glass transition temperature (
Tg) of emulsion polymers was determined using differential scanning calorimetry (DSC) on a Pyris 1 DSC (Perkin-Elmer, Waltham, MA, USA) under nitrogen, at a heating rate of 10 °C·min
−1, the second heating curve was used for
Tg determination and the In-Can antimicrobial efficiency of the self-crosslinking dispersions was tested by using Preventol
® Dipslides (LANXESS Deutschland GmbH, Cologne, Germany). The In-Can Preservation test of antimicrobial efficiency consisted of submerging the agar part of the DipSlide into the latex for 10 s, followed by incubation at 30 °C for 120 h. The result was evaluated by using standards [
48].
2.4. Description of the Pigments and Fillers
The specific weights (densities) were measured on an AutoPycnometer 1320 (Micrometritics, Norcross, GA, USA). The pigment and filler properties were as follows: Blanc Fixe filler, structure: barite, artificially precipitated BaSO4 crystalline powder, pH 8.5–10, density 4.502, manufacturer/supplier: Sigma-Aldrich; Litopon 30 white pigment, structure: mix of precipitated ZnS (30%) and BaSO4 pigments, pH: 6–7, density: 4.360, manufacturer/supplier: 3P-CHEM s.r.o.; Omyacarb-1VA filler, structure: calcite (CaCO3). pH: 9, density: 2.935, manufacturer/supplier: Omya CZ s.r.o.; Magnesium oxide nanoparticles: structure: periclase (MgO), particle size < 200 nm, pH 8–10, density 3.576, manufacturer/supplier: Xuancheng Jingrui New Materials Co.; Titanium oxide white pigment, structure: anatase (TiO2), pH 8–10, density 3.780, manufacturer/supplier: Prechezia a.s.; Zinc oxide (microparticles) white pigment, structure: 99.99% zincite (ZnO), pH 7–7.4, density 5.606, manufacturer/supplier: Sigma-Aldrich; Zinc oxide nanoparticles, structure: zincite (ZnO), pH 7–7.4, density 5.606, manufacturer/supplier: Sigma-Aldrich; Zinc sulfide pigment, structure: 99.99% ZnS, pH 10–10.75, density 4.104, manufacturer/supplier: Sigma-Aldrich; MicaCelia natural filler, structure: muscovite (K2Al2Si3O10(OH)2), pH 6–7.5, density 2.699, manufacturer/supplier: Ziegler & Co. GmbH; and Magnesium carbonate filler, structure: magnesite (MgCO3 powder), pH 9.5–10.5, density: 2.162, manufacturer/supplier: Lach-Ner.
2.5. Dispersing the Latexes with the Pigments and Antimicrobial Additives for Antimicrobial/Biocidal Testing
Typical pigments and fillers (
Table 3) were dispersed in the latex at concentrations of 2% and 10% (
w/
w) for antimicrobial efficiency testing. This enabled the contribution of the filler or pigment on the final antimicrobial efficiency of the paint film to be assessed. This implies that the paints tested contained inorganic pigments and fillers with different chemical compositions and particle structures—Mg, Zn, Ti, Ca, K, Al and Ba carbonates, oxides, sulfides, sulfites and silicates.
Zinc pyrithione (summary formula C10H8N2O2S2Zn, CAS No.: 13463-41-7, active substance content 52–56%, pH 6.5–8.5, density, 1.12 g/cm3, manufacturer/supplier: Lonza) at 0.1–0.3% (w/w) was also dispersed in the latex in order to examine the feasibility of achieving savings of the antimicrobial additive. The dispersions were prepared by using a Heidolph RK3 stirrer (Heidolph, Germany) at 2500 rpm for 30 min, whereby adequate conditions were attained for achieving uniform dispersion and pigment bonding to the liquid phase.
2.6. Preparation of the Paint Films: Latex Application to the Substrates
The latexes were applied to glass panels with a size of 200 mm × 100 mm × 5 mm to test the physical properties, chemical resistance and mechanical resistance (adhesion) of paint films. Mechanical (impact) resistance and corrosion resistance were tested on paint films applied to cold-rolled low-carbon steel panels with a size of 152 mm × 102 mm × 0.8 mm. Both the glass and steel panels were washed and cleaned thoroughly with chloroform before the tests [
49]. The liquid latexes were applied to the glass/steel substrates by using a film applicator coater (Bird type applicator with a constant slot width, product of Zehntner GmbH, Schwerzenbach, Switzerland). The slot width was 150 μm for application on the glass panels and 250 μm for application on the steel panels. The paint films on the panels were allowed to dry for 10 days (except for the panels intended for flash corrosion examination) in an air-conditioned room.
For determination of true nanoparticle content in the LM latex film, the latexes (both LM and L0) were cast into silicone molds to obtain loose films, which were then allowed to dry to a constant weight prior to ICP–OES (inductively coupled plasma–optical emission spectroscopy) measurement. For the antimicrobial efficiency tests, the latexes were applied to sterile filter paper (Munktell Filtrak 391, 15 cm in diameter, grammage 84 g/m2) squares with a size of 5 cm × 5 cm. The paint was applied to the filter paper with a brush in perpendicular directions in 4 layers, with a minimum drying time of 4 h between the layers; the tests themselves were performed after a drying period of 10 days.
Each test was performed in triplicate. All of the paint films—on glass, steel and filter paper and in silicone molds—were exposed to a temperature of 21 ± 2 °C and relative humidity of 55% in an air-conditioned room according to ČSN EN 23270 prior to the tests.
2.7. Paint Film Property Assessments
The true magnesium nanoparticle contents of the paint films were determined by ICP–OES (inductively coupled plasma–optical emission spectroscopy) on a Thermo Scientific iCAP 7000 Series instrument (Thermo Fisher Scientific, Waltham, MA, USA) [
50]. The paint film structure at a fracture plane was measured by scanning electron microscopy (SEM) on a LYRA 3 instrument (Tescan, Brno, Czech Republic).
The physical properties of the paint films were evaluated according to ČSN EN ISO 1522—Paints and varnishes—Pendulum damping test; Persoz type pendulum (3034M001 pendulum, Elcometer Instruments GmbH, Aalen, Germany).
The mechanical properties (mechanical resistance) of the paint films were assessed according to ČSN EN ISO 6272-2—Paints and varnishes—Rapid-deformation (impact resistance) tests, viz. through measurements on an Elcometer 1615 variable impact tester (Elcometer Instruments GmbH, Aalen, Germany). Tests according to ČSN ISO 2409, such as the cross-cut test, were also made. An Elcometer cross-cut system (Elcometer Instruments GmbH, Aalen, Germany) with 6 parallel knives 1 mm apart was used.
Chemical resistance of the paint films was assessed according to ASTM D-4752-10—rubbing test with methyl ethyl ketone (MEK).
Flash corrosion resistance of the paint films was assessed through a laboratory test to identify/measure any flash corrosion [
41]. The tests were made on paint films deposited on steel panels which, after drying at 21 ± 2 °C, RH 50 ± 5% for 2 h, were stored in a refrigerator at 5 °C for 16 h. After removal from the refrigerator, the entire paint film was uniformly covered with a filter paper that was pre-wetted with distilled water, and the system was covered with a heavy glass plate to achieve full contact between the paint film and water. The water was allowed to act for 2 h at room temperature. The filter paper was then removed, the paint film was dried, and corrosion phenomena were scored as per ASTM D 610-85; coloration was scored using Gardner’s iodometric scale (
Table 3) [
51].
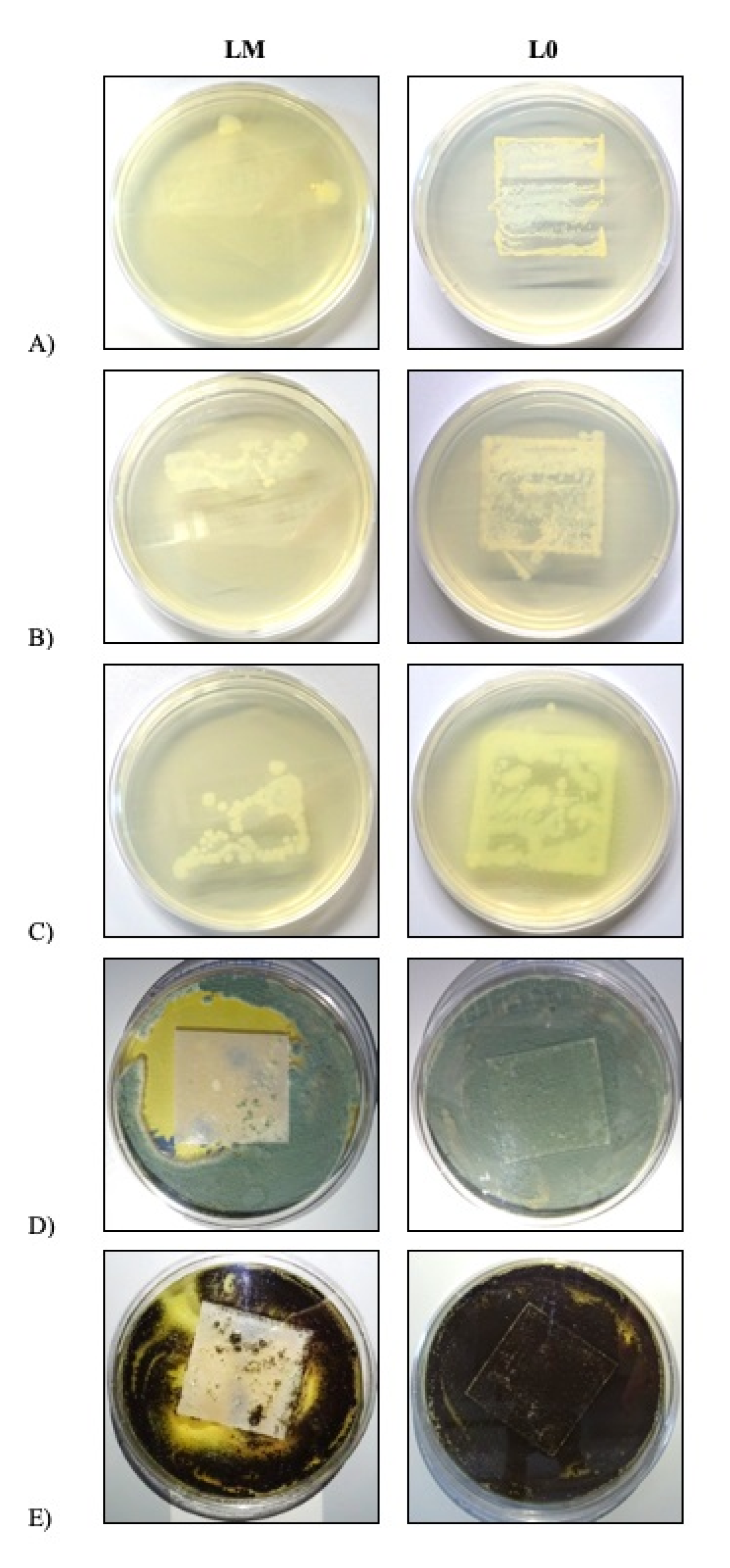
The antimicrobial efficiency of the paint films was assessed with respect to the collection of the strains Staphylococcus aureus CCM 2022, Escherichia coli CCM 3954 and Pseudomonas aeruginosa CCM 3955 and to the molds Penicillium chrysogenum CCM 8034 and Aspergillus brasiliensis CCM 8222 (Czech Collection of Microorganisms, Masaryk University, Brno, Czech Republic). Twenty-four-hour bacteria cultures and 5-day mold cultures were used for the testing. The microbial cultures were suspended in saline at concentrations of approximately 106 CFU/mL (bacteria) and 106 spores/mL (molds). A sterile sample of the smear was applied to the center of a Petri dish with agar, followed by 0.1 mL of the microbial suspension. The suspension was spread uniformly over the entire surface with an L-shaped hockey stick and the bacterial samples inoculated in this way were incubated at 37 °C for 24 h; the molds were incubated at 25 °C for 7 days. The microorganism density was evaluated by inoculating the suspension onto pure agar, which was expected to contain 300 bacterial colonies and 100% agar covering with the mold after the process. The mold increase on the material tested was evaluated after incubation. Where the bactericidal efficiency was examined, the sample was imprinted onto agar and incubation was conducted at 37 °C for 24 h. The percent bacterial colony increase was measured after the incubation procedure.
Each test was performed in triplicate and the final result was represented by the arithmetic mean of the 3 observed values.