Fabrication of Large Area Ag Gas Diffusion Electrode via Electrodeposition for Electrochemical CO2 Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipments
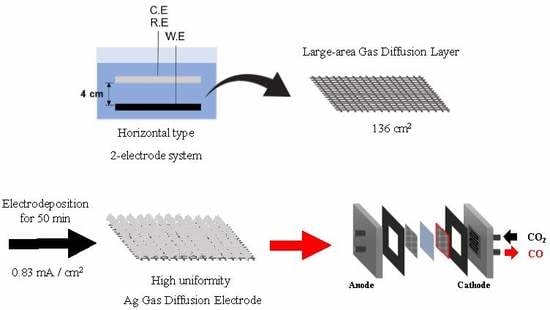
2.2. Fabrication of Ag GDE
2.3. Electrochemical CO2 Reduction to CO in Half-Cell
2.4. Electrochemical CO2 Reduction to CO in MEA-Type Electrolyzer
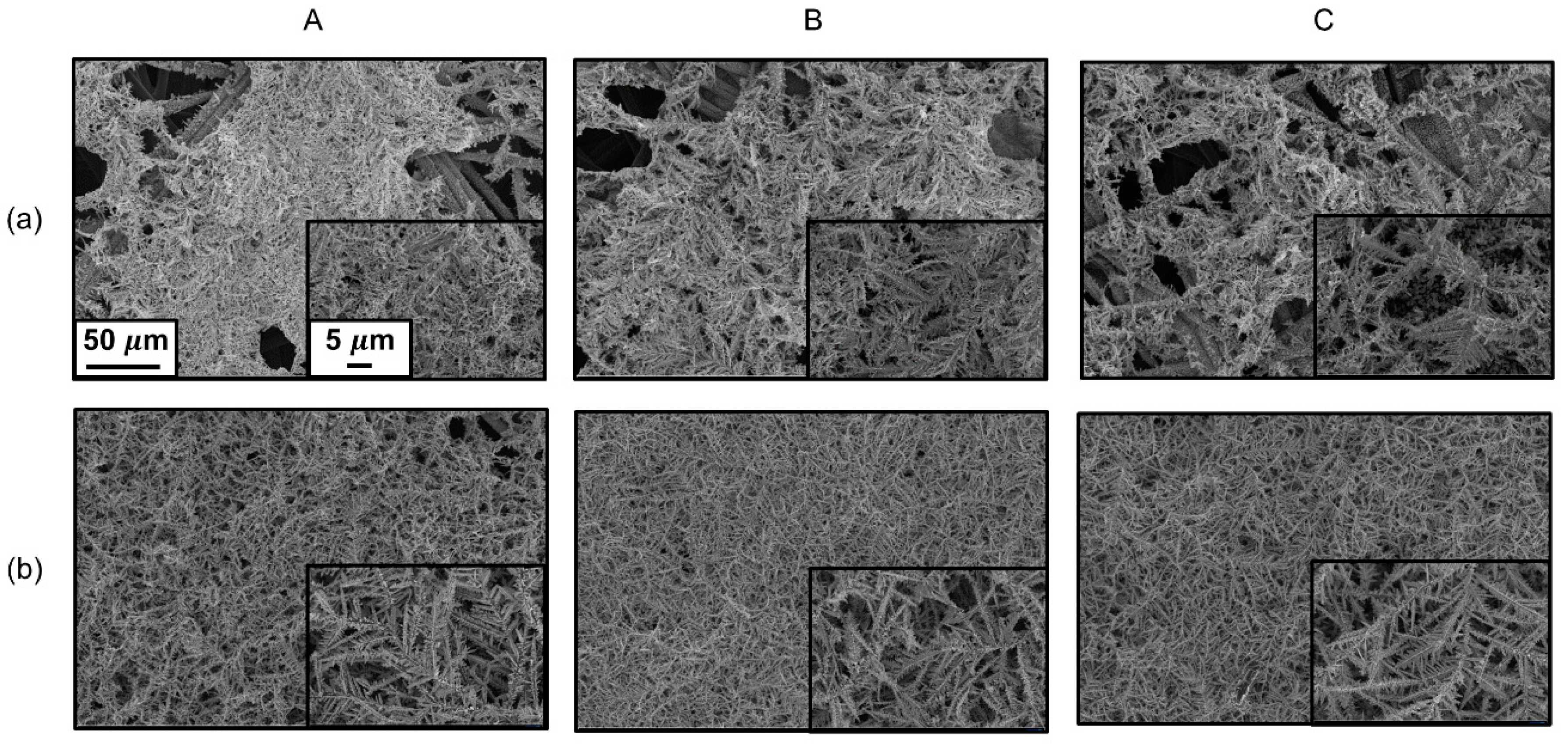
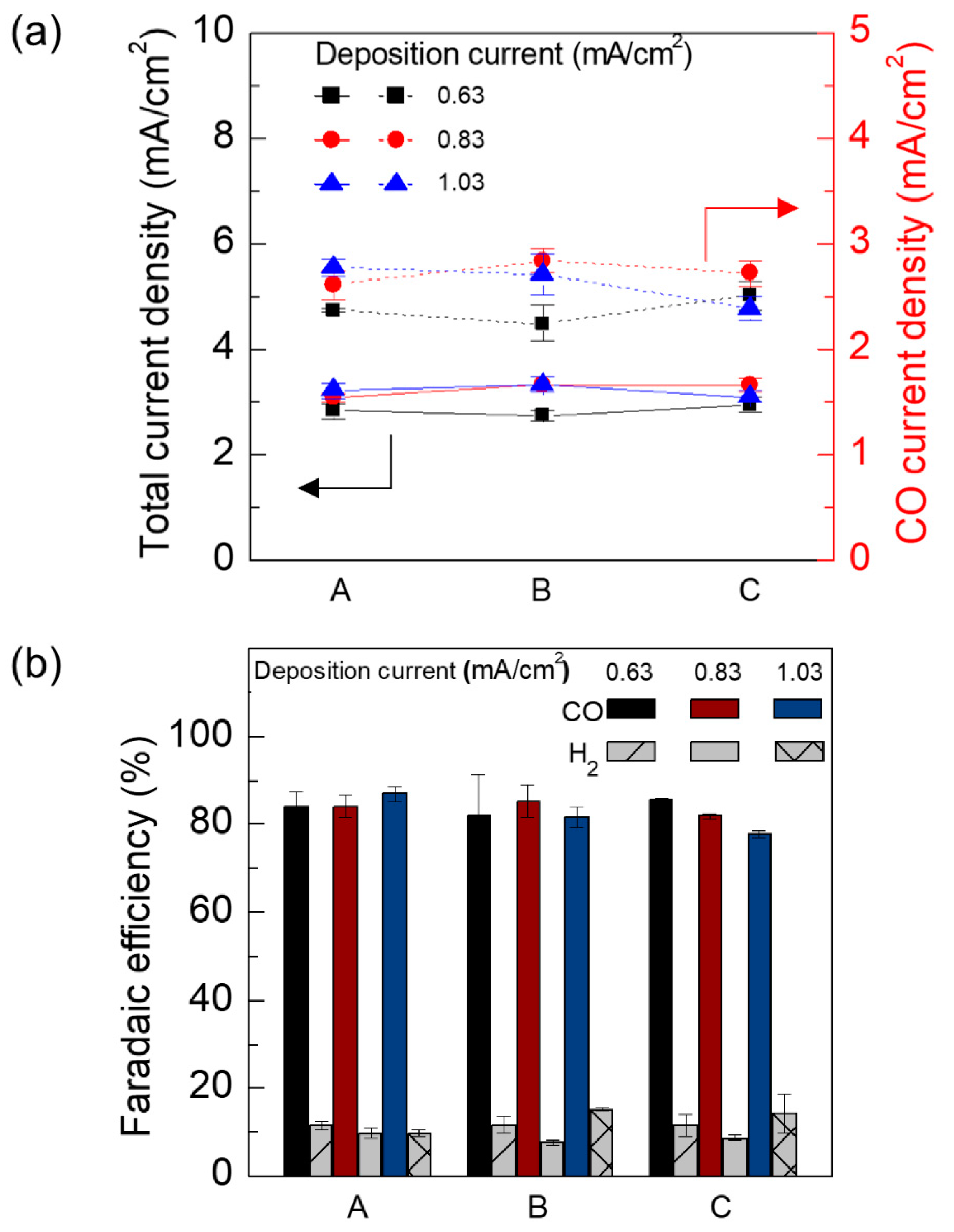
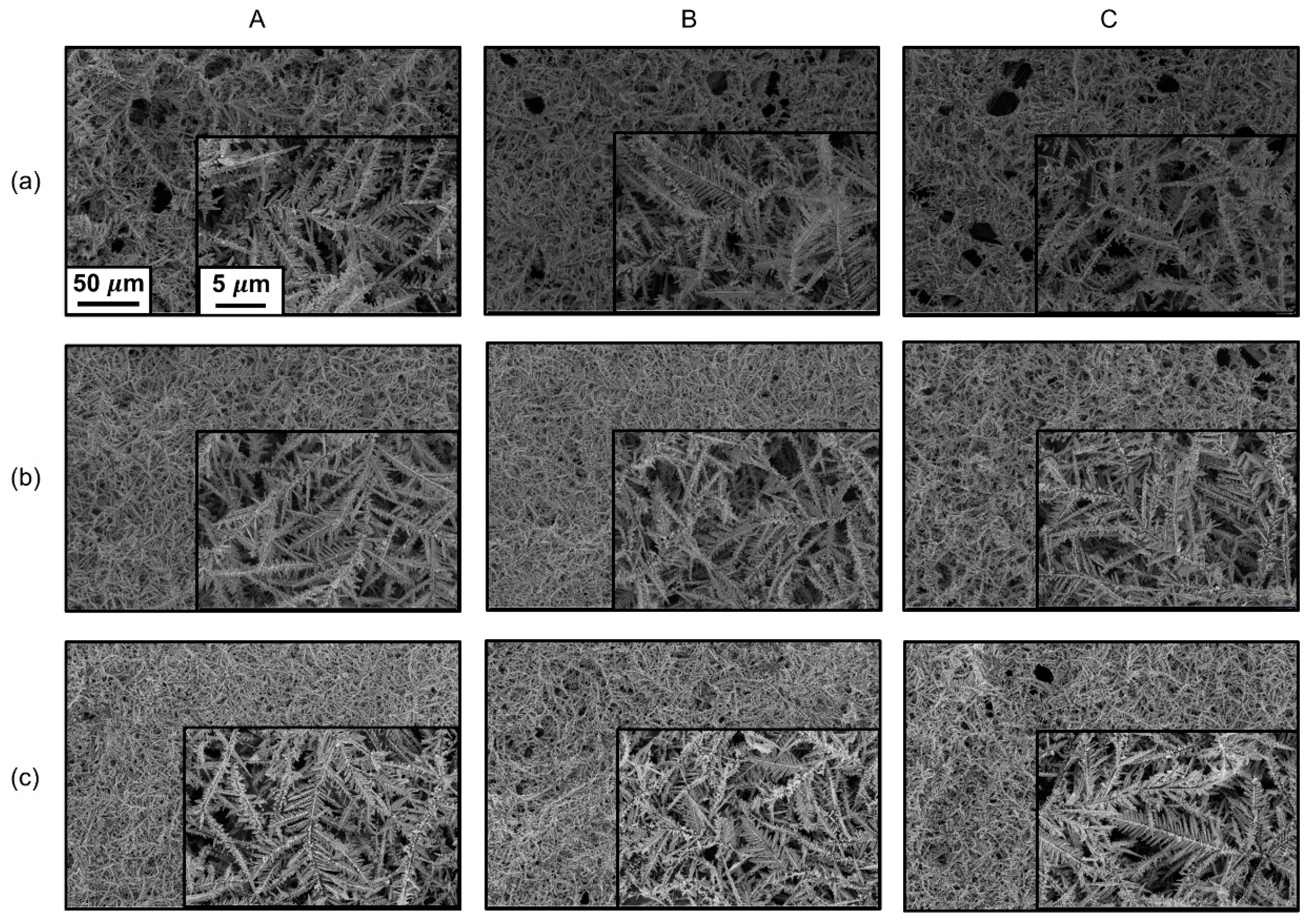
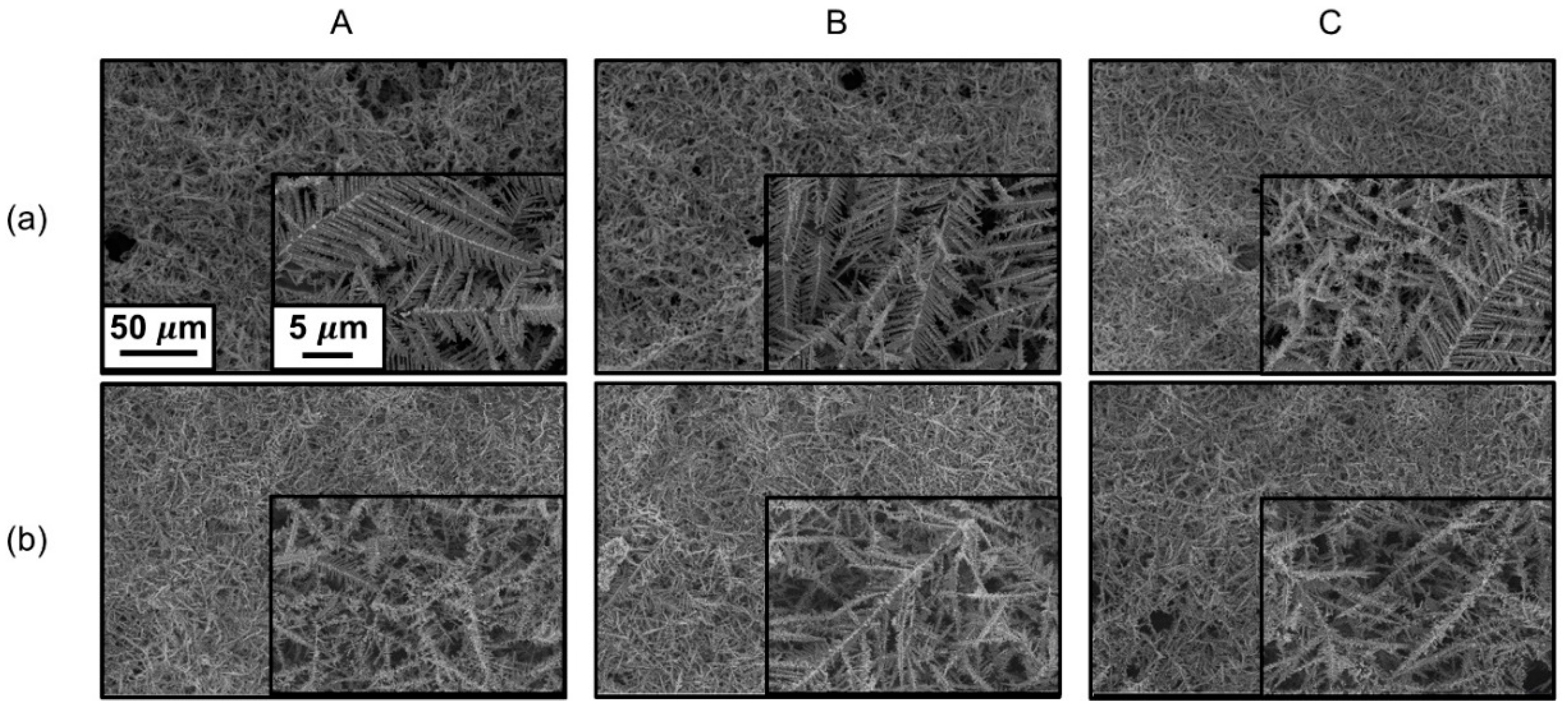
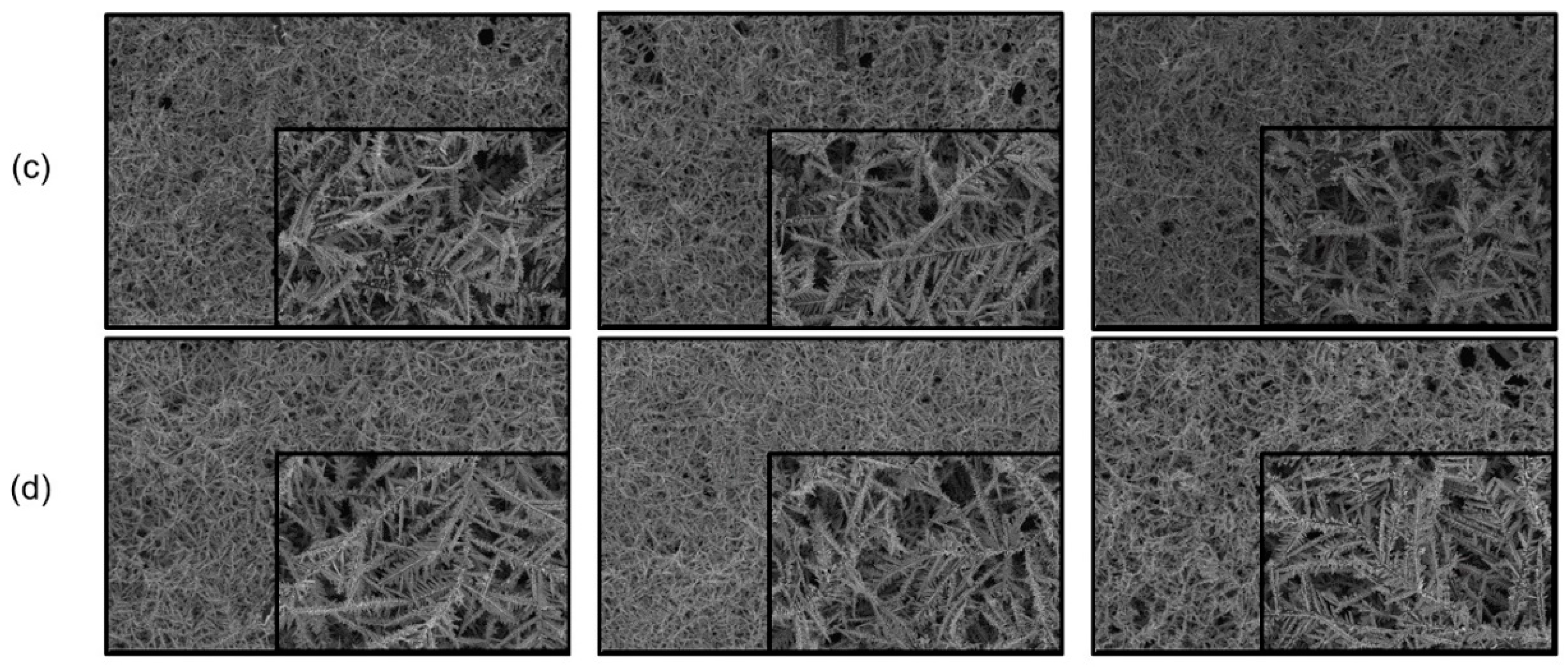
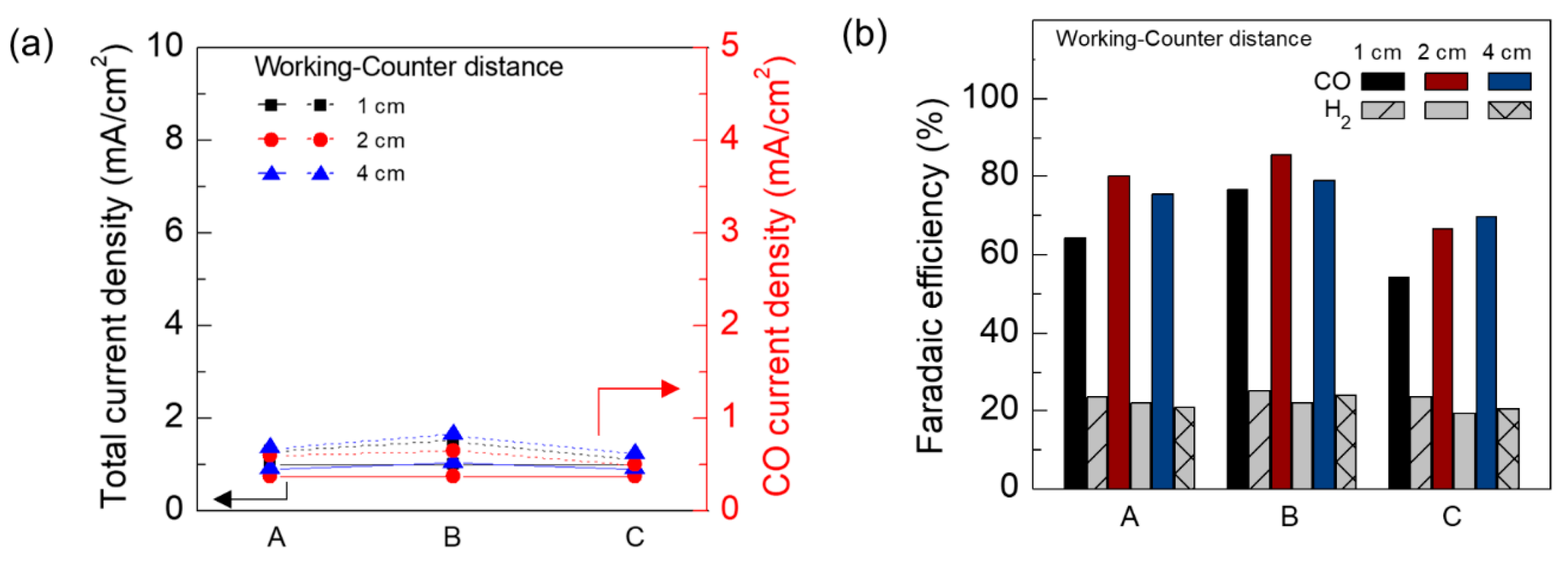
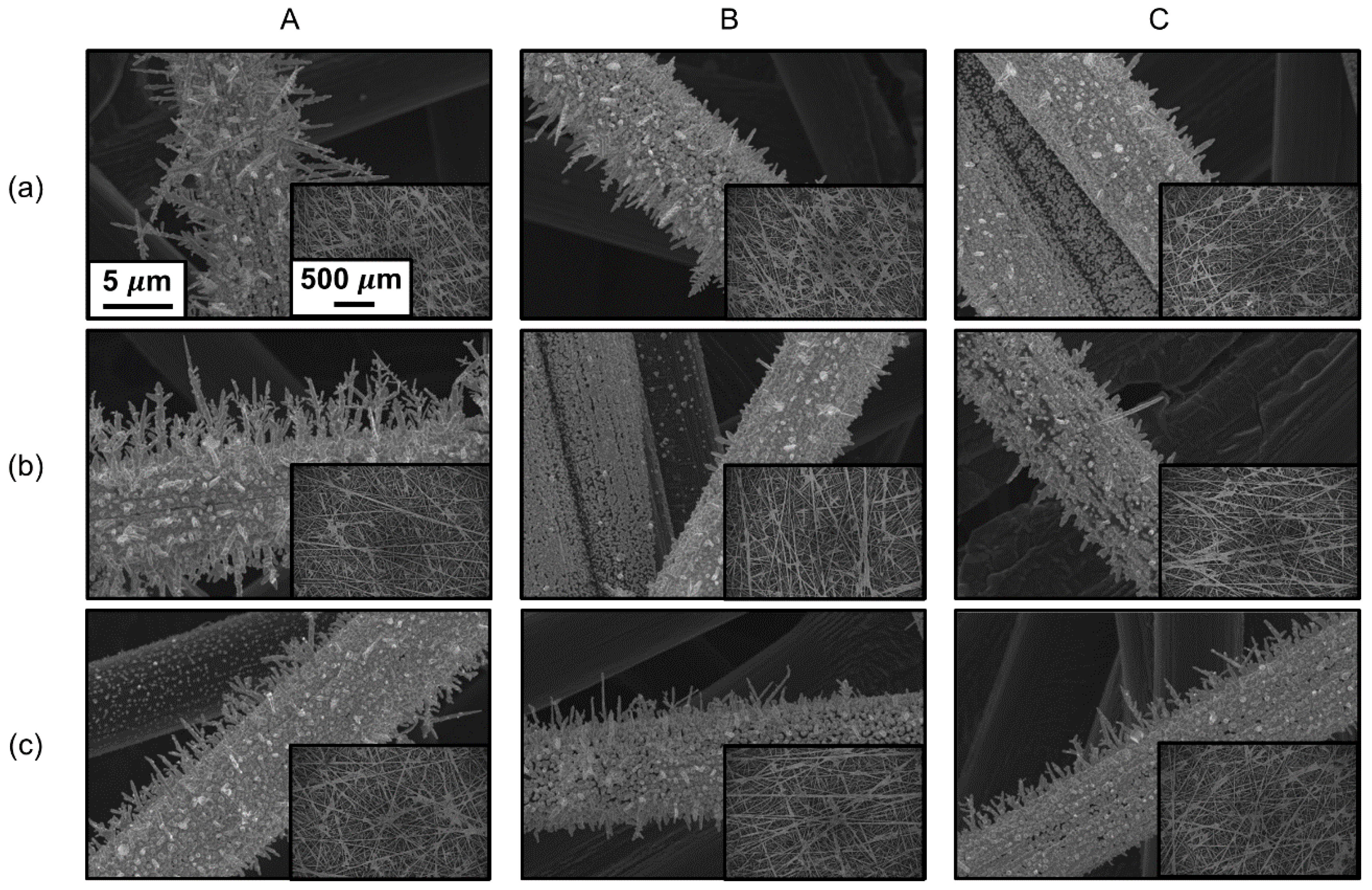
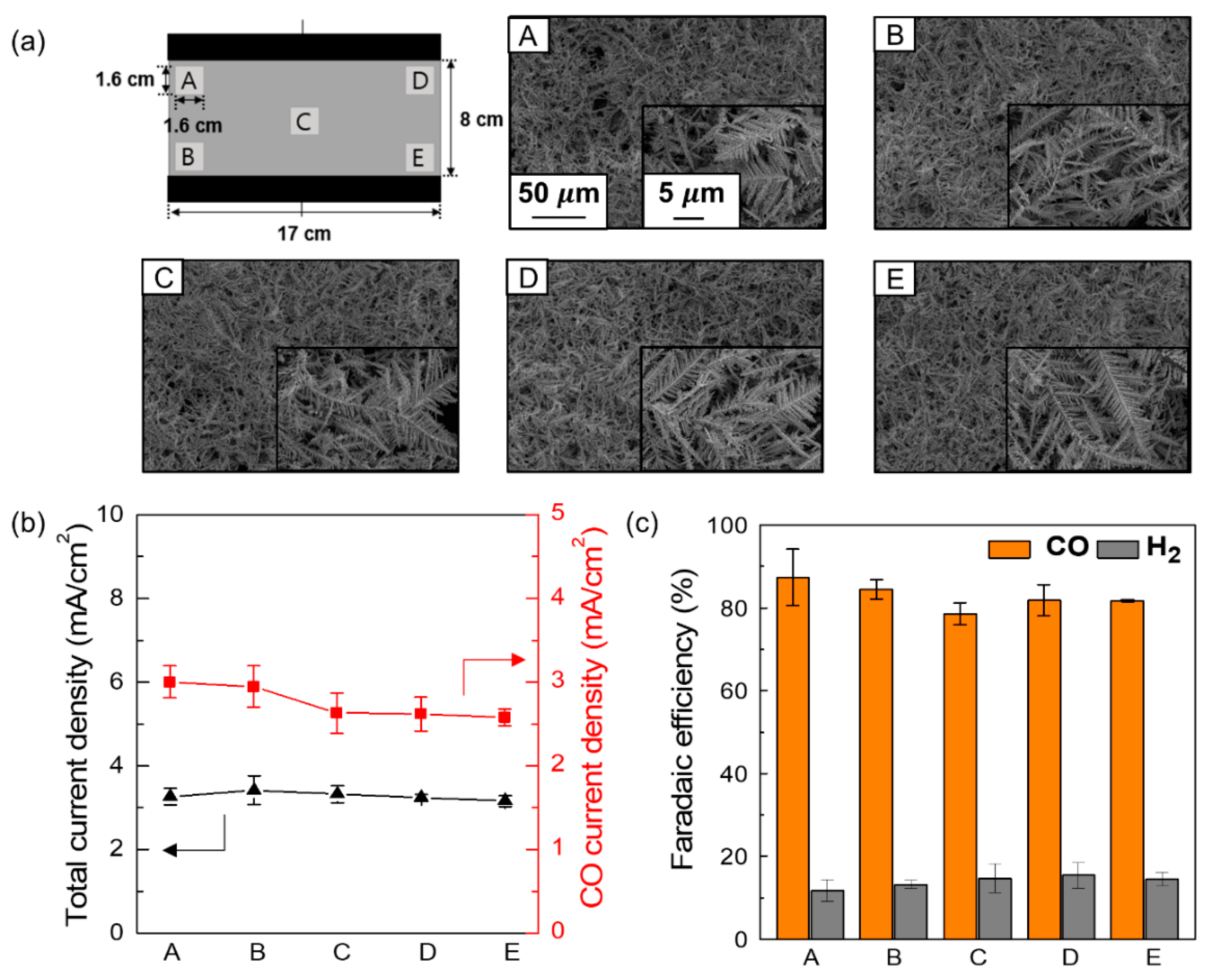
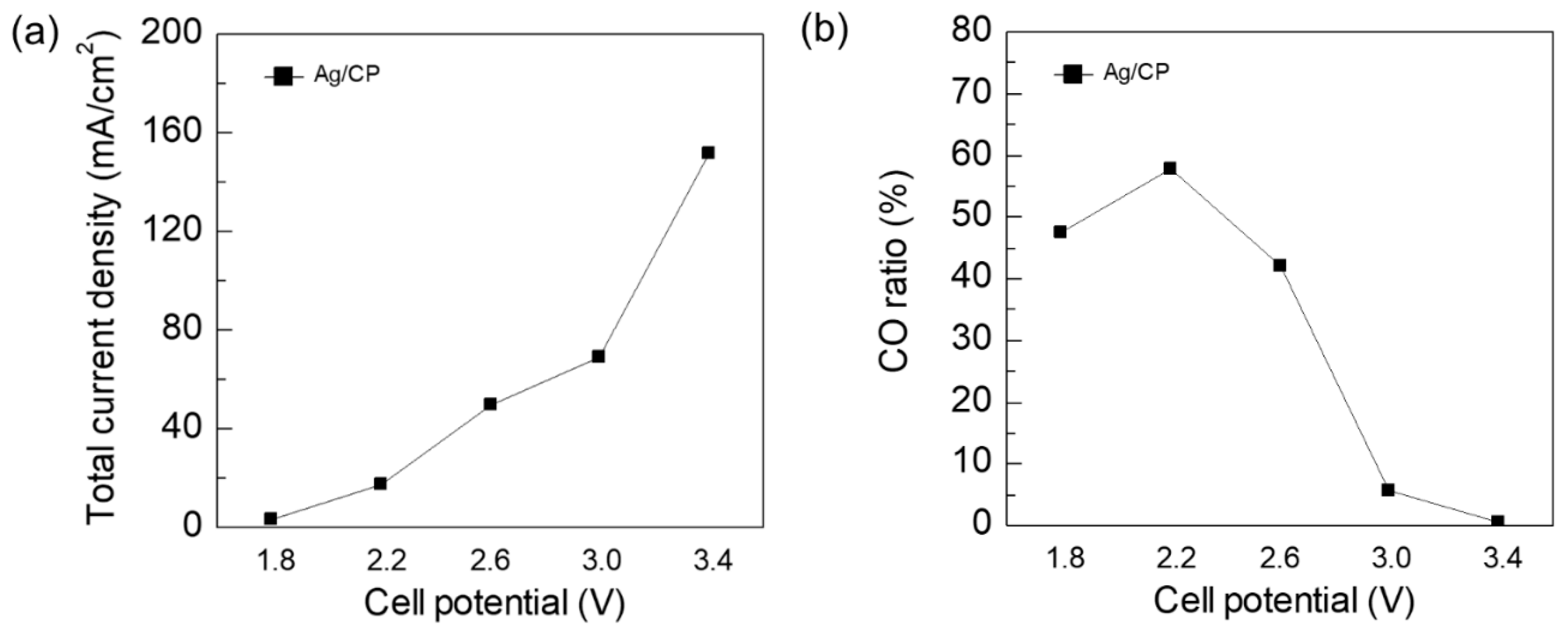
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbek, H.; DuBois, D.L.; Dupuis, M.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.A.; et al. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Llorente, M.; Froehlich, J.; Dang, T.; Sathrum, A.; Kubiak, C.P. Photochemical and Photoelectrochemical Reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Jorgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Mendoza, J.A.; Kim, H.K.; Park, H.K.; Park, K.Y. Photocatalytic reduction of carbon dioxide using Co3O4 nanoparticles under visible light irradiation. Korean J. Chem. Eng. 2012, 29, 1483–1486. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.P.; Ma, X.B.; Gong, J.L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes; Springer: New York, NY, USA, 2008; Volume 42, pp. 89–189. [Google Scholar]
- Singh, M.R.; Kwon, Y.; Lum, Y.; Ager, J.W.; Bell, A.T. Hydrolysis of Electrolyte Cations Enhances the Electrochemical Reduction of CO2 over Ag and Cu. ACS Catal. 2016, 138, 13006–13012. [Google Scholar] [CrossRef]
- Sun, K.; Wu, L.; Qin, W.; Zhou, J.; Hu, Y.; Jiang, Z.; Shen, B.; Wang, Z. Enhanced electrochemical reduction of CO2 to CO on Ag electrocatalysts with increased unoccupied density of states. J. Mater. Chem. A 2016, 4, 12616–12623. [Google Scholar] [CrossRef]
- Hirunsit, P.; Soodsawang, W.; Limtrakul, J. CO2 Electrochemical Reduction to Methane and Methanol on Copper-Based Alloys: Theoretical Insight. J. Phys. Chem. C 2015, 119, 8238–8249. [Google Scholar] [CrossRef]
- Peterson, A.A.; Norskov, J.K. Activity Descriptors for CO2 electroreduction to Methane on Transition-Metal Catalysts. J. Phys. Chem. Lett. 2012, 3, 251–258. [Google Scholar] [CrossRef]
- Chen, C.S.; Wan, J.H.; Yeo, B.S. Electrochemical Reduction of Carbon Dioxide to Ethane Using Nanostructured Cu2O-Derived Copper Catalyst and Palladium(II) Chloride. J. Phys. Chem. C 2015, 119, 26875–26882. [Google Scholar] [CrossRef]
- Yang, K.D.; Ko, W.R.; Lee, J.H.; Kim, S.J.; Lee, H.; Lee, M.H. Morphology-Directed Selective Production of Ethylene of Ethane from CO2 on a Cu Mesopore Electrode. Angew. Chem. Int. Ed. 2016, 56, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Kortlever, R.; Peters, I.; Koper, S.; Kopr, T.M. Electrochemical CO2 reduction to Formic Acid at Low Overpotential and with High Faradaic Efficiency on Carbon-Supported Bimetallic Pd-Pt Nanoparticles. ACS Catal. 2015, 5, 3916–3923. [Google Scholar] [CrossRef]
- Alvarex-Guerra, M.; Quintanilla, S.; Irabien, A. Conversion of carbon dioxide into formate using a continuous electrochemical reduction process in a lead cathode. Chem. Eng. J. 2012, 207–208, 278–284. [Google Scholar] [CrossRef]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Norskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Oh, W.; Rhee, C.K.; Han, J.W.; Shong, B. Atomic and Molecular Adsorption on the Bi(111) Surface: Insights into Catalytic CO2 Reduction. J. Phys. Chem. C 2018, 122, 23084–23090. [Google Scholar] [CrossRef]
- Li, W. Electrocatalytic Reduction of CO2 to Small Organic Molecule Fuels on Metal Catalysts; ACS Publications: Washington, DC, USA, 2010; pp. 55–76. [Google Scholar]
- Keim, W. Carbon monoxide: Feedstock for chemicals, present and future. J. Organomet. Chem. 1989, 372, 15–23. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Katayama, Y.; Nattino, F.; Giordano, L.; Hwang, J.; Rao, R.R.; Andreussi, O.; Marzari, N.; Shao-Horn, Y. An In Situ Surface-Enhanced Infrared Absorption Spectroscopy Study of Electrochemical CO2 Reduction: Selectivity Dependence on Surface C-Bound and O-Bound Reaction Intermediates. J. Phys. Chem. C 2019, 123, 5951–5963. [Google Scholar] [CrossRef]
- Kim, C.; Jeon, H.S.; Eom, T.; Jee, M.S.; Kim, H.; Friend, C.M.; Min, B.K.; Hwang, Y.J. Achieving Selective and Efficient Electrocatalytic Activity for CO2 Reduction Using Immobilized Silver Nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Senanayake, S.D.; Wenqian, Y.Z.; Xu, W.; Polyansky, D.E. Effect of Chloride Anions on the Synthesis and Enhanced Catalytic Activity of Silver Nanocoral Electrodes for CO2 Electroreduction. ACS Catal. 2015, 5, 5349–5356. [Google Scholar] [CrossRef]
- Liu, S.; Tao, H.; Zeng, L.; Liu, Q.; Xu, Z.; Liu, Q.; Luo, J.-L. Shape-Dependent Electrocatalytic Reduction of CO2 to CO on Triangular Silver Nanoplates. J. Am. Chem. Soc. 2017, 139, 2160–2163. [Google Scholar] [CrossRef] [PubMed]
- Back, S.; Yeom, M.S.; Jung, Y. Active Sites of Au and Ag Nanoparticle Catalysts for CO2 Electroreduction to CO. ACS Catal. 2015, 5, 5089–5096. [Google Scholar] [CrossRef]
- Tornow, C.E.; Thorson, M.R.; Ma, S.; Gewirth, A.A.; Kenis, P.J.A. Nitrogen-Based Catalysts for the Electrochemical Reduction of CO2 to CO. J. Am. Chem. Soc. 2012, 134, 19520–19523. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Forest, R.V.; Moore, A.; Jiao, F. Electrodeposited Zn Dendrites with Enhanced CO Selectivity for Electrocatalytic CO2 Reduction. ACS Catal. 2015, 5, 4586–4591. [Google Scholar] [CrossRef]
- Zhu, W.; Michalsky, R.; Metin, O.; Lv, H.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef]
- Luc, W.; Collins, C.; Wang, S.; Xin, H.; He, K.; Kang, Y.; Jiao, F. Ag-Sn Bimetallic Catalyst with a Core-Shell Structure for CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 1885–1893. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. Am improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Dufek, E.J.; Lister, T.E.; Stone, S.G.; Mcllwain, M.E. Operation of a pressurized system for continuous reduction of CO2. J. Electrochem. Soc. 2012, 159, F514–F517. [Google Scholar] [CrossRef]
- Dufek, E.J.; Lister, T.E.; Mcllwain, M.E. Influence of S Contamination on CO2 Reduction at Ag Electrodes. J. Electrochem. Soc. 2011, 158, B1384–B1390. [Google Scholar] [CrossRef]
- Kim, B.; Ma, S.; Jhong, H.-R.M.; Kenis, J.A. Influence of dilute feed and pH on electrochemical reduction of CO2 to CO on Ag in a continuous flow electrolyzer. Electrochim. Acta 2015, 166, 271–276. [Google Scholar] [CrossRef]
- Delacourt, C.; Ridgway, P.L.; Kerr, J.B.; Newman, J. Design of an Electrochemical Cell Making Syngas (CO + H2) from CO2 and H2O Reduction at Room Temperature. J. Electrochem. Soc. 2008, 155, B42–B49. [Google Scholar] [CrossRef]
- Wu, J.; Risalvato, F.G.; Sharma, P.P.; Pellechia, P.J.; Ke, F.S.; Zhou, X.D. Electrochemical Reduction of Carbon Dioxide II. Design, Assembly, and Performance of Low Temperature Full Electrochemical Cells. J. Electrochem. Soc. 2013, 160, F953–F957. [Google Scholar] [CrossRef]
- Ham, Y.S.; Park, Y.S.; Jo, A.; Jang, J.H.; Kim, S.-K.; Kim, J.J. Proton-exchange membrane CO2 electrolyzer for CO production using Ag catalyst directly electrodeposited onto gas diffusion layer. J. Power Sources 2019, 437, 226898. [Google Scholar] [CrossRef]
- Echendu, O.K.; Okeoma, K.B.; Oriaku, C.I.; Dharmadasa, I.M. Electrochemical Deposition of CdTe Semiconductor Thin Films for Solar Cell Application Using Two-Electrode and Three-Electrode Configurations: A Comparative study. Adv. Mater. Sci. Eng. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Rajkumar, S.R.; Alagar, M.; Kanagasabapathy, M. Current Density Distribution Studies in Manifold Dimensions. J. Chem. Pharm. Res. 2012, 4, 1173–1178. [Google Scholar]
- Park, H.; Choi, J.; Kim, H.; Hwang, E.; Ha, D.-H.; Ahn, S.H.; Kim, S.-K. AgIn dendrite catalysts for electrochemical reduction of CO2 to CO. Appl. Catal. B 2017, 219, 123–131. [Google Scholar] [CrossRef]
- Ham, Y.S.; Choe, S.; Kim, M.J.; Lim, T.; Kim, S.-K.; Kim, J.J. Electrodeposited Ag catalysts for the electrochemical reduction of CO2 to CO. Appl. Catal. B 2017, 208, 35–43. [Google Scholar] [CrossRef]
- Oh, S.; Park, Y.S.; Park, H.; Kim, H.; Jang, J.H.; Choi, I.; Kim, S.-K. Ag-deposited Ti gas diffusion electrode in proton exchange membrane CO2 electrolyzer for CO production. J. Ind. Eng. Chem. 2020, 82, 374–382. [Google Scholar] [CrossRef]
- Choi, J.; Kim, M.J.; Ahn, S.H.; Choi, I.; Jang, J.H.; Ham, Y.S.; Kim, J.J.; Kim, S.-K. Electrochemical CO2 reduction to CO on dendritic Ag-Cu electrocatalysts prepared by electrodeposition. Chem. Eng. J. 2016, 299, 37–44. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Park, H.; Kim, H.; Park, Y.S.; Ha, M.G.; Jang, J.H.; Kim, S.-K. Fabrication of Large Area Ag Gas Diffusion Electrode via Electrodeposition for Electrochemical CO2 Reduction. Coatings 2020, 10, 341. https://doi.org/10.3390/coatings10040341
Oh S, Park H, Kim H, Park YS, Ha MG, Jang JH, Kim S-K. Fabrication of Large Area Ag Gas Diffusion Electrode via Electrodeposition for Electrochemical CO2 Reduction. Coatings. 2020; 10(4):341. https://doi.org/10.3390/coatings10040341
Chicago/Turabian StyleOh, Seonhwa, Hyanjoo Park, Hoyoung Kim, Young Sang Park, Min Gwan Ha, Jong Hyun Jang, and Soo-Kil Kim. 2020. "Fabrication of Large Area Ag Gas Diffusion Electrode via Electrodeposition for Electrochemical CO2 Reduction" Coatings 10, no. 4: 341. https://doi.org/10.3390/coatings10040341
APA StyleOh, S., Park, H., Kim, H., Park, Y. S., Ha, M. G., Jang, J. H., & Kim, S.-K. (2020). Fabrication of Large Area Ag Gas Diffusion Electrode via Electrodeposition for Electrochemical CO2 Reduction. Coatings, 10(4), 341. https://doi.org/10.3390/coatings10040341