Zinc/Silver Particle (Zn/AgP) Composite Coatings: Evaluation of Corrosion in Physiological Environments and Antibacterial Activity against P. aeruginosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrodeposition of Zinc/Silver Particle (Zn/AgP) Composites Coatings
2.2. Chemical Composition and Morphological Characterization
2.3. Antibacterial Activity of the Zn/AgP Composite Coatings
2.4. Corrosion Test
2.5. Immersion Test
3. Results
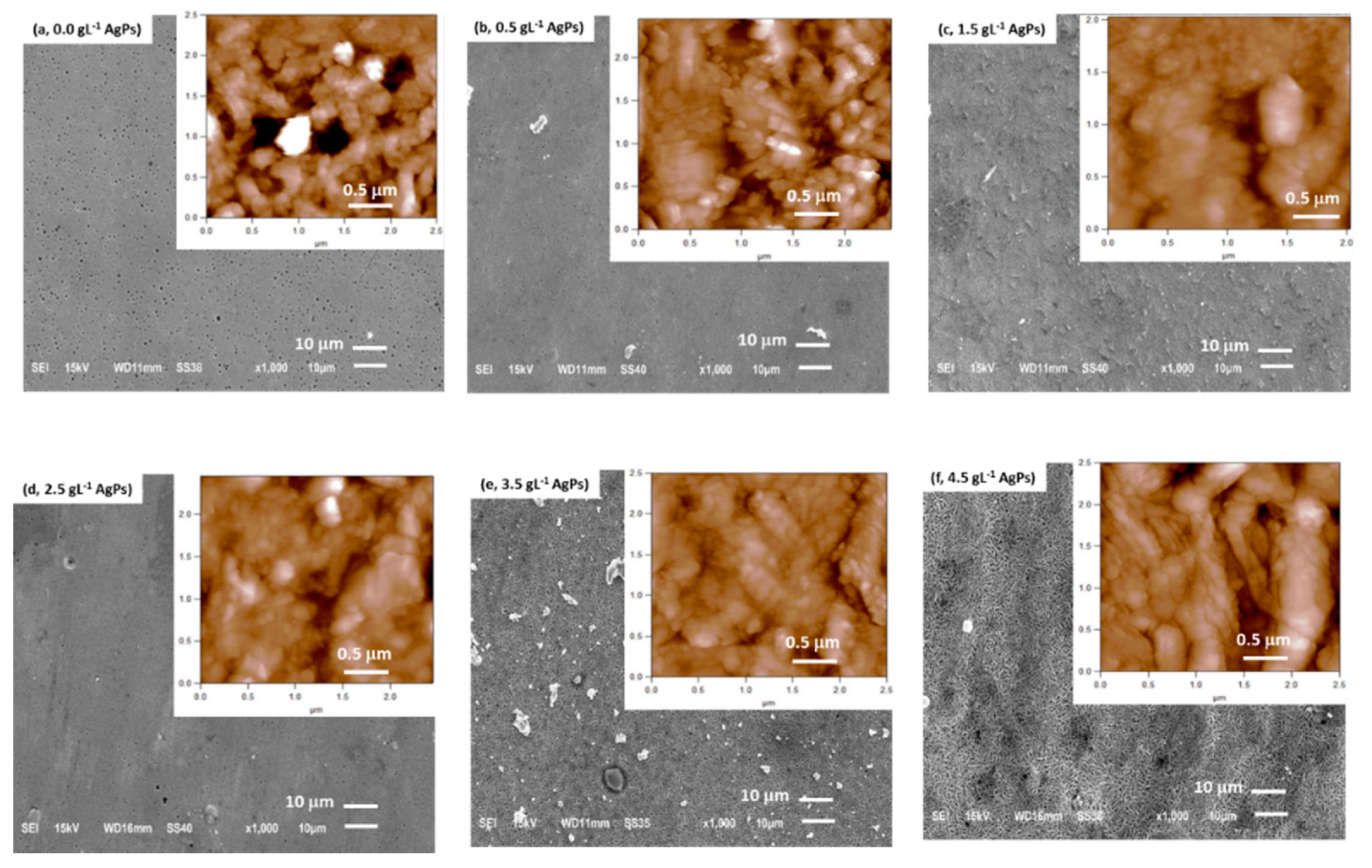
3.1. Chemical Composition and Morphology of Zn/AgP Composite Coatings
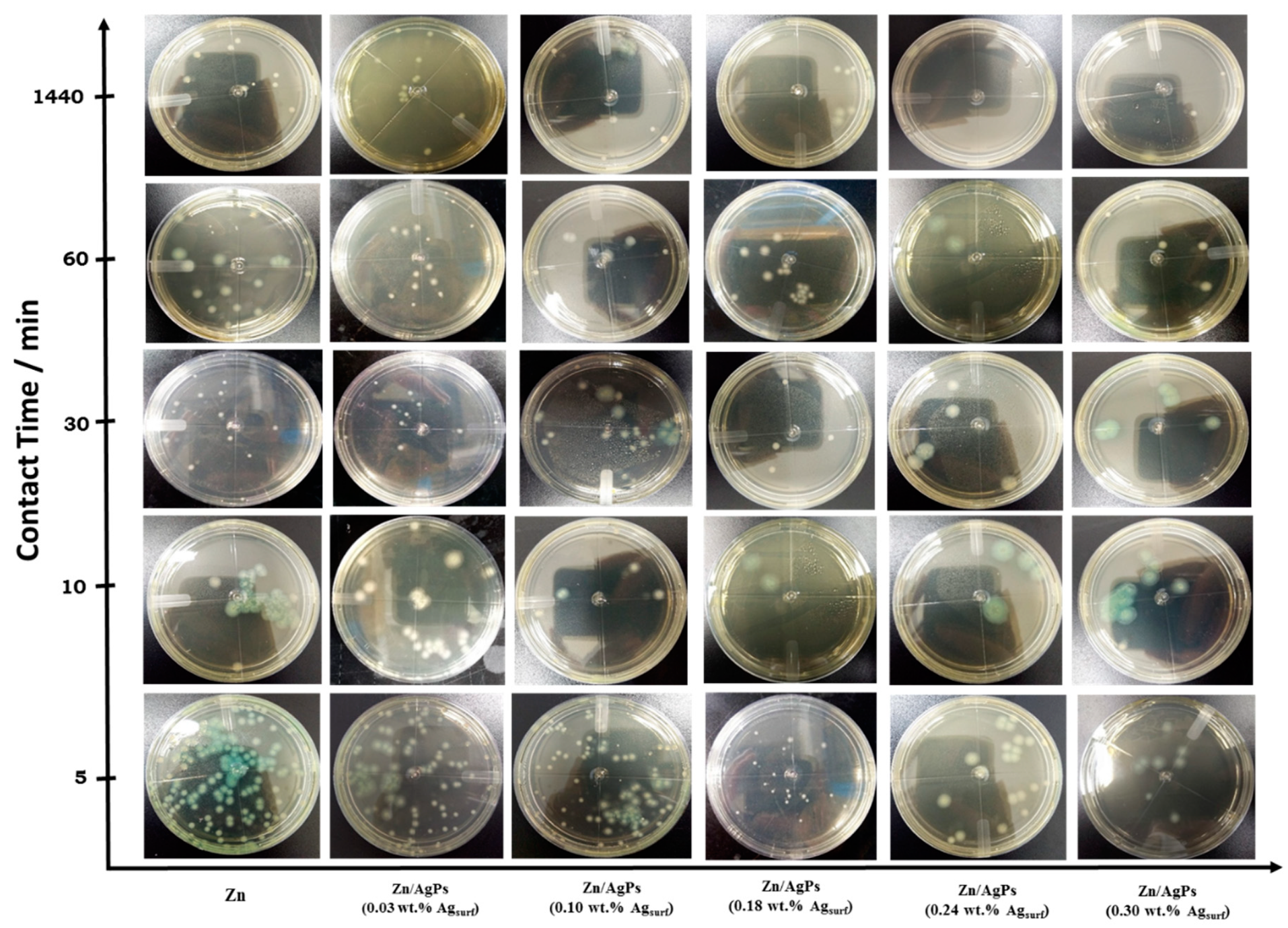
3.2. Antibacterial Activity of the Zn/AgP Composite Coatings
3.3. Corrosion Tests
3.3.1. Polarization Curves
3.3.2. Immersion Test
3.4. Characterization of Corrosion Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative in vitro study on pure metals (Fe, Mn, Mg. Zn and W) as biodegradable metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y. Novel Magnesium alloys developed for biomedical applications: A review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Vojtech, D.; Kubásek, J.; Serák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart 2003, 89, 651–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, A.; Von der Höh, N.; Bormann, D.; Krause, C.; Bach, F.W.; Windhagen, H.; Meyer-Lindenberg, A. Degradation behavior and mechanical properties of magnesium implants in rabbit tibiae. J. Mater. Sci. 2010, 45, 624–632. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.A.; Song, Y.; Zhao, C.L.; Zhang, X.; Xie, C.; Zhang, Y.; Tao, H.; He, Y.; Jiang, Y.; et al. In vitro degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable Mg–Zn alloy. Mater. Sci. Eng. C 2009, 29, 1907–1912. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.; Wang, Y.; Geng, L.; Jiao, X. Preparation and characterization of a new biomedical Mg–Zn–Ca alloy. Mater. Des. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Peuster, M.; Wohlsein, P.; Brugmann, M.; Ehlerding, M.; Seidler, K.; Fink, C. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal—Results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Hermawan, H.; Purnama, A.; Dube, D.; Couet, J.; Montovani, D. Fe–Mn alloys for metallic biodegradable stents: Degradation and cell viability studies. Acta Biomater. 2010, 6, 1852–1860. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable Fe-Mn-C (-Pd) alloys. Mater. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Törne, K.; Larsson, M.; Norlin, A.; Weissenrieder, J. Degradation of zinc in saline solutions, plasma, and whole blood. J. Biomed. Mater. Res. Part B 2016, 104, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioadsorbables stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Tapiro, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Niu, J.; Tang, Z.; Huang, H.; Pei, H.J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents applications. Mater. Sci. Eng. C 2016, 69, 407–413. [Google Scholar] [CrossRef]
- Törne, K.B.; Khan, F.A.; Örberg, A.; Weissenrieder, J. Zn-Mg and Zn-Ag degradation mechanism under biologically relevante conditions. Surf. Innov. 2018, 6, 81–82. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed Zn-Ag alloys for degradable implants applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.G.; Previtali, B.; Mantovani, D.; Beuland, R.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef]
- Hehrlein, C.; Schorch, B.; Kress, N.; Arab, A.; Von zur Mühlen, C.; Bode, C. Zn-alloy provides a novel platform for mechanically stable bioresorbable vascular stents. PLoS ONE. 2019, 14, e0209111. [Google Scholar] [CrossRef]
- Alabbasi, A.; Liyanaarachchi, S.; Kannan, M.B. Polylactic acid coating on a biode-gradable magnesium alloy: An in vitro degradation study by electrochemical impedance spectroscopy. Thin Solid Films 2012, 520, 6841–6844. [Google Scholar] [CrossRef]
- Park, M.; Eun, J.; Chun, L.; Park, G.; Ho, S.; Hyun, L.; Seok, K.; Bin Choy, Y. Polycaprolactone coating with varying thicknesses for controlled corrosion of magnesium. J. Coat. Technol. Res. 2013, 10, 695–706. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. Corrosion behaviour of polypyrrole-coated WE43 Mg alloy in a modified simulated body fluid solution. Corr. Sci. 2018, 133, 261–275. [Google Scholar] [CrossRef]
- Chang, L.; Tian, L.; Liu, W.; Duan, X. Formation of dicalcium phosphate dihydrate on magnesium alloy by micro-arc oxidation coupled with hydrothermal treatment. Corr. Sci. 2013, 72, 118–124. [Google Scholar] [CrossRef]
- da Conceição, T.F.; Scharnagl, N.; Dietzel, W.; Kainer, K.U. Controlled degradation of a magnesium alloy in simulated body fluid using hydrofluoric acid treatment followed by polyacrylonitrile coating. Corr. Sci. 2012, 62, 83–89. [Google Scholar] [CrossRef]
- Rasouli, M.R.; Restrepo, C.; Maltenfort, M.; Purtill, J.J.; Parvizi, J. Risk factors for surgical site infection following total joint arthroplasty. J. Bone Jt. Surg. Am. 2014, 96, e158. [Google Scholar] [CrossRef] [Green Version]
- Hernández, A.; Yagüe, G.; Vázquez, E.G.; Simón, M.; Parrado, L.M.; Canteras, M.; Gómez, J. Infecciones nosocomiales por Pseudomonas aeruginosa multiresistente incluido carbapenémicos: Factores predictivos y pronósticos. Estudio prospectivo 2016–2017. Rev. Esp. Quimioter. 2018, 31, 123–130. [Google Scholar]
- Fazzeli, H.; Akbari, R.; Moghim, S.; Narimani, T.; Arabestani, M.R.; Ghoddousi, A.R. Pseudomonas aeruginosa infections in patients, hospital means, and personnel’s specimens. J. Res. Med. Sci. 2012, 17, 332–337. [Google Scholar]
- ISO 22196. Measurement of antibacterial activity on plastics and other non-porous surfaces. In International Organization for Standardization; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- ASTM E2149-13a. Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions. In American Society for Testing and Materials; ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM G59-97. Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. In American Society for Testing and Materials; ASTM: West Conshohocken, PA, USA, 2014. [Google Scholar]
- NACE TMO169/G31-12a. Standard Guide for laboratory Immersion corrosion testing of metals; NACE: Houston, TE, USA, 2012. [Google Scholar]
- Trejo, G.; Ortega, R.; Meas, Y. The effect of polyethylene glicol 8000 additive on the deposition mechanism and morphology of Zn deposits. Plat. Surf. Finish. 2002, 89, 84–87. [Google Scholar]
- Kazimierczak, H.; Morgiel, J.; Swiatek, Z.; Vega, J.M. Effect of Mo addition on corrosion of Zn coatings electrodeposited on Steel. Corr. Sci. 2018, 135, 107–119. [Google Scholar] [CrossRef]
- Kazimierczak, H.; Szymkiewics, K.; Gileadi, E.; Eliaz, N. The effect of direct and pulsed current in the presence of surfactants on the electrodeposition of Zn-SiC nanocomposite coatings. Coatings 2019, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Albores, A.; González-Arellano, S.G.; Reyes-Vidal, Y.; Torres, J.; Ţălu, Ş.; Cercado, B.; Trejo, G. Electrodeposited chrome/silver nanoparticle (Cr/AgNPs) composite coatings: Characterization and antibacterial activity. J. Alloy. Compd. 2017, 710, 302–311. [Google Scholar] [CrossRef]
- Masaadeh, H.A.; Jaran, A.S. Incident of Pseudomonas aeruginosa in post-operative wound infection. Am. J. Infect. Dis. 2009, 5, 1–6. [Google Scholar] [CrossRef]
- Ohlin, A.; Mattsson, E.; Mörgelin, M.; Davies, J.R.; Esvensâter, G.; Corvec, S.; Tengvall, P.; Riesbeck, K. Titanium granules pre-treated with hydrogen peroxide inhibit growth of bacteria associated with post-operative infections in spine surgery. Eur. Spine J. 2018, 27, 2463–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundy, K.J.; Butler, M.F.; Hochman, R.F. An investigation of the bacteriostatic properties of pure metals. J. Biomed. Mater. Res. 1980, 14, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Xi, H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H.; Huang, L.; Tian, L.; et al. Development of Biodregradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Sankara Narayanan, T.S.N. Corrosion behavior of commercially pure Mg and ZM21 Mg alloy in Ringer’s solution-Long term evaluation by EIS. Corr. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]
- Punith Kumar, M.K.; Srivastava, C. Morphological and electrochemical characterization of electrodeposited Zn-Ag nanoparticle composite coatings. Mater. Charact. 2013, 85, 82–91. [Google Scholar] [CrossRef]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and coloidal silver, a rewiew. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]







| Physiological | Composition (g L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| CaCl2·2H2O | MgCl2·6H2O | KCl | NaHCO3 | NaCl | NaH2PO4 | Glucose | KH2PO4 | |
| Hank’s | 0.265 | 0.214 | 0.2 | 1 | 8 | 0.05 | 1 | - |
| Ringer’s | 0.12 | - | 0.105 | 0.05 | 2.25 | - | - | - |
| PBS | - | - | - | - | - | 17.385 | - | 10.764 |
| AgP Concentration in the Electrollytic Solution/ g L−1 | wt.% Ag Surface Composition (wt.% Agsurf) |
|---|---|
| 0 | 0 |
| 0.5 | 0.03 ± 0.01 |
| 1.5 | 0.10 ± 0.04 |
| 2.5 | 0.18 ± 0.03 |
| 3.5 | 0.24 ± 0.02 |
| 4.5 | 0.30 ± 0.03 |
| Ag Surface Concentration in the Coating (wt.% Agsurf) | Initial ConcentrationP. aeruginosa/CFU mL−1 (×109) | Final Concentration of Pseudomonas aeruginosa/ CFU mL−1 (×109) | ||||
|---|---|---|---|---|---|---|
| Contact Time (min) | ||||||
| 5 | 10 | 30 | 60 | 1440 | ||
| 0.0 | 84 | 79.1 ± 6 | 74.5 ± 1 | 66.5 ± 13 | 61.3 ± 7 | 53.3 ± 11 |
| 0.03 ± 0.02 | 88 | 76.0 ± 8 | 61.2 ± 2 | 57.3 ± 13 | 47.7 ± 13 | 14.2 ± 0.7 |
| 0.10 ± 0.02 | 420 | 150.0 ± 6 | 112.4 ± 4 | 70.2 ± 1.3 | 7.6 ± 1 | 5.8 ± 0.2 |
| 0.18 ± 0.03 | 160 | 45.2 ± 4 | 29.0 ± 3 | 6.7 ± 2.6 | 2.3 ± 0.2 | 2.1 ± 0.1 |
| 0.24 ± 0.03 | 110 | 15.5 ± 1 | 11.8 ± 2 | 3.6 ± 0.5 | 2.9 ± 0.6 | 1.8 ± 0.4 |
| 0.30 ± 0.02 | 450 | 60.2 ± 7 | 38.6 ± 3 | 9.8 ± 1 | 11.2 ± 2 | 7.7 ± 1 |
| Physiological Solution | Coatings | Ecorr (V·vs SCE) | Jcorr (μA·cm−2) | C.R. (mm yr−1) | Vdiss (mg·cm−2·day−1) |
|---|---|---|---|---|---|
| PBS | Zn | −0.827 ± 0.012 | 13.06 ± 2.10 | 0.19 ± 0.03 | 0.37 ± 0.06 |
| Zn/AgP (0.30 wt.% Agsurf) | −0.848 ± 0.010 | 5.18 ± 0.72 | 0.08 ± 0.01 | 0.15 ± 0.02 | |
| Ringer’s | Zn | −0.966 ± 0.010 | 23.02 ± 2.87 | 0.34 ± 0.04 | 0.66 ± 0.08 |
| Zn/AgP (0.30 wt.% Agsurf) | − 0.985 ± 0.012 | 17.77 ± 2.50 | 0.26 ± 0.04 | 0.50 ± 0.08 | |
| Hank’s | Zn | −1.017 ± 0.008 | 18.33 ± 3.13 | 0.27 ± 0.05 | 0.52 ± 0.09 |
| Zn/AgP (0.30 wt.% Agsurf) | −1.032 ± 0.013 | 10.10 ± 2.74 | 0.15 ± 0.04 | 0.29 ± 0.08 |
| Physiological Solution | Coatings | C.R. (mm y−1) | Vdiss (mg cm−2 day−1) |
|---|---|---|---|
| PBS | Zn | 0.10 ± 0.033 | 0.19 ± 0.06 |
| Zn/AgPs (0.30 wt.% Agsurf) | 0.06 ± 0.02 | 0.12 ± 0.03 | |
| Ringer | Zn | 1.07 ± 0.29 | 2.09 ± 0.58 |
| Zn/AgPs (0.30 wt.% Agsurf) | 0.88 ± 0.18 | 1.72 ± 0.35 | |
| Hanks | Zn | 0.55 ± 0.18 | 1.07 ± 0.34 |
| Zn/AgPs (0.30 wt.% Agsurf) | 0.41 ± 0.12 | 0.80 ± 0.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Rodríguez, B.; Terán-López, A.; Reyes-Vidal, Y.; Bácame-Valenzuela, F.J.; Flores, J.G.; Ortega, R.; Mojica, J.; Acosta-Smith, E.; Vázquez-Arenas, J.; Lara, R.H.; et al. Zinc/Silver Particle (Zn/AgP) Composite Coatings: Evaluation of Corrosion in Physiological Environments and Antibacterial Activity against P. aeruginosa. Coatings 2020, 10, 337. https://doi.org/10.3390/coatings10040337
Castro-Rodríguez B, Terán-López A, Reyes-Vidal Y, Bácame-Valenzuela FJ, Flores JG, Ortega R, Mojica J, Acosta-Smith E, Vázquez-Arenas J, Lara RH, et al. Zinc/Silver Particle (Zn/AgP) Composite Coatings: Evaluation of Corrosion in Physiological Environments and Antibacterial Activity against P. aeruginosa. Coatings. 2020; 10(4):337. https://doi.org/10.3390/coatings10040337
Chicago/Turabian StyleCastro-Rodríguez, Berenice, Arnulfo Terán-López, Yolanda Reyes-Vidal, Francisco J. Bácame-Valenzuela, José G. Flores, Raúl Ortega, José Mojica, Erika Acosta-Smith, Jorge Vázquez-Arenas, René H. Lara, and et al. 2020. "Zinc/Silver Particle (Zn/AgP) Composite Coatings: Evaluation of Corrosion in Physiological Environments and Antibacterial Activity against P. aeruginosa" Coatings 10, no. 4: 337. https://doi.org/10.3390/coatings10040337
APA StyleCastro-Rodríguez, B., Terán-López, A., Reyes-Vidal, Y., Bácame-Valenzuela, F. J., Flores, J. G., Ortega, R., Mojica, J., Acosta-Smith, E., Vázquez-Arenas, J., Lara, R. H., & Trejo, G. (2020). Zinc/Silver Particle (Zn/AgP) Composite Coatings: Evaluation of Corrosion in Physiological Environments and Antibacterial Activity against P. aeruginosa. Coatings, 10(4), 337. https://doi.org/10.3390/coatings10040337





