Formulation of Lipoprotein Microencapsulated Beadlets by Ionic Complexes in Algae-Based Carbohydrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of Lipoproteins
- Three phases—mainly a dry part, a paste and a clear juice were obtained in the first step. The organic sea buckthorn fruits seeds and skin remained in the dry part.
- The paste was centrifuged further. The three additional fractions resulted on the second step. The three fractions were called: the first fraction, which was the pellet, or the “butter” fraction; the second fraction called the lipoprotein fraction; the third fraction, called the juicy fraction.
2.3. Fractions Analysis and β-Carotene Quantification
2.3.1. Acidity Analysis
2.3.2. HPLC Analysis
2.3.3. UV-Vis Analysis
2.4. Preparation of Lipoproteins Self-Emulsion
2.5. Evaluation of Lipoproteins Self-Emulsion
2.5.1. Organoleptic Properties
2.5.2. Emulsion Stability
2.5.3. Stability Under Centrifugation
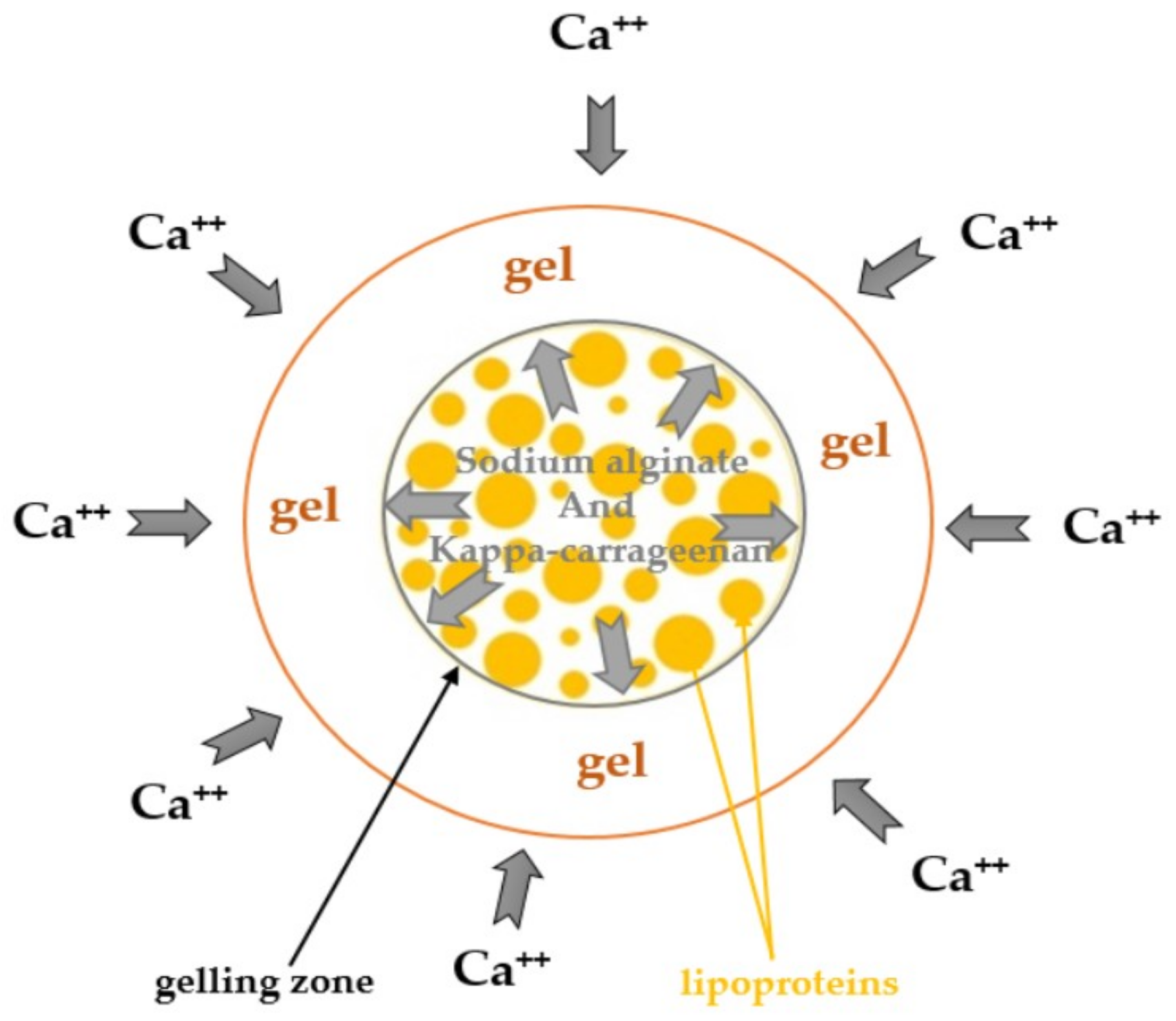
2.6. Microbeadlets and HMPC Capsules Containing Microbeadlets Preparation
2.7. Encapsulation Efficiency
2.8. Analysis of Microbeadlets
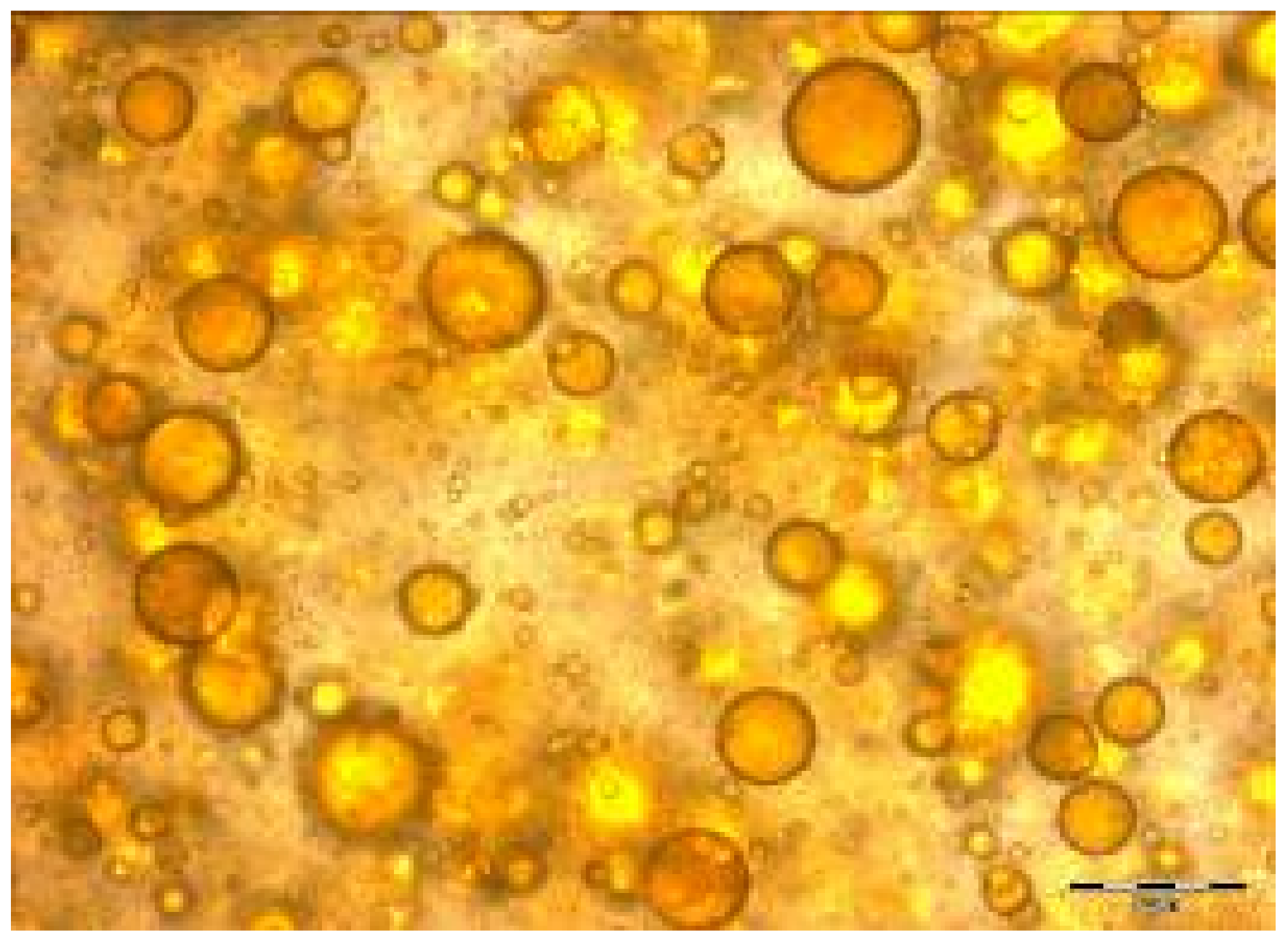
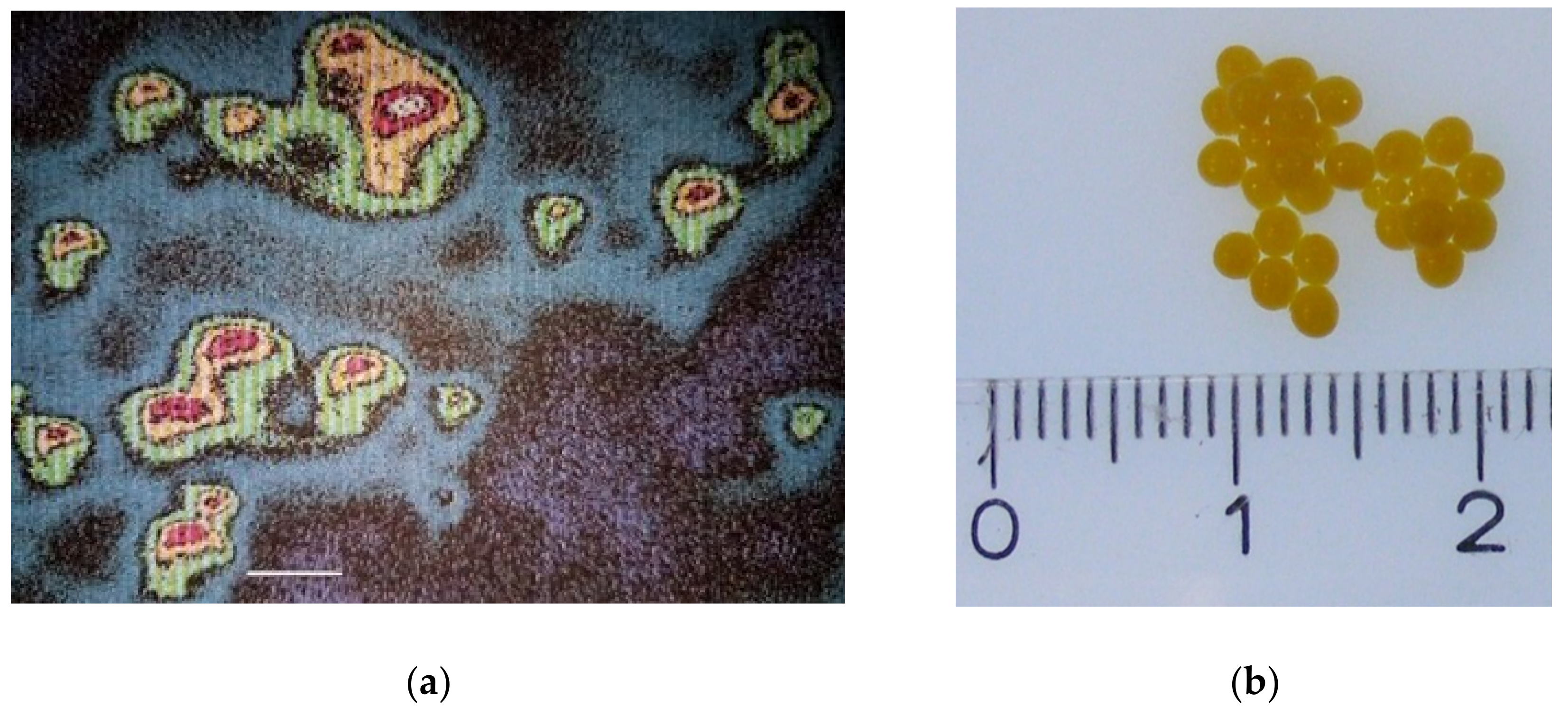
2.8.1. Microscopically
2.8.2. Fluorescence Method
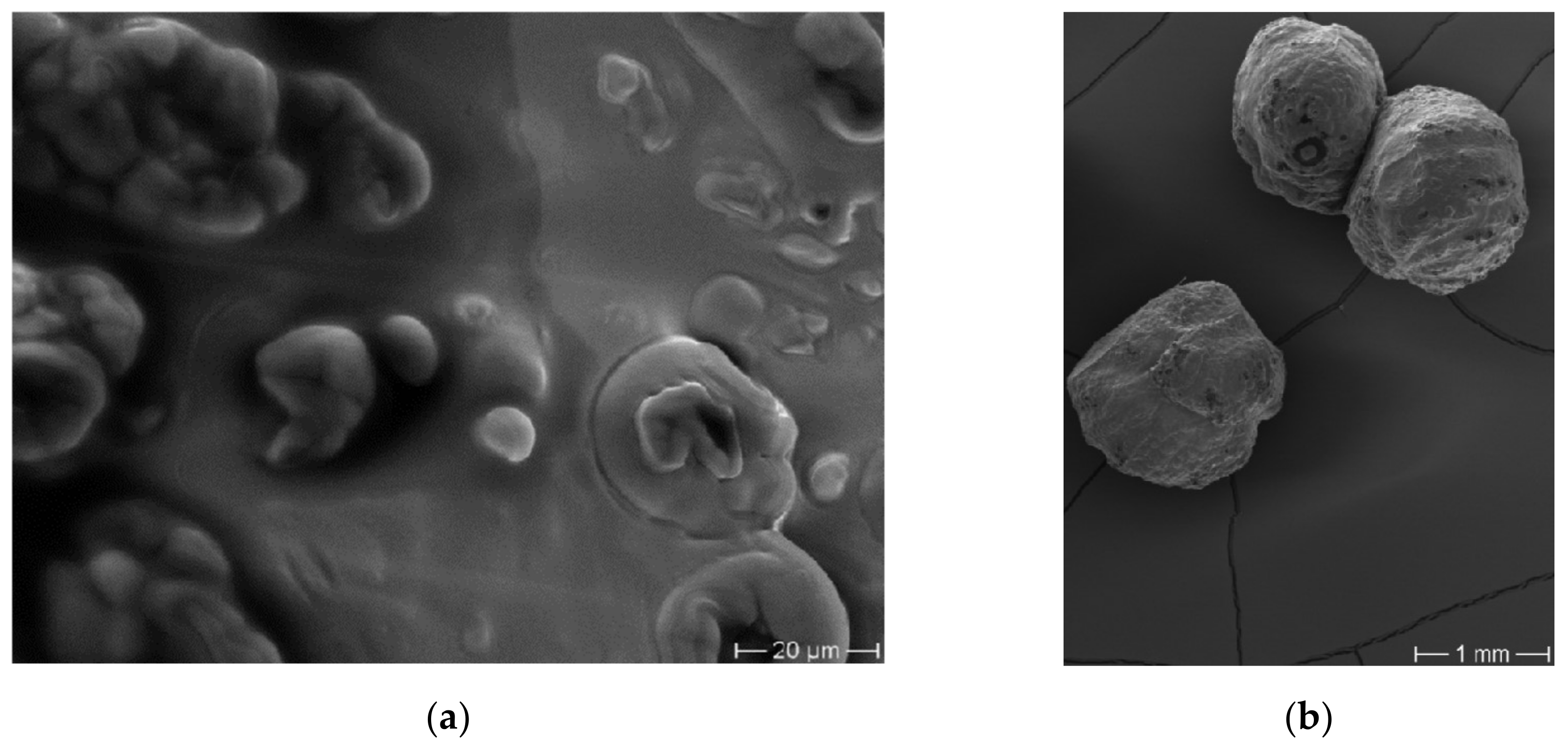
2.8.3. Scanning Electron Microscopy
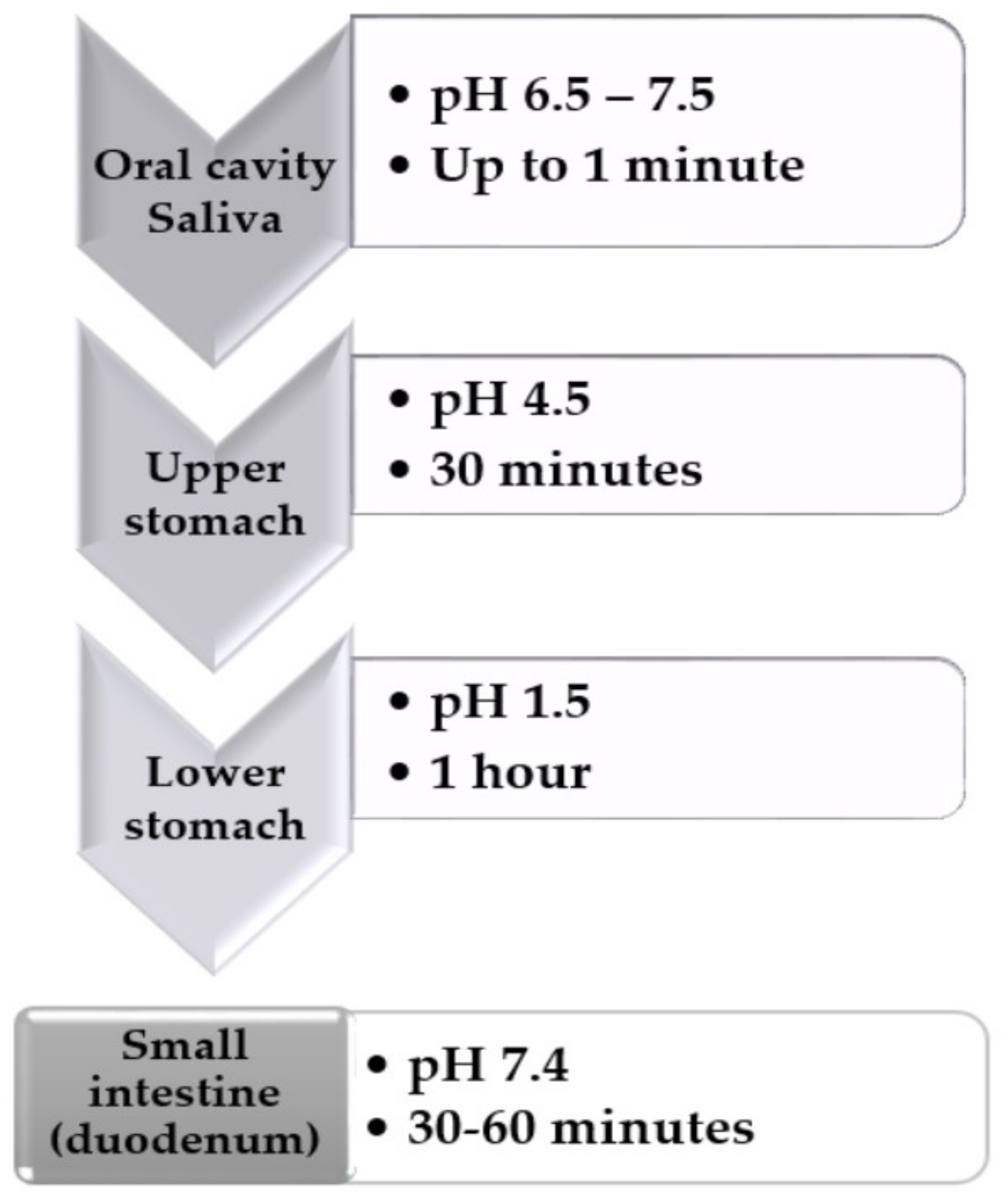
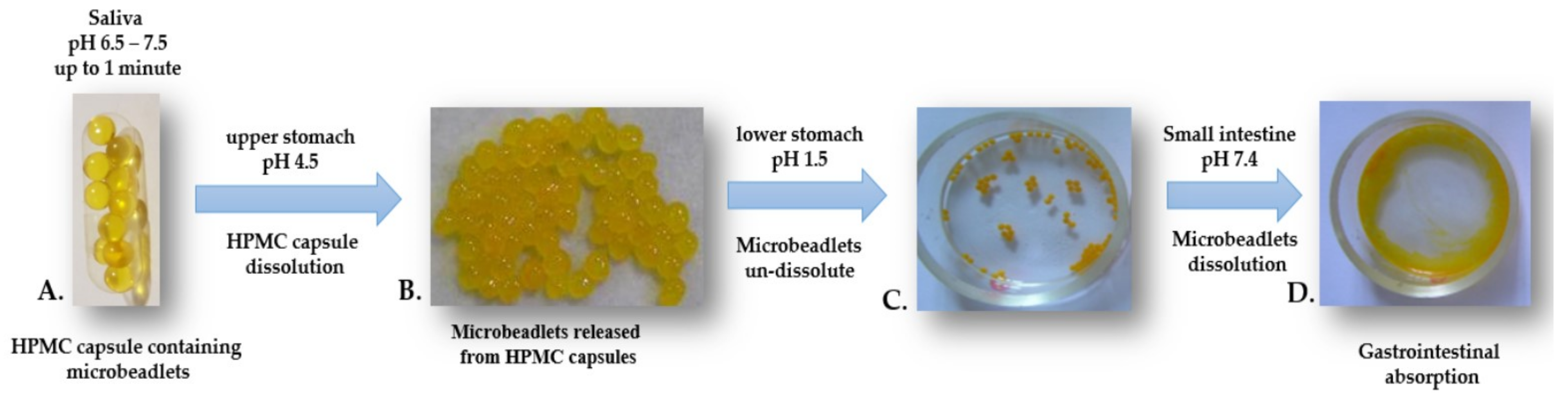
2.9. In Vitro simulation of Gastrointestinal Model
- pH 1.5 - consisted of 0.1 N HCl and 5 mL Sanzyme (enzyme syrup containing 80 mg papain, 40 mg pepsin and 10 mg Sanzyme 2000); pH adjusted to 1.5 ± 0.1.
- pH 4.5 - prepared by mixing SGF pH 1.5 and SIF
- pH 7.4 in a ratio 39:61; pH adjusted to 4.5 ± 0.1. pH 7.4—consisted of KH2PO4 1.074 g in 30 mL of 0.2 N NaOH, and one tablet of Triferment (containing pancreatin 275 mg, equivalent to a minimum enzyme activity of 2970 amylase units, 3720 lipase units and 250 protease units); pH adjusted to 7.4 ± 0.1.
2.10. Statistical Analysis
3. Results
3.1. Characterization of Fractions
3.1.1. Acidity Analysis
3.1.2. Composition of Lipoproteins Fraction Used for Encapsulation by HPLC
3.2. Self Emulsions Characterization
3.3. Characterization of Microbeadlets Containing Lipoproteins Encapsulated
3.3.1. Encapsulation Efficiency (EE%)
3.3.2. Microbeadlets Characterization
3.4. HMPC Capsules Containing Microbeadlets with Lipoproteins
3.5. Stability in Different Simulated Gastrointestinal Fluids under Laboratory Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Irimie, A.I.; Sonea, L.; Jurj, A.; Mehterov, N.; Zimta, A.A.; Budisan, L.; Braicu, C.; Berindan-Neagoe, I. Future trends and emerging issues for nanodelivery systems in oral and oropharyngeal cancer. Int. J. Nanomed. 2017, 26, 4593–4606. [Google Scholar] [CrossRef] [PubMed]
- Trif, M.; Socaciu, C. Evaluation of effiency, release and oxidation stability of seabuckthorn microencapsulated oil using Fourier transformed infrared spectroscopy. Chem. Listy 2008, 102, s1198–s1199. [Google Scholar]
- Zanoaga, O.; Braicu, C.; Jurj, A.; Rusu, A.; Buiga, R.; Berindan-Neagoe, I. Progress in Research on the Role of Flavonoids in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 4291. [Google Scholar] [CrossRef] [PubMed]
- Obasi, T.C.; Moldovan, R.; Toiu, A.; Braicu, C.; Bodoki, E.; Berindan-Neagoe, I.; Oniga, I.; Sandulescu, R.; Oprean, R. Molecular-trapping in Emulsion’s Monolayer: A New Strategy for Production and Purification of Bioactive Saponins. Sci. Rep. 2017, 6, 14511. [Google Scholar] [CrossRef] [PubMed]
- Trif, M.; Vodnar, D.C.; Mitrea, L.; Rusu, A.V.; Socol, C.T. Design and Development of Oleoresins Rich in Carotenoids Coated Microbeads. Coatings 2019, 9, 235. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, P.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2016, 34, 754–771. [Google Scholar] [CrossRef]
- Trif, M.; Csutak, E.; Perez-Moral, N.; Gagyi, T.; Pintori, D.; Bethke, M.; Wilde, P. TeRiFiQ EU Project: Multiple Gel in Oil in Water Emulsions as Fat Replacers in Sauces and Ready Prepared Foods. Bull. UASVM Food Sci. Technol. 2016, 73, 47–48. [Google Scholar] [CrossRef]
- Braicu, C.; Gulei, D.; Raduly, L.; Harangus, A.; Rusu, A.; Berindan-Neagoe, I. Altered expression of miR-181 affects cell fate and targets drug resistance-related mechanisms. Mol. Aspects Med. 2019, 70, 90–105. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Strasdat, B.; Bunjes, H. Incorporation of lipid nanoparticles into calcium alginate beads and characterization of the encapsulated particles by differential scanning calorimetry. Food Hydrocoll. 2013, 30, 567–575. [Google Scholar] [CrossRef]
- Rusu, A.; Randriambelonoro, M.; Perrin, C.; Valk, C.; Alvarez, B.; Schwarze, A.-K. Aspects Influencing Food Intake and Approaches towards Personalising Nutrition in the Elderly. J. Popul. Ageing 2020. [Google Scholar] [CrossRef]
- Trif, M.; Muresan, L.; Bethke, M. Personalised Nutritional Powder for Elderly Developed in OPTIFEL European Project. Bull. UASVM Food Sci. Technol. 2016, 73. [Google Scholar] [CrossRef]
- Qin, Y. Health Benefits of Bioactive Seaweed Substances. Bioact. Seaweeds Food Appl. 2018. [Google Scholar] [CrossRef]
- Henao, E.; Delgado, E.; Contreras, H.; Quintana, G. Polyelectrolyte Complexation versus Ionotropic Gelation for Chitosan-Based Hydrogels with Carboxymethylcellulose, Carboxymethyl Starch, and Alginic Acid. Inter. J. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Pandey, S.P.; Sudheesh, M. Site-specific drug delivery, targeting, and gene therapy. In Nanoarchitectonics in Biomedicine; Elsevier BV: San Diego, CA, USA, 2019; pp. 473–505. [Google Scholar] [CrossRef]
- Braicu, C.; Mehterov, N.; Vladimirov, B.; Sarafian, V.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. Nutrigenomics in cancer: Revisiting the effects of natural compounds. Semin Cancer Biol. 2017, 46, 84–106. [Google Scholar] [CrossRef]
- Harisa, G.I.; Alanazi, F.K. Low density lipoprotein bionanoparticles: From cholesterol transport to delivery of anti-cancer drugs. Saudi Pharm. J. 2014, 22, 504–515. [Google Scholar] [CrossRef]
- Chaudhary, J.; Bower, J.; Corbin, I.R. Lipoprotein Drug Delivery Vehicles for Cancer: Rationale and Reason. Int J. Mol. Sci. 2019, 20, 6327. [Google Scholar] [CrossRef]
- Cojocneanu Petric, R.; Braicu, C.; Raduly, L.; Zanoaga, O.; Dragos, N.; Monroig, P.; Dumitrascu, D.; Berindan-Neagoe, I. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. Onco Targets Ther. 2015, 8, 2053–2066. [Google Scholar] [CrossRef]
- Bindea, M.; Rusu, B.; Rusu, A.; Trif, M.; Leopold, L.F.; Dulf, F.; Vodnar, D.C. Valorification of crude glycerol for pure fractions of docosahexaenoic acid and β-carotene production by using Schizochytrium limacinum and Blakeslea trispora. Microb. Cell Factories 2018, 17, 97. [Google Scholar] [CrossRef]
- Dietrich, T.; Del Carmen Villaran Velasco, M.; Echeverría, P.J.; Pop, B.; Rusu, A. Crop and Plant Biomass as Valuable Material for BBB. Alternatives for Valorization of Green Wastes. In Biotransformation of Agricultural Waste and By-Products: The Food, Feed, Fibre, Fuel (4F) Economy; Elsevier: San Diego, CA, USA, 2016. [Google Scholar]
- Petrut, R.F.; Danthine, S.; Blecker, C. Assessment of partial coalescence in whippable oil-in-water food emulsions. Adv. Colloid Interface Sci. 2016, 229, 25–33. [Google Scholar] [CrossRef]
- Gangurde, A.B.; Amin, P.D. Microencapsulation by Spray Drying of Vitamin A Palmitate from Oil to Powder and Its Application in Topical Delivery System. J. Encapsulation Adsorpt. Sci. 2017, 7, 10–39. [Google Scholar] [CrossRef]
- Patil, A.K.; Gangurde, A.B.; Amin, P.D. Comparative Study of Different Formulations of Aceclofenac as a Topical Drug Delivery System and It’s in Vitro and in Vivo Characterization. Int. J. Pharm. Sci. Res. 2014, 5, 3401. [Google Scholar]
- Yang, K.-M.; Chiang, P.-Y. Preparation and Evaluation of Release Formulation of γ-Oryzanol/Algae Oil Self-Emulsified with Alginate Beads. Mar. Drugs 2019, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnia, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Jamshidi, A.; Mobedi, H. Ionically cross-linked carrageenan-alginate hydrogel beads. J. Biomater. Sci. Polym. Ed. 2008, 19, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Gullapalli, R.P. Dissolution of Liquid-Filled Capsules Based Formulations. Ch. 18 in Poorly Soluble Drugs: Dissolution and Drug Release. In PanStanford Series on Pharmaceutical Analysis; Webster, G.K., Jackson, J.D., Bell, R.G., Eds.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2017. [Google Scholar]
- Sutapa, M. HPMC as capsule shell material: Physicochemical, pharmaceutical and biopharmaceutical and biopharmaceutical properties. Int. J. Pharm. Pharm. Sci. 2017, 9. [Google Scholar] [CrossRef]
- Chiwele, I.; Jones, B.E.; Podczeck, F. The shell dissolution of various empty hard capsules. Chem. Pharm. Bull. (Tokyo) 2000, 48, 951–956. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Kyomugasho, C.; Salvia-Trujillo, L.; Denis, S.; Bourgeois, M.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion stabilizing properties of citrus pectin and its interactions with conventional emulsifiers in oil-in-water emulsions. Food Hydrocoll. 2018, 85, 144–157. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. (Eds.) Carotenoids Volume 5: Nutrition and Health; Birkhäuser: Basel, Switzerland, 2009. [Google Scholar] [CrossRef]
- Fuller, C.J.; Butterfoss, M.S.; Failla, M.L. Relative bioavailability of betacarotene from supplement sources. Nutr. Res. 2001, 21, 1209–1215. [Google Scholar] [CrossRef]
- Jayant, D.; Laxman, K.; Prakash, B. Stable Oil Suspensions Comprising Lipophilic Nutrient with Enhanced Bioavailability and Process of Preparation. WO2016083874A1, 2 June 2016. [Google Scholar]
- Sanidad, K.Z.; Zhu, J.; Wang, W.; Du, Y.; Zhang, G. Effects of Stable Degradation Products of Curcumin on Cancer Cell Proliferation and Inflammation. J. Agric. Food Chem. 2016, 64, 9189–9195. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 22, 1618. [Google Scholar] [CrossRef] [PubMed]
- Jutkova, A.; Chorvat, D.; Miskovsky, P.; Jancura, D.; Datta, S. Encapsulation of anticancer drug curcumin and co-loading with photosensitizer hypericin into lipoproteins investigated by fluorescence resonance energy transfer. Int. J. Pharm. 2019, 564, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.R.; Macierzanka, A.; Aarak, K.; Rigby, N.M.; Parker, R.; Channell, G.A.; Harding, S.E.; Bajka, B.H. Sodium alginate decreases the permeability of intestinal mucus. Food Hydrocoll. 2016, 52, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Dettmar, P.W.; Strugala, V.; Craig Richardson, J. The key role alginates play in health. Food Hydrocoll. 2011, 25, 263–266. [Google Scholar] [CrossRef]
- Qin, Y. (Ed.) 9-Health Benefits of Bioactive Seaweed Substances. In Bioactive Seaweeds for Food Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 179–200. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petrut, R.; Olive, G.; Anastas, P.; Blecker, C.; Richel, A. Structure impact of two galactomannan fractions on their viscosity properties in dilute solution, unperturbed state and gel state. Int. J. Biol. Macromol. 2017, 96, 550–559. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Szabo, K.; Teleky, B.-E.; Mureșan, V.; Rusu, A.-V.; Socol, C.-T.; Vodnar, D.-C. Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products. Polymers 2020, 12, 532. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Mierlita, D. Fatty acids profile and oxidative stability of eggs from laying hens fed diets containing hemp seed or hempseed cake. S. Afr. J. Anim. Sci. 2019, 49. [Google Scholar] [CrossRef]
- Forstner, S.; Rusu, A. Development of personalised food for the nutrition of elderly consumers. In Know Your Food: Food Ethics and Innovation; Academic Publishers: Wageningen, The Netherlands, 2015; pp. 24–27. [Google Scholar] [CrossRef]
- Criste, F.L.; Mierlita, D.; Simeanu, D.; Boisteanu, P.C.; Pop, I.M.; Georgescu, B.; Nacu, G. Study of Fatty Acids Profile and Oxidative Stability of Egg Yolk from Hens Fed a Diet Containing White Lupine Seeds Meal. Revista Chim. 2018, 69, 2454–2460. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, A.V.; Criste, F.L.; Mierliţă, D.; Socol, C.T.; Trif, M. Formulation of Lipoprotein Microencapsulated Beadlets by Ionic Complexes in Algae-Based Carbohydrates. Coatings 2020, 10, 302. https://doi.org/10.3390/coatings10030302
Rusu AV, Criste FL, Mierliţă D, Socol CT, Trif M. Formulation of Lipoprotein Microencapsulated Beadlets by Ionic Complexes in Algae-Based Carbohydrates. Coatings. 2020; 10(3):302. https://doi.org/10.3390/coatings10030302
Chicago/Turabian StyleRusu, Alexandru Vasile, Florin Leontin Criste, Daniel Mierliţă, Claudia Terezia Socol, and Monica Trif. 2020. "Formulation of Lipoprotein Microencapsulated Beadlets by Ionic Complexes in Algae-Based Carbohydrates" Coatings 10, no. 3: 302. https://doi.org/10.3390/coatings10030302
APA StyleRusu, A. V., Criste, F. L., Mierliţă, D., Socol, C. T., & Trif, M. (2020). Formulation of Lipoprotein Microencapsulated Beadlets by Ionic Complexes in Algae-Based Carbohydrates. Coatings, 10(3), 302. https://doi.org/10.3390/coatings10030302






