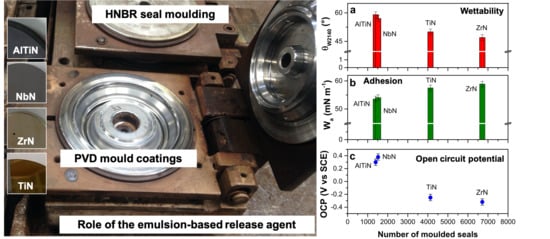
Insight into the Release Agents/PVD Coatings Interaction for Plastic Mold Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. PVD Coatings
2.2. Mold Release Agents
2.3. Interaction Releasing Agents-PVD Coatings Analyses
2.4. Industrial Molding Set-Up
3. Results and Discussion
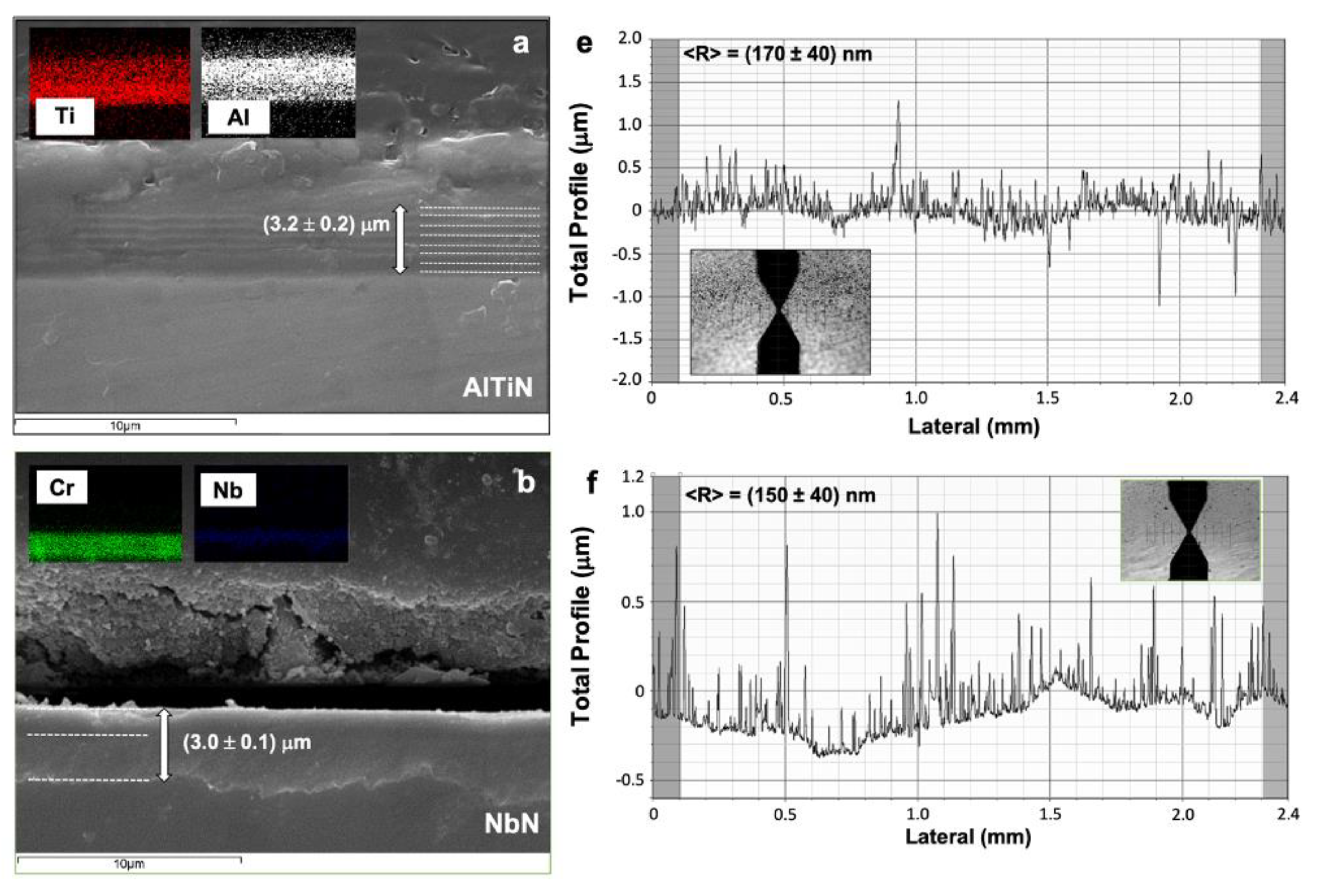
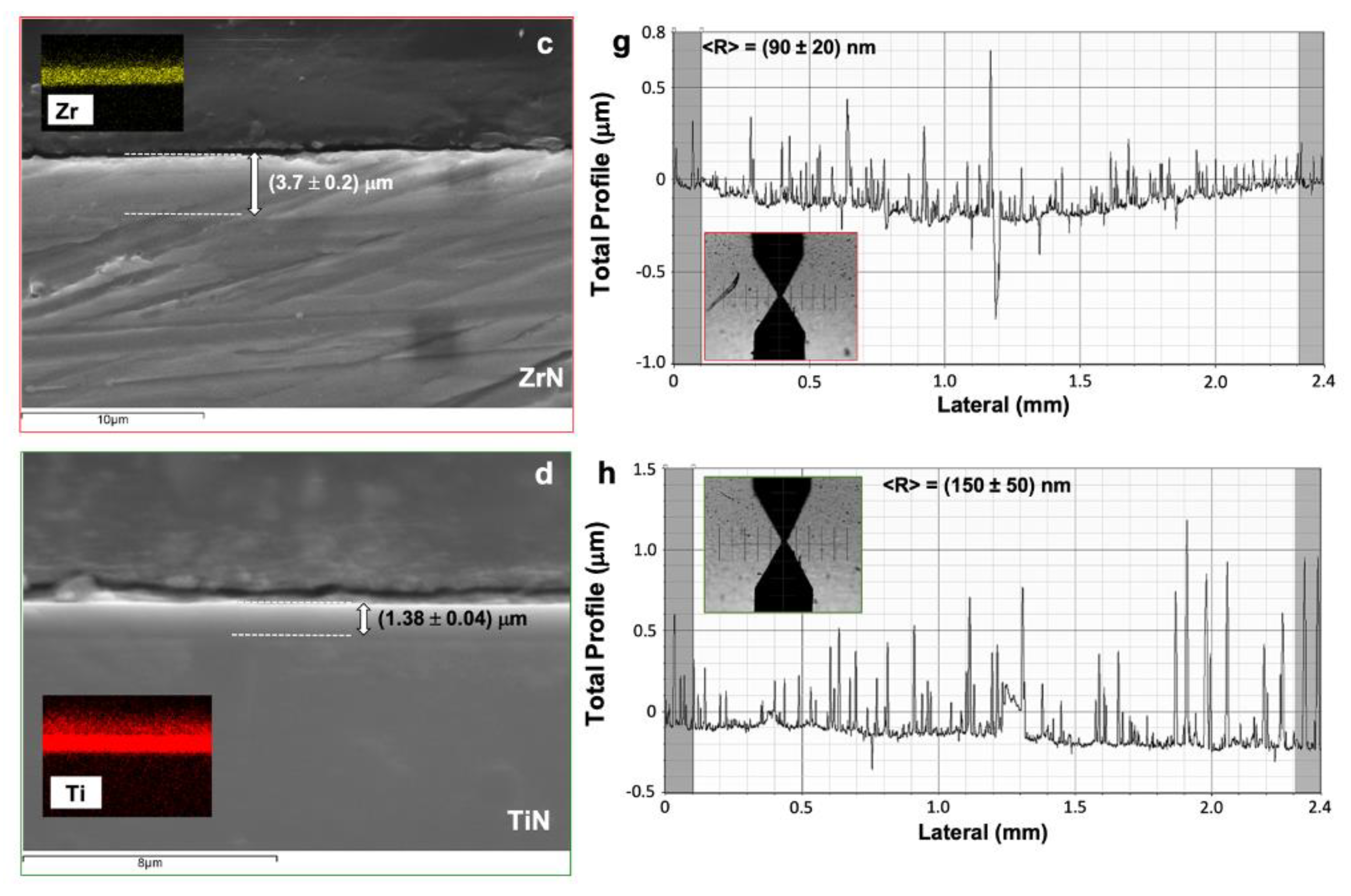
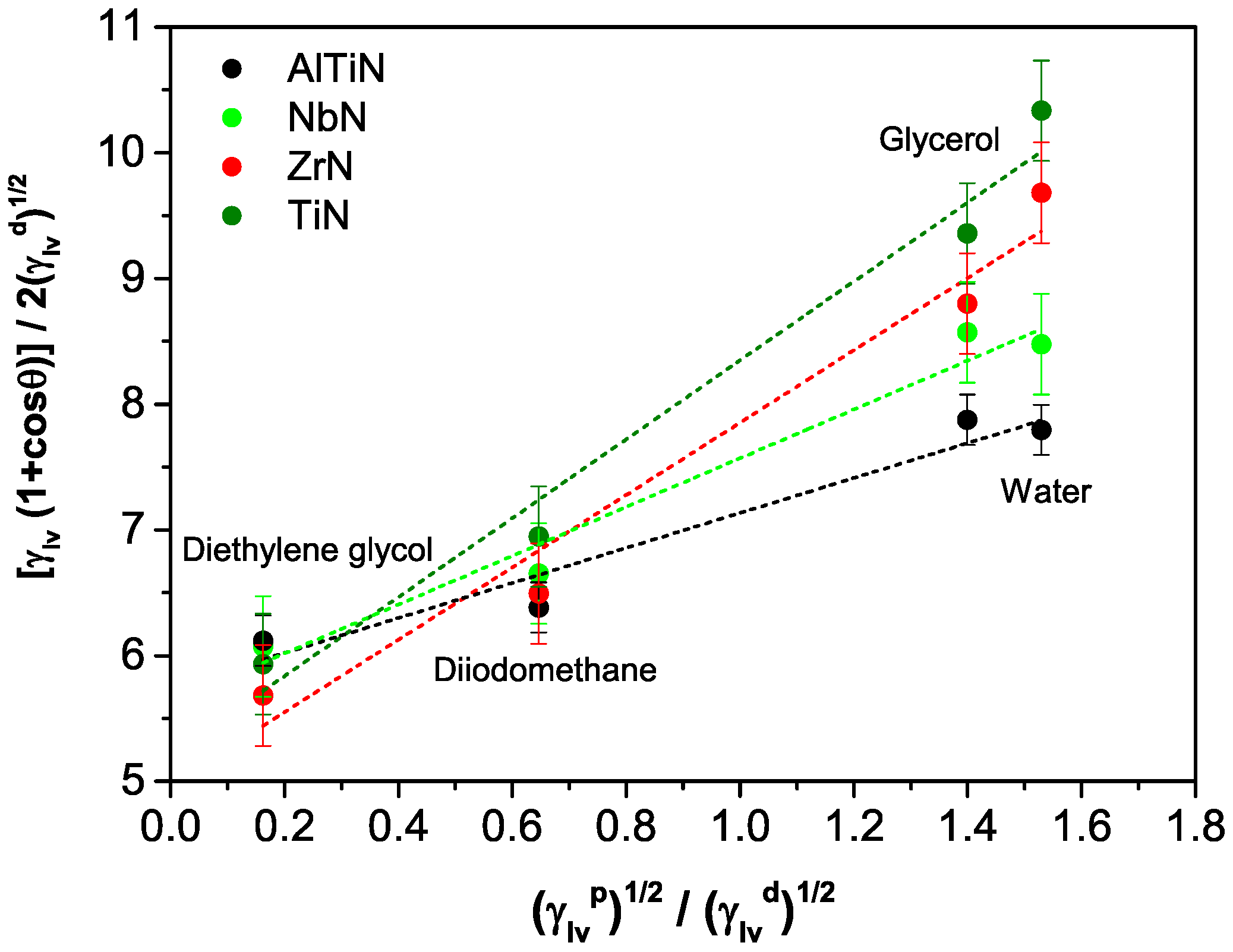
3.1. PVD Coatings Properties
3.2. Release Agents Characterization
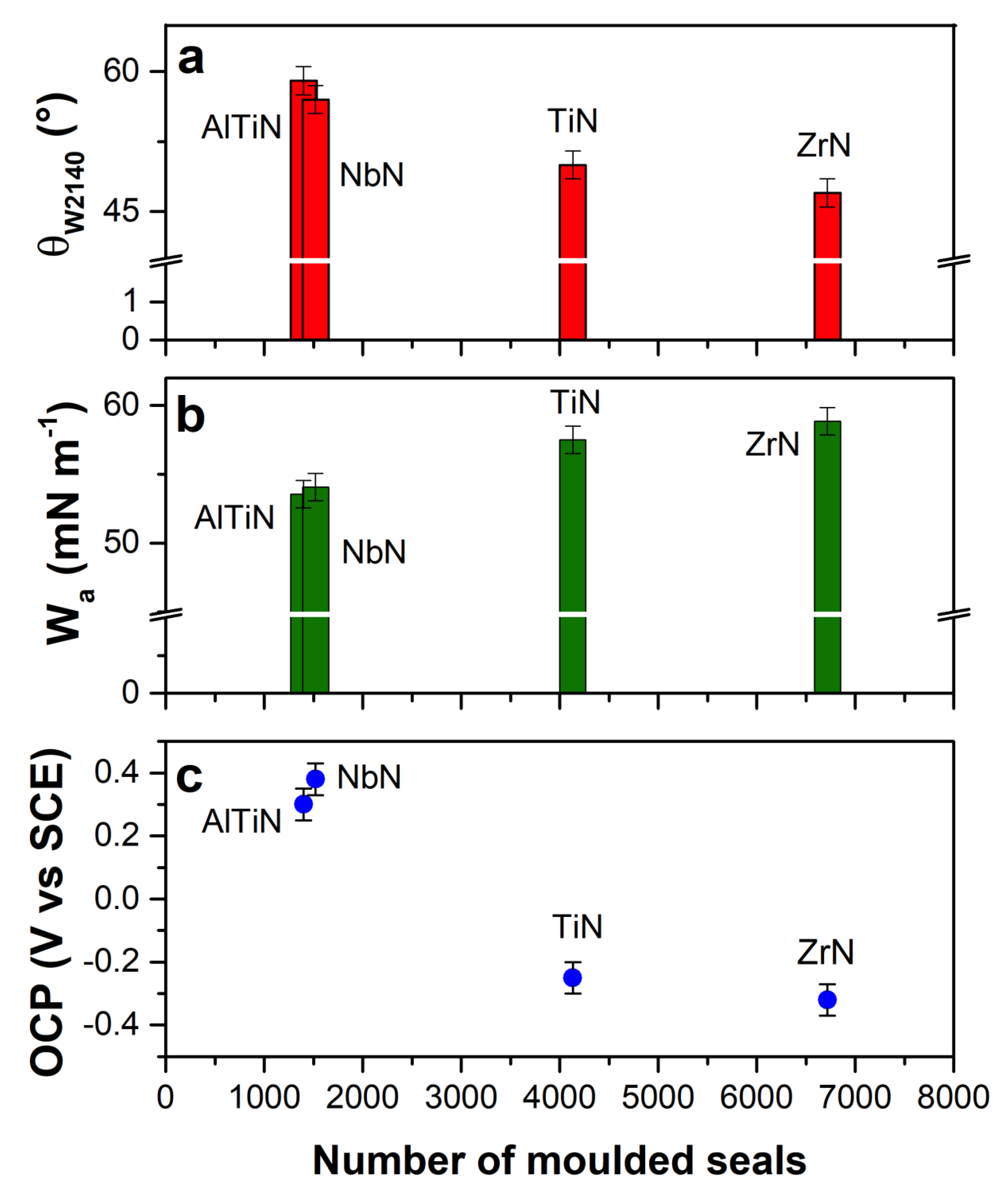
3.3. Work of Adhesion (Wa) and Spreading Coefficient (S)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kutz, M. Applied Plastics Engineering Handbook, 2nd ed.; Elsevier Inc.: Oxford, UK, 2016; ISBN 9780323390408. [Google Scholar]
- Meyer, R.W. Handbook of Polyester Molding Compounds and Molding Technology, 2nd ed.; Chapman and Hall: London, UK, 2012; ISBN 0-412-00771-1. [Google Scholar]
- Pruner, H.; Nesch, W. Understanding Injection Molds; Carl Hanser Verlag: Munich, Germany, 2013; ISBN 978-1-56990-527-2. [Google Scholar]
- Navabpour, P.; Teer, D.G.; Hitt, D.J.; Gilbert, M. Evaluation of non-stick properties of magnetron-sputtered coatings for molds used for the processing of polymers. Surf. Coat. Technol. 2006, 201, 3802–3809. [Google Scholar] [CrossRef]
- Lugscheider, E.; Bobzin, K.; Möller, M. The Effect of PVD layer constitution on surface free energy. Thin Solid Films 1999, 355, 367–373. [Google Scholar] [CrossRef]
- Sun, C.C.; Lee, S.C.; Dai, S.B.; Tien, S.L.; Chang, C.C.; Fu, Y.S. Surface free energy of non-stick coatings deposited using closed field unbalanced magnetron sputter ion plating. Appl. Surf. Sci. 2007, 253, 4094–4098. [Google Scholar] [CrossRef]
- Hornsby, P.R.; Singh, I.; Daley, J.R.; Firth, J. Mold fouling of elastomers during injection molding. Plast. Rubber Compos. 2006, 35, 331–339. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, S.; Lu, T.; Li, R.; Zhong, T.; Zhu, X. Design and synthesis of ethoxylated esters derived from waste frying oil as anti-ultraviolet and efficient primary plasticizers for poly(vinyl chloride). J. Clean. Prod. 2019, 229, 1274–1282. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Plasticizers, 3rd ed.; ChemTec Publishing: Scarborough, ON, Canada, 2017; ISBN 978-1-895198-97-3. [Google Scholar]
- Wesala, R.J. Mold Release Agents and Means of Application. U.S. Patent 4,491,607, 1 January 1985. [Google Scholar]
- Schneider, A.; Schneider, V.; Wochnowski, H.; Niemeyer, P. Release Agent. U.S. Patent 6,162,290, 19 December 2000. [Google Scholar]
- Olietti, A.; Pargoletti, E.; Diona, A.; Cappelletti, G. A novel optimized mold release oil-in-water emulsion for polyurethane foams production. J. Mol. Liq. 2018, 261, 199–207. [Google Scholar] [CrossRef]
- Althoff, R.; Henning, T.; Lammerting, H. Aqueous Release Agent and Its Use in the Production of Polyurethane Moldings. U.S. Patent 7,811,502, 12 October 2010. [Google Scholar]
- D’Avico, L.; Beltrami, R.; Lecis, N.; Trasatti, S. Corrosion Behavior and Surface Properties of PVD Coatings for Mold Technology Applications. Coatings 2018, 9, 7. [Google Scholar] [CrossRef]
- Shugurov, A.R.; Kazachenok, M.S. Mechanical properties and tribological behavior of magnetron sputtered TiAlN/TiAl multilayer coatings. Surf. Coat. Technol. 2018, 353, 254–262. [Google Scholar] [CrossRef]
- Holleck, H.; Schier, V. Multilayer PVD coatings for wear protection. Surf. Coat. Technol. 1995, 76–77, 328–336. [Google Scholar] [CrossRef]
- Sproul, W.D. Physical vapor deposition tool coatings. Surf. Coat. Technol. 1996, 81, 1–7. [Google Scholar] [CrossRef]
- Mo, J.L.; Zhu, M.H.; Lei, B.; Leng, Y.X.; Huang, N. Comparison of tribological behaviours of AlCrN and TiAlN coatings—Deposited by physical vapor deposition. Wear 2007, 263, 1423–1429. [Google Scholar] [CrossRef]
- Lotierzo, A.; Pifferi, V.; Ardizzone, S.; Pasqualin, P.; Cappelletti, G. Insight into the role of amines in Metal Working Fluids. Corros. Sci. 2016, 110, 192–199. [Google Scholar] [CrossRef]
- Rosen, M.J. Wetting and Its Modification by Surfactants. In Surfactants Interfacial Phenom; John Wiley & Sons Inc.: New York, NY, USA, 1978; pp. 174–199. [Google Scholar]
- Meroni, D.; Ardizzone, S.; Cappelletti, G.; Ceotto, M.; Ratti, M.; Annunziata, R.; Benaglia, M.; Raimondi, L. Interplay between Chemistry and Texture in Hydrophobic TiO2 Hybrids. J. Phys. Chem. C 2011, 115, 18649–18658. [Google Scholar] [CrossRef]
- Soliveri, G.; Pifferi, V.; Panzarasa, G.; Ardizzone, S.; Cappelletti, G.; Meroni, D.; Sparnacci, K.; Falciola, L. Self-cleaning properties in engineered sensors for dopamine electroanalytical detection. Analyst 2015, 140, 1486–1494. [Google Scholar] [CrossRef]
- Macy, R. Surface tension by the ring method. Applicability of the du Nouy apparatus. J. Chem. Educ. 1935, 12, 573. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Pargoletti, E.; Motta, L.; Comite, V.; Fermo, P.; Cappelletti, G. The hydrophobicity modulation of glass and marble materials by different Si-based coatings. Prog. Org. Coat. 2019, 136, 105260. [Google Scholar] [CrossRef]
- Keller, R. Practical Guide to Hydrogenated Nitrile Butadiene Rubber Technology Practical Guide to Hydrogenated Nitrile Butadiene Rubber Technology; Smithers Rapra: Akron, OH, USA, 2012; ISBN 978-1-84735-521-8. [Google Scholar]
- Lakel, S.; Almi, K.; Berriche, Y. Micro-scale abrasive wear testing of Cr-Nx coatings. Rom. Rep. Phys. 2007, 59, 113. [Google Scholar]
- Cappelletti, G.; Ardizzone, S.; Meroni, D.; Soliveri, G.; Ceotto, M.; Biaggi, C.; Benaglia, M.; Raimondi, L. Wettability of bare and fluorinated silanes: A combined approach based on surface free energy evaluations and dipole moment calculations. J. Colloid Interface Sci. 2013, 389, 284–291. [Google Scholar] [CrossRef]
- Lugscheider, E.; Bobzin, K.U. The influence on surface free energy of PVD coatings. Surf. Coat. Technol. 2001, 142, 755–760. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Z.; Xing, W.; Bao, L.; Wang, P.; Xu, J.; Zhang, Y. A Release Agent, the Preparation and Use Thereof. WO2012103808A1, 9 August 2012. [Google Scholar]
- Cambiella, A.; Benito, J.M.; Pazos, C.; Coca, J.; Ratoi, M.; Spikes, H.A. The effect of emulsifier concentration on the lubricating properties of oil-in-water emulsions. Tribol. Lett. 2006, 22, 53–65. [Google Scholar] [CrossRef]
- Mollet, H.; Grubernmann, A. Formulation Technology; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Arslan, E.; Totik, Y.; Efeoglu, I. The investigation of the tribocorrosion properties of DLC coatings deposited on Ti6Al4V alloys by CFUBMS. Prog. Org. Coat. 2012, 74, 768–771. [Google Scholar] [CrossRef]
- Caiazzo, F.; Sisti, V.; Trasatti, S.; Trasatti, S. Electrochemical Characterization of Multilayer Cr/CrN-Based Coatings. Coatings 2014, 4, 508–526. [Google Scholar] [CrossRef]
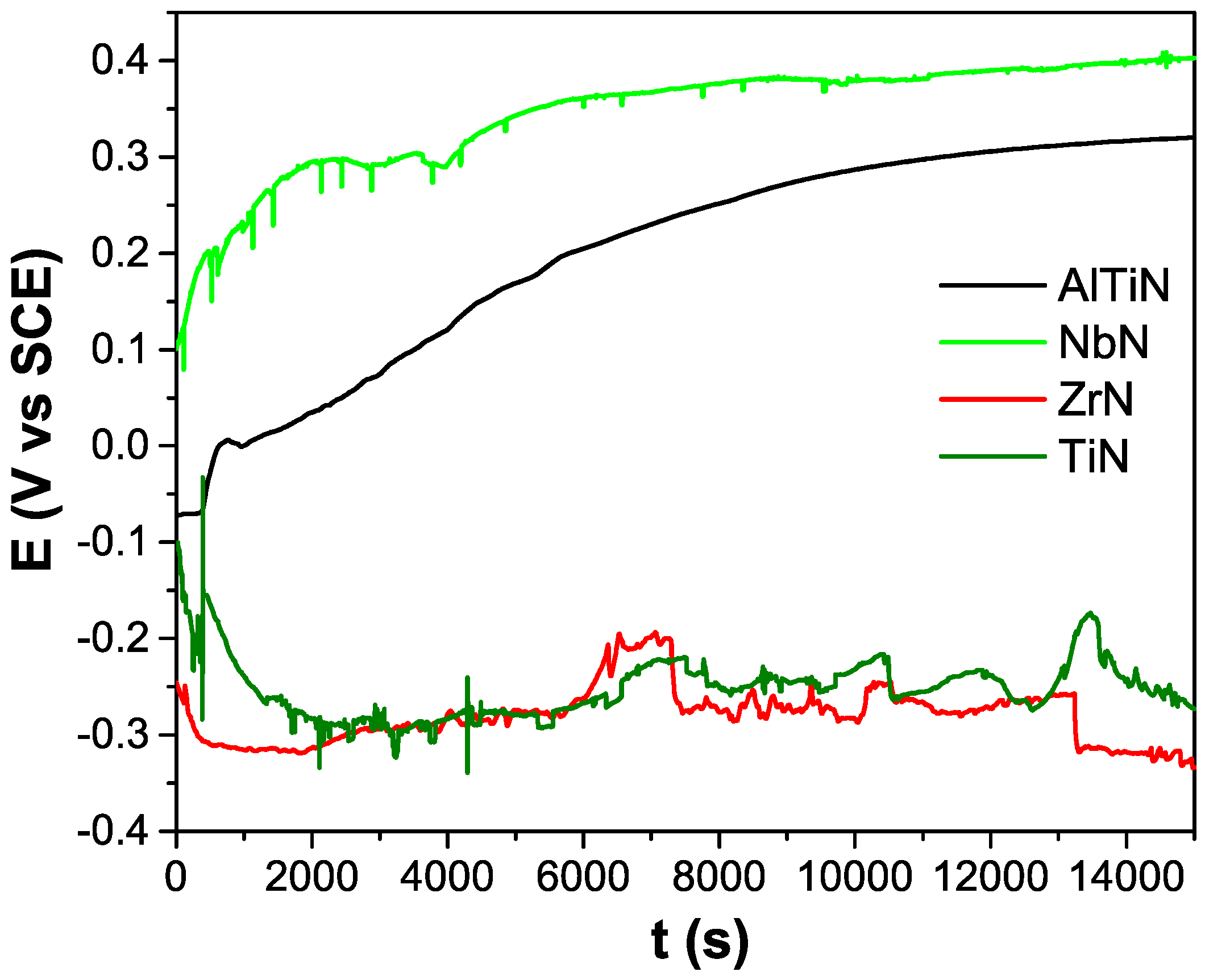
| Coatings | HV | Ecorr (V vs SCE) | <R> (nm) |
|---|---|---|---|
| AlTiN | 3000 ± 200 | −(0.028 ± 0.005) | 170 ± 40 |
| NbN | 2600 ± 190 | −(0.22 ± 0.02) | 150 ± 40 |
| ZrN | 2900 ± 180 | −(0.145 ± 0.009) | 90 ± 20 |
| TiN | 2800 ± 120 | −(0.021 ± 0.005) | 150 ± 50 |
| Coatings | θ (°) | SFE (mN m−1) | |||||
|---|---|---|---|---|---|---|---|
| Water | Glycerol | Diethylene Glycol | Diiodomethane | SFEp | SFEd | SFETOT | |
| AlTiN | 90 ± 3 | 81 ± 2 | 53 ± 2 | 46 ± 1 | 2 ± 1 | 33 ± 2 | 35 ± 3 |
| NbN | 85 ± 1 | 75 ± 1 | 48 ± 1 | 47 ± 2 | 4 ± 2 | 31 ± 1 | 35 ± 3 |
| ZrN | 76 ± 2 | 73 ± 3 | 51 ± 2 | 55 ± 1 | 8 ± 1 | 25 ± 2 | 33 ± 3 |
| TiN | 71 ± 2 | 68 ± 1 | 42 ± 3 | 50 ± 2 | 10 ± 1 | 27 ± 2 | 37 ± 3 |
| Emulsion | 25% γlv (mN m−1) | 50% γlv (mN m−1) | 100% γlv (mN m−1) |
|---|---|---|---|
| Marbocote® W2140 | 35 | 34 | 33 |
| EP | 43 | 40 | 38 |
| EV-333 | 47 | 44 | 43 |
| Release Agent | θ (°) | Wa (mN m−1) | S (mN m−1) |
|---|---|---|---|
| Marbocote® W2140 | |||
| AlTiN | 49 ± 4 | 58 ± 4 | −12 ± 3 |
| NbN | 49 ± 4 | 58 ± 2 | −12 ± 3 |
| ZrN | 50 ± 3 | 57 ± 2 | −13 ± 3 |
| TiN | 47 ± 3 | 59 ± 2 | −11 ± 3 |
| EP | |||
| AlTiN | 53 ± 3 | 56 ± 3 | −14 ± 3 |
| NbN | 51 ± 3 | 57 ± 2 | −13 ± 3 |
| ZrN | 48 ± 3 | 58 ± 2 | −12 ± 3 |
| TiN | 52 ± 3 | 57 ± 2 | −13 ± 3 |
| EV-333 | |||
| AlTiN | 73 ± 4 | 45 ± 3 | −25 ± 3 |
| NbN | 69 ± 2 | 48 ± 2 | −22 ± 3 |
| ZrN | 72 ± 4 | 46 ± 2 | −24 ± 3 |
| TiN | 69 ± 3 | 48 ± 2 | −22 ± 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Avico, L.; Beltrami, R.; Pargoletti, E.; Trasatti, S.P.M.; Cappelletti, G. Insight into the Release Agents/PVD Coatings Interaction for Plastic Mold Technology. Coatings 2020, 10, 281. https://doi.org/10.3390/coatings10030281
D’Avico L, Beltrami R, Pargoletti E, Trasatti SPM, Cappelletti G. Insight into the Release Agents/PVD Coatings Interaction for Plastic Mold Technology. Coatings. 2020; 10(3):281. https://doi.org/10.3390/coatings10030281
Chicago/Turabian StyleD’Avico, Luigi, Ruben Beltrami, Eleonora Pargoletti, Stefano P.M. Trasatti, and Giuseppe Cappelletti. 2020. "Insight into the Release Agents/PVD Coatings Interaction for Plastic Mold Technology" Coatings 10, no. 3: 281. https://doi.org/10.3390/coatings10030281
APA StyleD’Avico, L., Beltrami, R., Pargoletti, E., Trasatti, S. P. M., & Cappelletti, G. (2020). Insight into the Release Agents/PVD Coatings Interaction for Plastic Mold Technology. Coatings, 10(3), 281. https://doi.org/10.3390/coatings10030281