Sol-Gel Processing of Bismuth Germanate Thin-Films
Abstract
1. Introduction
2. Materials and Methods
Sol-Gel Synthesis of Bismuth Germanate Oxides
3. Results
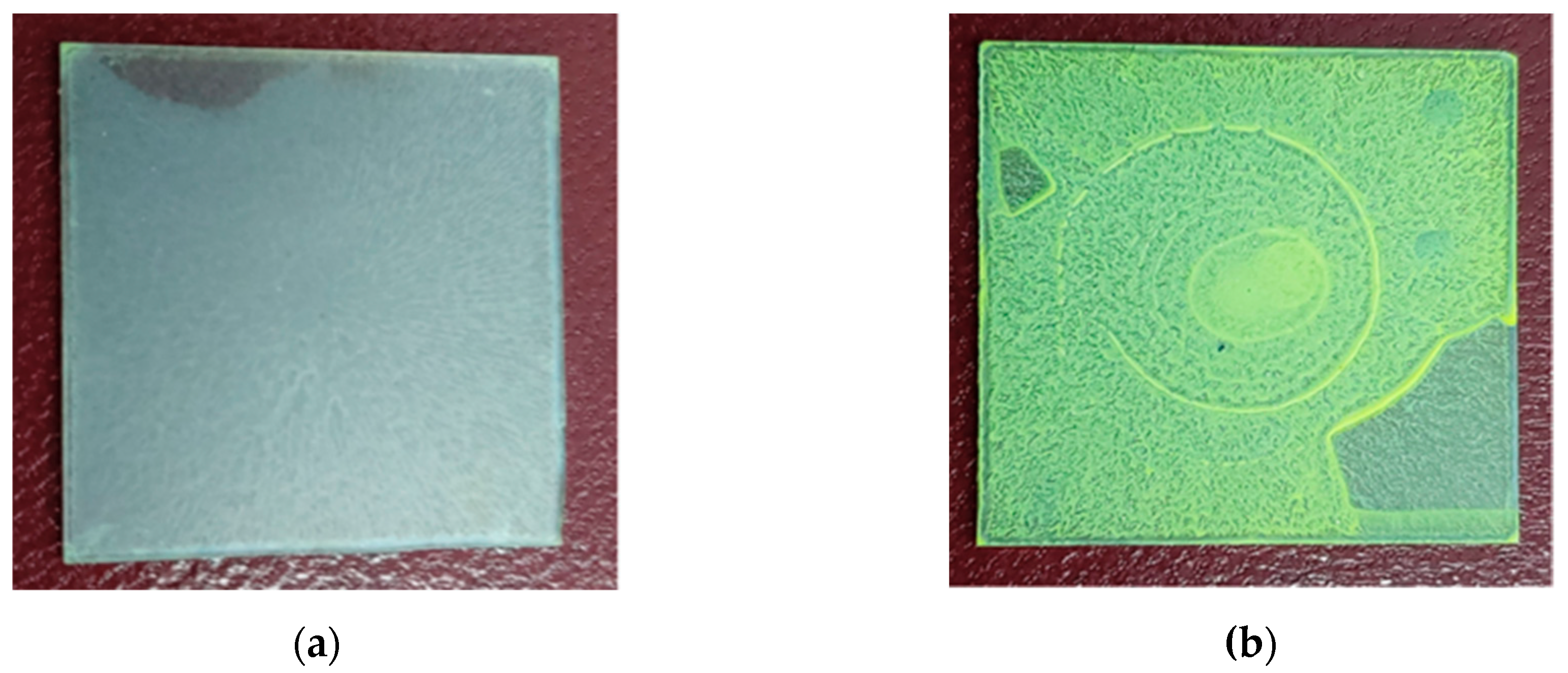
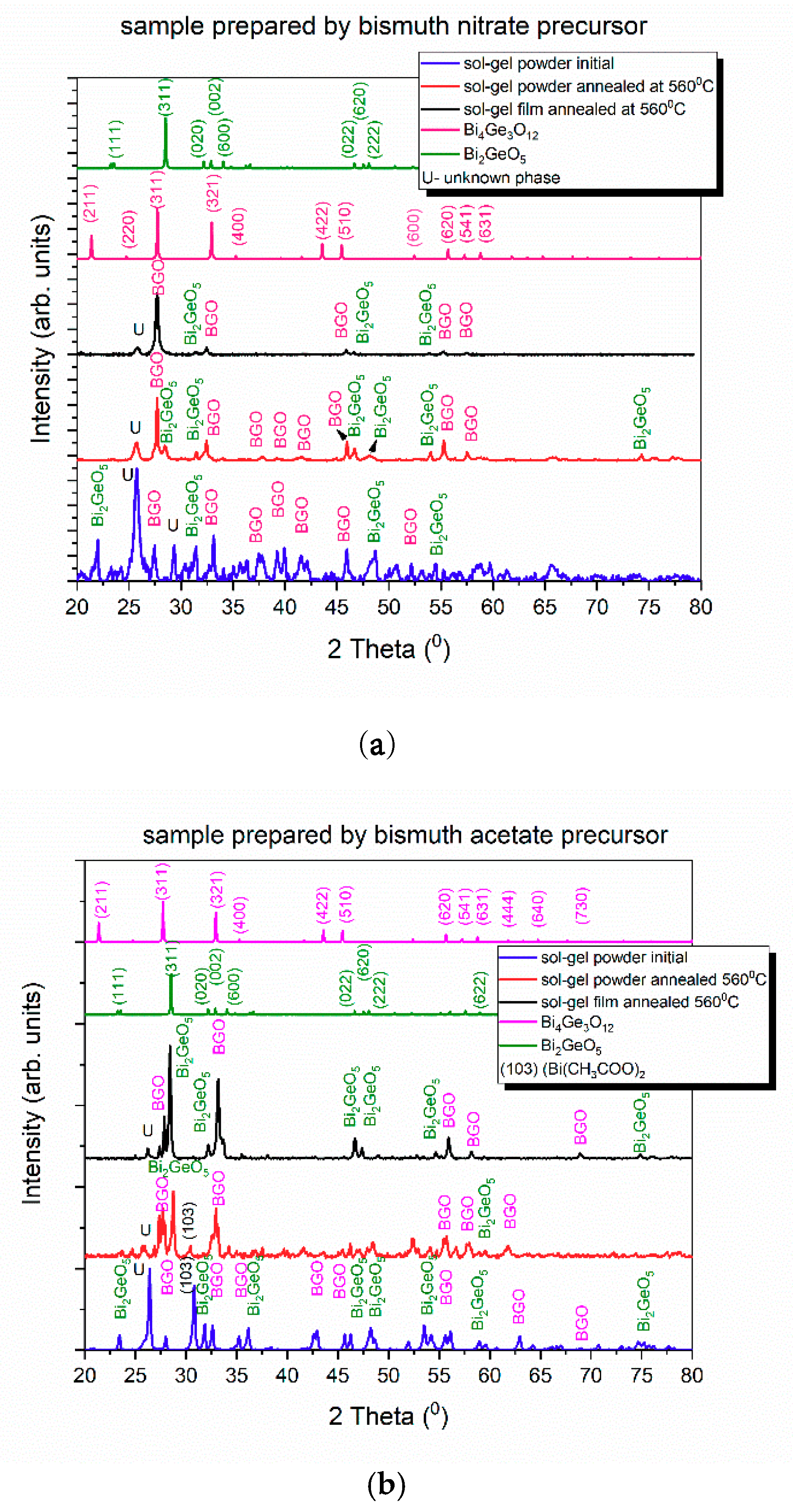
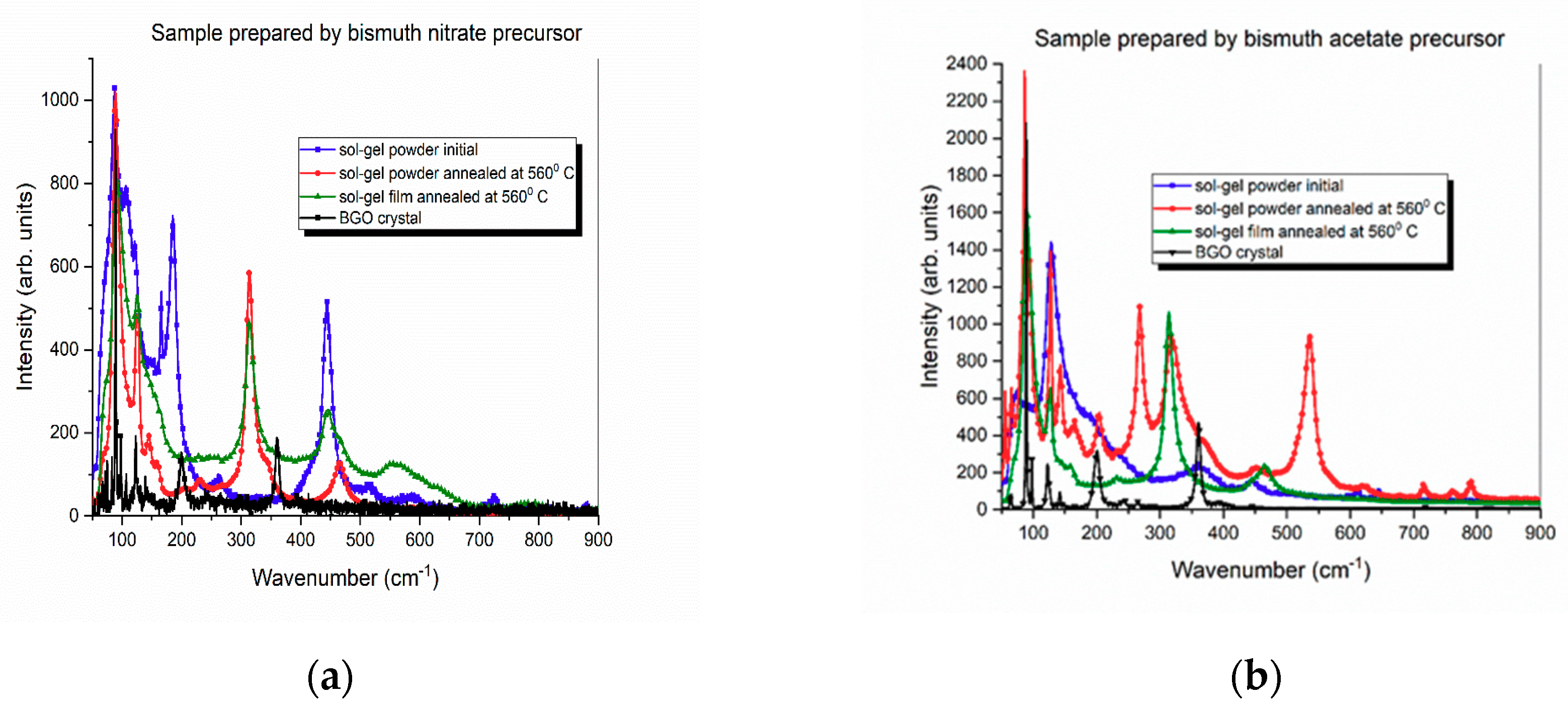
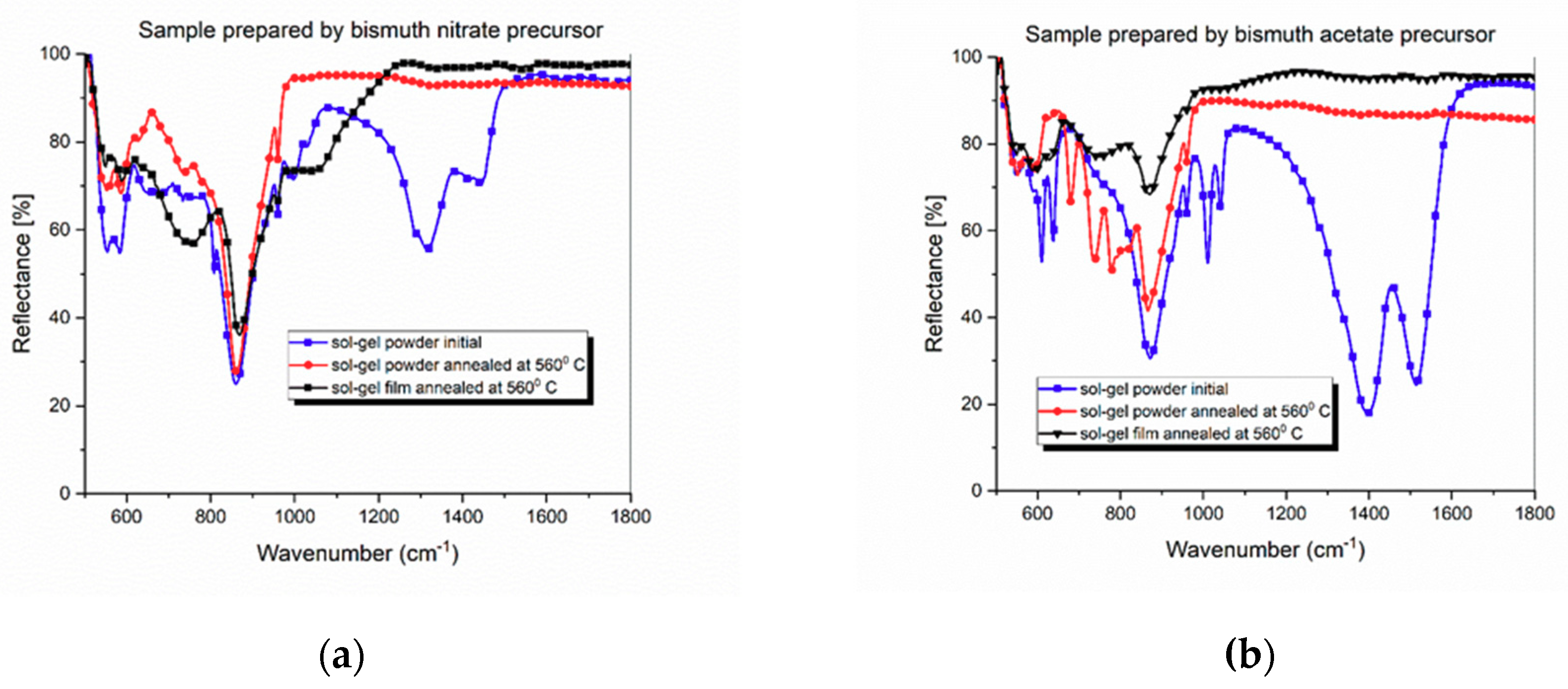
3.1. Structural Analysis by XRD
3.2. Structural Analysis by Elemental Analysis with the Energy-Dispersive X-ray Spectroscopy (EDS) Method
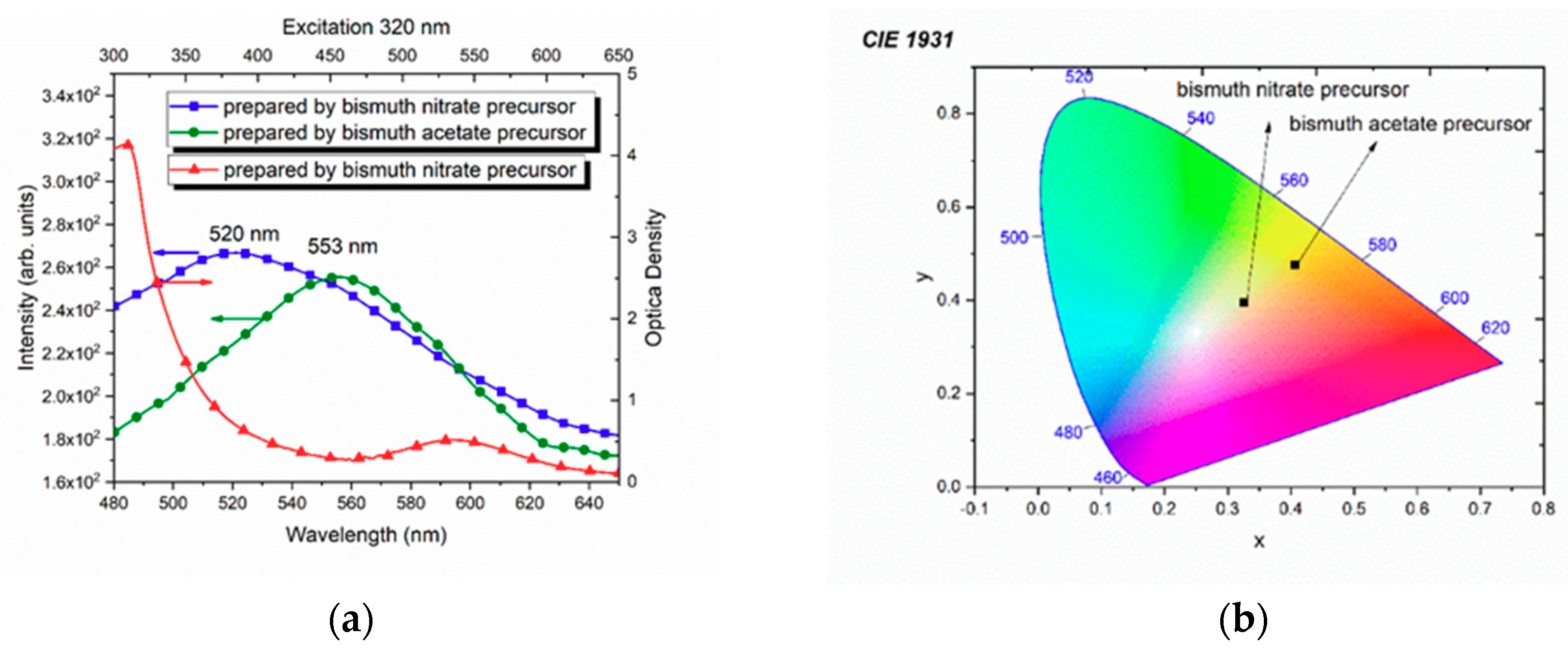
3.3. Optical Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ihantola, S.; Tengblad, O.; Toivonen, H.; Toivonen, H.; Peräjärvi, K.; Csome, C.; Borg, J.; Paepen, J.; Tagziria, H.; Gattinesi, P. Novel Detection Technologies for Nuclear Security; JRC Technical Reports; European Reference Network for Critical Infrastructure Protection: Radiological and Nuclear Threats to Critical Infrastructure; Publications Office of the European Union: Luxembourg City, Luxembourg, 2018; ISBN 978-92-79-87925-8. [CrossRef]
- An, Q.; Asfandiyarov, R.; Azzarello, P.; Bernardini, P.; Bi, X.J.; Cai, M.S.; Chang, J.; Chen, D.Y.; Chen, H.F.; et al. Measurement of the cosmic ray proton spectrum from 40 GeV to 100 TeV with the DAMPE satellite. Sci. Adv. 2019, 5, eaax3793. [Google Scholar] [PubMed]
- Chapman, Y.P.; Courchesne, E.B.; Derenzo, S.E. Bi3+ luminescence in ABiO2Cl (A=Sr, Ba) and BaBiO2Br. J. Lumin. 2008, 128, 87–91. [Google Scholar] [CrossRef]
- Arshak, K.; Korostynska, O.; Harris, J.; Morris, D.; Arshak, A.; Jafer, E. Properties of BGO thin films under the influence of gamma radiation. Thin Solid Films 2008, 516, 1493–1498. [Google Scholar] [CrossRef]
- Richard, M.H.; Dahoumane, M.; Dauvergne, D.; De Rydt, M.; Dedes, G.; Freud, N.; Krimmer, J.; Létang, M.J.; Lojacono, X.; Maxim, V.; et al. Design Study of the Absorber Detector of a Compton Camera for On-Line Control in Ion Beam Therapy. IEEE Trans. Nucl. Sci. 2012, 59, 1850–1855. [Google Scholar] [CrossRef]
- Oviedo, M.J.; Contreras, O.E.; Rosenstein, Y.; Vazquez-Duhalt, R.; Macedo, Z.S.; Carbajal-Arizaga, G.G.; Hirata, G.A. New Bismuth Germanate Oxide Nanoparticle Material for Biolabel Applications in Medicine. J. Nanomat. 2016, 2016, 9782625. [Google Scholar] [CrossRef]
- Macedo, Z.S.; Martinez, A.L.; Hernandes, A.C. Characterization of Bi4 Ge3 O12 Single Crystal by Impedance Spectroscopy. Mat. Res. 2003, 6, 577–581. [Google Scholar] [CrossRef]
- Bordun, O.M.; Butynskaia, O.B.; Novosad, S.S. Dispersion of light in thin Bi4Ge3O12 films. J. Appl. Spectr. 1996, 63, 651–655. [Google Scholar] [CrossRef]
- Polosan, S. Dynamics of energy absorption versus crystallization in Bi4Ge3O12 (BGO) amorphous materials. Mater. Res. Bull. 2010, 45, 1492–1495. [Google Scholar] [CrossRef]
- Polosan, S.; Apostol, E.; Secu, M.; Aldica, G. BGO glasses: structural and optical characterization. Phys. Stat. Sol. 2005, 2, 93–96. [Google Scholar] [CrossRef]
- Polosan, S.; Secu, M. X-ray excited luminescence and photoluminescence of Bi4(GeO4)3 glass-ceramics. Rad. Meas. 2010, 45, 409–411. [Google Scholar] [CrossRef]
- Polosan, S.; Nastase, F.; Secu, M. Structural changes during the crystallization of the Bi4Ge3O12 glasses. J. Non-Cryst. Sol. 2011, 357, 1110–1113. [Google Scholar] [CrossRef]
- Polosan, S. Crystallization processes in europium-doped Bi4Ge3O12 glass materials. J. Lum. 2019, 213, 235–240. [Google Scholar] [CrossRef]
- Oviedo, M.J.; Contreras, O.; Rodriguez, C.E.; Macedo, Z.S.; Hirata, G.A.; McKittrick, J. Photo- and radioluminescence characteristics of bismuth germanate nanoparticles by sol–gel and pressure-assisted combustion synthesis. Opt. Mater. 2012, 34, 1116–1119. [Google Scholar] [CrossRef]
- Mitsuyu, T.; Wasa, K.; Hayakawa, S. Structures and Optical Properties of Rf-Sputtered Bi12GeO20 Films. J. Electrochem. Soc. 1976, 123, 94–96. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ikeda, T.; Mihara, S.; Hirai, K.; Akashi, T.; Sakka, Y. Room-temperature synthesis of Bi4Ge3O12 from aqueous solution. Jpn. J. Appl. Phys. 2015, 54, 06FJ03. [Google Scholar] [CrossRef]
- Danks, E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Secu, C.E.; Negrila, C.; Secu, M. Investigation of sol-gel derived BaCl2:Eu2+ luminescent nanophosphor and the corresponding PVP@BaCl2:Eu2+ polymer. J. Phys. D Appl. Phys. 2018, 51, 305302. [Google Scholar] [CrossRef]
- Bartha, C.; Secu, C.E.; Matei, E.; Secu, M. Crystallization kinetics mechanism investigation of sol–gel-derived NaYF4:(Yb,Er) up-converting phosphors. Cryst. Eng. Comm. 2017, 19, 4992–5113. [Google Scholar] [CrossRef]
- Oviedo, M.J.; Han, J.K.; Contreras, O.; Macedo, Z.S.; Hirata, G.A.; McKittrick, J. Photoluminescence of bismuth germanate phosphors with a silica-shell structure. Phys. Proc. 2012, 29, 91–96. [Google Scholar] [CrossRef][Green Version]
- Aldica, G.; Polosan, S. Investigations of the non-isothermal crystallization of Bi4Ge3O12 (2:3) glasses. J. Non-Cryst. Sol. 2012, 358, 1221–1227. [Google Scholar] [CrossRef]
- Xie, H.; Jia, C.; Jiang, Y.; Wang, X. Synthesis of Bi4Si3O12 powders by a sol–gel method. Mater. Chem. Phys. 2012, 133, 1003–1005. [Google Scholar] [CrossRef]
- Polosan, S.; Galca, A.C.; Secu, M. Band-gap correlations in Bi4Ge3O12 amorphous and glass–ceramic materials. Sol. State Sci. 2011, 13, 49–53. [Google Scholar] [CrossRef]
- Radaev, S.F.; Muradyan, L.A.; Kargin, Y.F.; Sarin, V.A.; Kanepit, V.N.; Simonov, V.I. Neutron-diffraction investigation of single crystals of Bi4Ge3O12 with the eulytine structure. Sov. Phys. Cryst. 1990, 35, 204–206. [Google Scholar]
- Zhang, X.; Yin, S.T.; Wan, S.M.; You, J.L.; Chen, H.; Zao, S.J.; Zhang, Q.L. Raman Spectrum Analysis on the Solid--Liquid Boundary Layer of BGO Crystal Growth. Chin. Phys. Lett. 2007, 24, 1898–1900. [Google Scholar]
- Edgar, A.; Williams, G.V.M.; Secu, M.; Schweizer, S.; Spaeth, J.M. Optical properties of a high-efficiency glass ceramic X-ray storage phosphor. Radiat. Meas. 2004, 38, 413–416. [Google Scholar] [CrossRef]
- Lima, A.F.; Souza, S.O.; Lalic, M.V. Electronic structure and optical absorption of the Bi4Ge3O12 and the Bi4Si3O12 scintillators in ultraviolet region: An ab initio study. J. Appl. Phys. 2009, 106, 013715. [Google Scholar] [CrossRef]
- Lalic, M.V.; Souza, S.O. The first-principles study of electronic and optical properties of BGO and BSO scintillators. Opt. Mater. 2008, 30, 1189–1192. [Google Scholar] [CrossRef]
- Vaithianathan, V.; Kesavamoorthy, R.; Kannan, C.; Santhanaraghavan, P.; Ramasamy, P. Raman study of gaseous bubble inclusions in bismuth germanate and bismuth germanium silicon oxide single crystals. J. Mater. Res. 2003, 18, 762–767. [Google Scholar] [CrossRef]
- Titorenkova, R.; Mihailova, B.; Petrova, R.; Gospodinov, M.; Konstantinova, L. Effect of Doping on the Structure and Raman Spectra of Bi4Ge3O12. AIP Conf. Proc. 2010, 1203, 289–293. [Google Scholar]
- Bordun, O.M. Vibrational spectra of thin eulytine films. J. Appl. Spect. 1997, 64, 476–479. [Google Scholar] [CrossRef]
- Couzi, M.; Vignalou, J.R.; Boulon, G. Infrared and Raman study of the optical phonons in Bi4Ge3O12 single crystal. Sol. State Comm. 1976, 20, 461–465. [Google Scholar] [CrossRef]
- Polosan, S.; Negrea, R.; Ciobotaru, I.C.; Schinteie, G.; Kuncser, V. Ferromagnetic behaviour of bismuth germanate oxides glass-ceramic materials. J. Alloys Comp. 2015, 623, 192–196. [Google Scholar] [CrossRef]
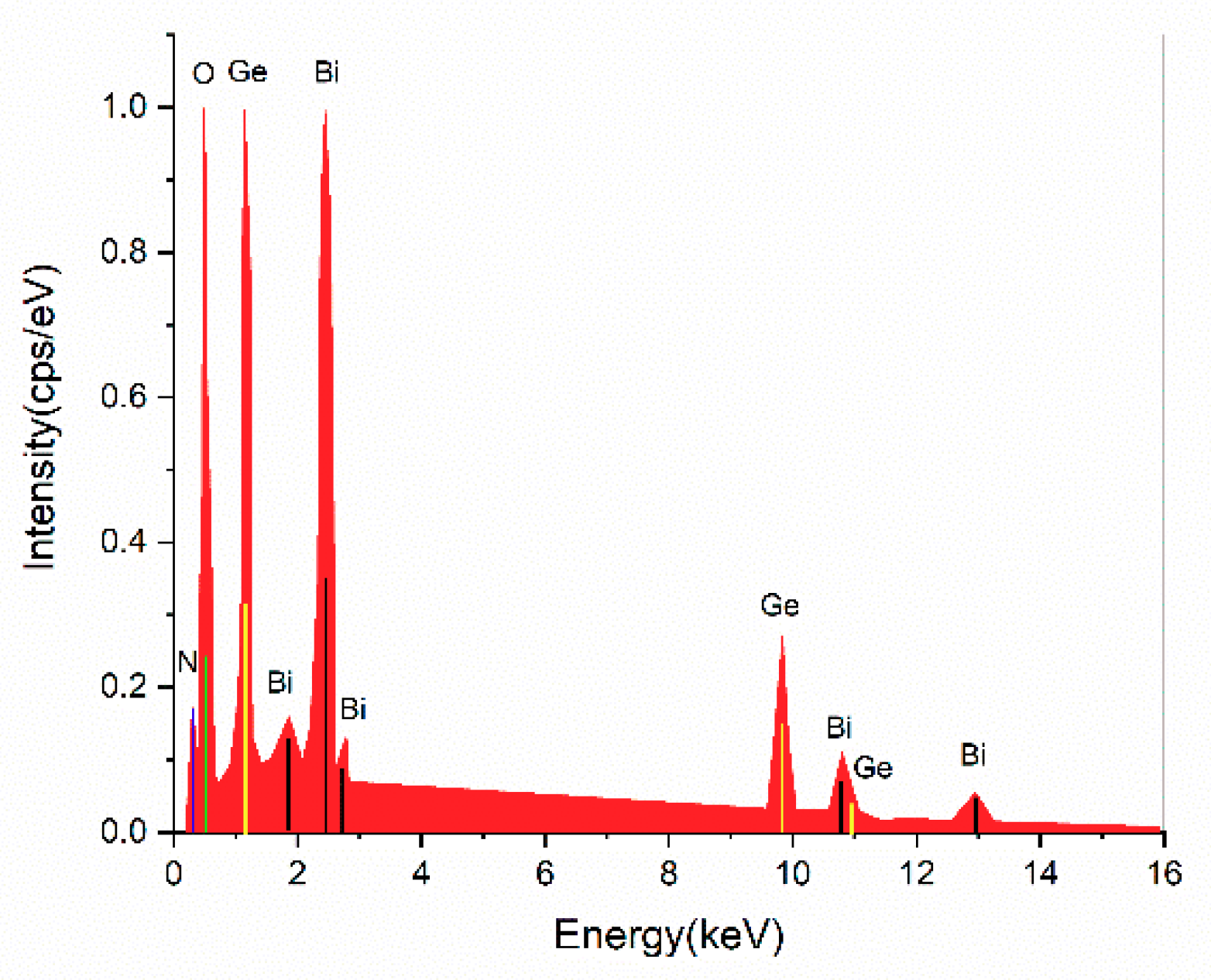
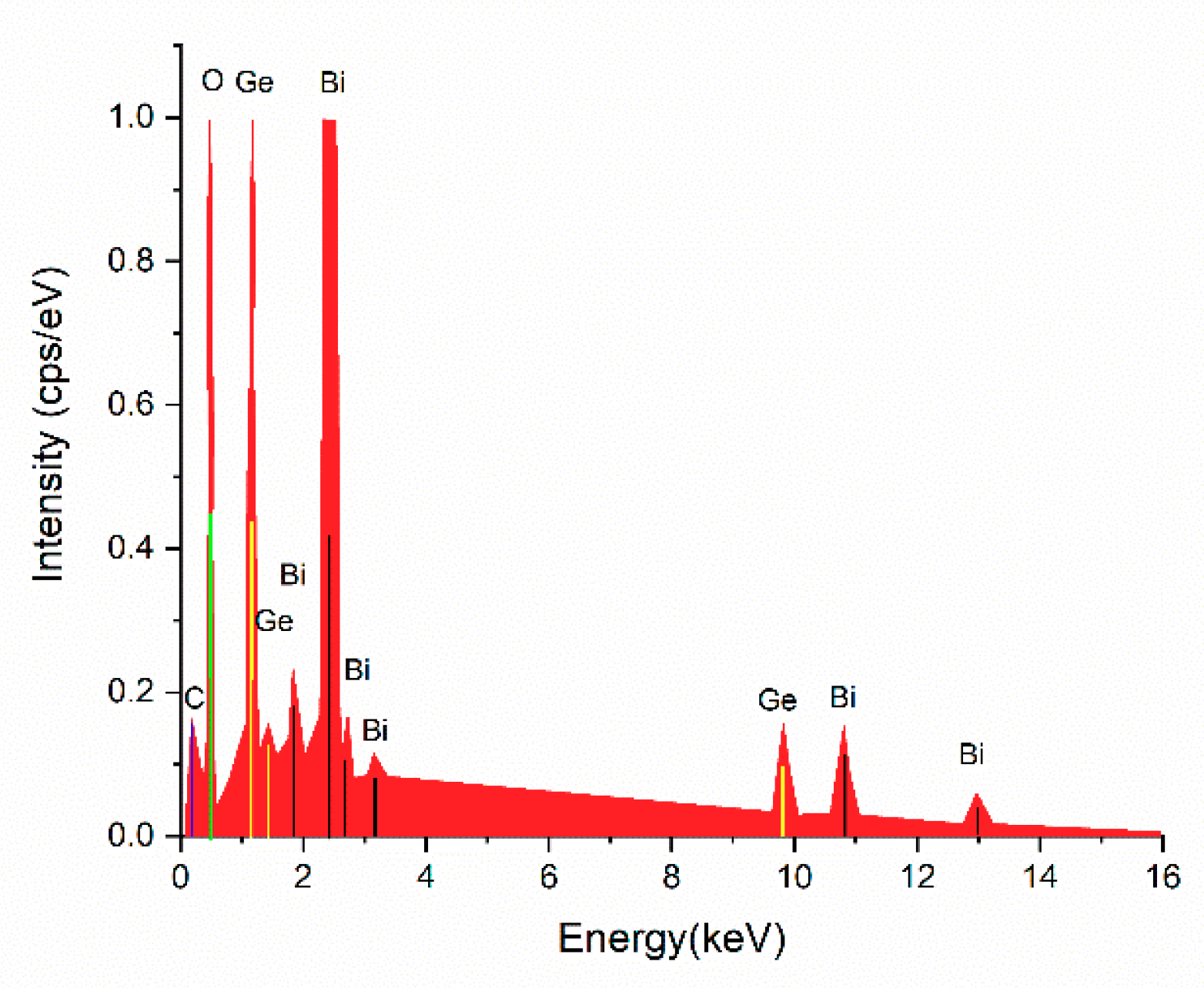
| Element | Atomic Number | Atomic Concentration (wt %) | Error (%) |
|---|---|---|---|
| O | 8 | 85.92 | 5.5 |
| Ge | 32 | 8.56 | 0.6 |
| Bi | 83 | 5.52 | 1.2 |
| Total | 100 |
| Element | Atomic Number | Atomic Concentration (wt %) | Error (%) |
|---|---|---|---|
| O | 8 | 88.82 | 5.4 |
| Ge | 32 | 4.25 | 0.3 |
| Bi | 83 | 6.93 | 1.4 |
| Total | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secu, M.; Secu, C.E.; Tite, T.; Polosan, S. Sol-Gel Processing of Bismuth Germanate Thin-Films. Coatings 2020, 10, 255. https://doi.org/10.3390/coatings10030255
Secu M, Secu CE, Tite T, Polosan S. Sol-Gel Processing of Bismuth Germanate Thin-Films. Coatings. 2020; 10(3):255. https://doi.org/10.3390/coatings10030255
Chicago/Turabian StyleSecu, Mihail, Corina Elisabeta Secu, Teddy Tite, and Silviu Polosan. 2020. "Sol-Gel Processing of Bismuth Germanate Thin-Films" Coatings 10, no. 3: 255. https://doi.org/10.3390/coatings10030255
APA StyleSecu, M., Secu, C. E., Tite, T., & Polosan, S. (2020). Sol-Gel Processing of Bismuth Germanate Thin-Films. Coatings, 10(3), 255. https://doi.org/10.3390/coatings10030255





