Effects of the Origin and Deacetylation Degree of Chitosan on Properties of Its Coatings on Titanium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan Membranes and Coatings
2.3. Examinations of Surface Topography and Morphology
2.4. Measurements of Wettability of Chitosan Membranes
2.5. Measurements of Wear Resistance
2.6. Biological Tests
3. Results
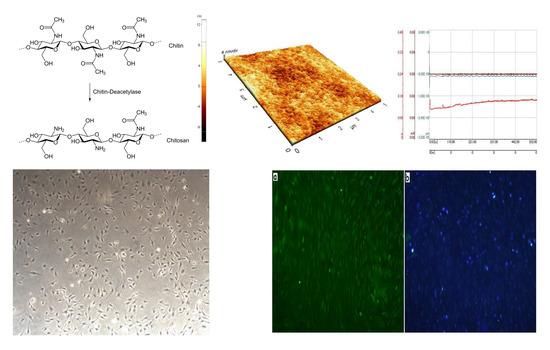
3.1. Surface Topography of Oxidized Titanium
3.2. Surface Topography of Chitosan Membranes
3.3. Wettability of Chitosan Membranes
3.4. Wear Resistance
3.5. Biological Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Lei, C.; Meng, L.; Wang, C.; Song, Y. Chitosan as a barrier membrane material in periodontal tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Naira, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 329, 762–798. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2012, 76, 167–182. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 2, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, H.; Qiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its microbial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Lieder, R. Chitosan and Chitosan Derivatives in Tissue Engineering and Stem Cell Biology. Ph.D. Thesis, School of Science and Engineering, Reykjavik University, Reykjavik, Iceland, 2013. [Google Scholar]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, M.; DeMarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Liu, X.; Ma, L.; Mao, Z.W.; Gao, C. Chitosan-based biomaterials for tissue repair and regeneration. Adv. Polym. Sci. 2011, 244, 81–128. [Google Scholar]
- Bottino, M.C.; Thomas, V.; Schmidt, H.; Vohra, Y.K.; Chu, T.M.G.; Kolowik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V. Membranes for Periodontal Regeneration—A Materials Perspective. Front. Oral Biol. 2015, 17, 90–100. [Google Scholar]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and modification: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Fernandes, I.P.; Amaral, J.S.; Pinto, V.; Ferreira, M.J. Development of chitosan-based antimicrobial leather coatings. Carbohydr. Polym. 2013, 98, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X.Y.; Feng, Y.F.; Wang, L.; Wang, C. A novel composite scaffold consisted of porous titanium and chitosan sponge for load-bearing applications: Fabrication, characterization and cellular activity. Compos. Sci. Technol. 2015, 117, 78–84. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Thein-Han, W.W.; Misra, R.D.K. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M. Review Paper: Chitosan Derivatives as Promising Materials for Controlled Drug Delivery. J. Biomater. Appl. 2008, 23, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Busilacchi, A.; Gigante, A.; Mattioli-Belmonte, M.; Manzotti, S.; Muzarelli, S.A. Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr. Polym. 2013, 98, 665–676. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Weselucha-Birczynska, A.; Stodolak-Zych, E.; Hasik, M. 2D IR correlation analysis of chitosan-MMT nanocomposite system. Vib. Spectrosc. 2012, 5, 185–188. [Google Scholar] [CrossRef]
- Abueva, C.D.G.; Padalhin, A.R.; Min, Y.K.; Lee, B.T. Preformed chitosan cryogel-biphasic calcium phosphate: A potential injectable biocomposite for pathologic fracture. J. Biomater. Appl. 2015, 30, 182–192. [Google Scholar] [CrossRef]
- Chang, C.; Peng, N.; He, M.; Teramoto, Y. Fabrication and properties of chitin/hydroxyapatite hybrid hydrogels as scaffold nano-materials. Carbohydr. Polym. 2013, 91, 7–13. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Greco, F.; Busilacchi, A.; Solazzo, V. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef]
- Ma, S.; Chen, Z.; Qiao, F.; Sun, Y. Guided bone regeneration with tripolyphosphate cross-linked asymmetric chitosan membrane. J. Dent. 2014, 42, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, Y.; Zuo, Y.; Zhang, L. Properties and in vitro biological evaluation of nano-hydroxyapatite/chitosan membranes for bone guided regeneration. Mater. Sci. Eng. C 2009, 29, 29–35. [Google Scholar]
- Nagahama, H.; Maeda, H.; Kasjiki, T.; Jayakumar, R.; Furuike, H.; Tamura, H. Preparation and characterization on novel chitosan/gelatin membranes using chitosan hydrogel. Carbohydr. Polym. 2009, 76, 255–260. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Elder, S.H.; Jouett, R.; Yang, Y.; Ong, J.L. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J. Biomater. Sci. Polym. Ed. 2003, 14, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Bumgardner, J.D.; Wiser, R.; Gerard, P.D.; Bergin, P.; Chestnutt, B.; Marin, M.; Ramsey, V.; Elder, S.H.; Gilbert, J.A. Chitosan: Potential use as a bioactive coating for orthopaedic and craniofacial/dental implants. J. Biomater. Sci. Polym. Ed. 2003, 14, 423–428. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Wright, L.; Haggard, W.O.; Bumgardner, J.D. Mechanical property, degradation rate, and bone cell growth of chitosan coated on titanium influenced by degree of deacetylation of chitosan. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 245–252. [Google Scholar] [CrossRef]
- Martin, H.J.; Schulz, K.H.; Bumgardner, J.D.; Walters, K.B. An XPS study on the attachment of triethoxsilylbutyraldehyde to two titanium surfaces as a way to bond chitosan. Appl. Surf. Sci. 2008, 254, 4599–4605. [Google Scholar] [CrossRef]
- Martin, H.J.; Schulz, K.H.; Bumgardner, J.D.; Schneider, J.A. Enhanced bonding of chitosan to implant quality titanium via four treatment combinations. Thin Solid Films 2008, 516, 6277–6286. [Google Scholar] [CrossRef]
- Jun, S.H.; Lee, E.J.; Yook, S.W.; Kim, H.E.; Kim, H.W.; Koh, Y.H. A bioactive coating of a silica xerogel/chitosan hybrid on titanium by a roomtemperature sol–gel process. Acta Biomater. 2010, 6, 302–307. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kannan, S. Development, mechanical evaluation and surface characteristics of chitosan/polyvinyl alcohol based polymer composite coatings on titanium metal. J. Mech. Behav. Biomed. Mater. 2014, 40, 314–324. [Google Scholar] [CrossRef]
- Ghimire, N.; Luo, J.; Tang, R.; Sun, Y.; Deng, Y. Novel anti-infective activities of chitosan immobilized titaniumsurface with enhanced osteogenic properties. Colloids Surf. B Biointerfaces 2014, 122, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Lieder, R.; Darai, M.; Thor, M.B.; Ng, C.H.; Einarsson, J.M.; Gudmundsson, S.; Helgason, B.; Gaware, V.S.; Másson, M.; Gíslason, J.; et al. In Vitro bioactivity of different degree of deacetylation chitosan, a potential coating material for titanium implants. J. Biomed. Res. A 2012, 100, 3392–3399. [Google Scholar]
- Chatelet, C.; Damour, O.; Domar, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- ASTM 265-20 Standard Specification for Titanium and Titanium Alloy Strip, Sheet, and Plate; ASTM: West Conshohocken, PA, USA, 2019.
- Ellingsen, J.E.; Johansson, C.B.; Wennerberg, A.; Holmén, A. Improved retention and bone-to-implant contact with fluoride-modified titanium implants. Int. J. Oral Maxillofac. Implants 2004, 19, 659–666. [Google Scholar] [PubMed]














| Chitosan Form | Roughness ± Standard Deviation, nm |
|---|---|
| DD 87% sigma | 1.73 ± 0.10 |
| DD 87% | 1.56 ± 0.16 |
| DD 94% | 0.91 ± 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supernak-Marczewska, M.; Zielinski, A. Effects of the Origin and Deacetylation Degree of Chitosan on Properties of Its Coatings on Titanium. Coatings 2020, 10, 99. https://doi.org/10.3390/coatings10020099
Supernak-Marczewska M, Zielinski A. Effects of the Origin and Deacetylation Degree of Chitosan on Properties of Its Coatings on Titanium. Coatings. 2020; 10(2):99. https://doi.org/10.3390/coatings10020099
Chicago/Turabian StyleSupernak-Marczewska, Milena, and Andrzej Zielinski. 2020. "Effects of the Origin and Deacetylation Degree of Chitosan on Properties of Its Coatings on Titanium" Coatings 10, no. 2: 99. https://doi.org/10.3390/coatings10020099
APA StyleSupernak-Marczewska, M., & Zielinski, A. (2020). Effects of the Origin and Deacetylation Degree of Chitosan on Properties of Its Coatings on Titanium. Coatings, 10(2), 99. https://doi.org/10.3390/coatings10020099