Abstract
NRL-AgNP was developed bringing important properties of natural rubber as occlusive membrane with antimicrobial activity of silver nanoparticles. Biological aspects, such as cell viability, tissue reaction, and occlusive membrane performance of NRL-AgNP, are presented. In addition, in vivo degradation was investigated by Fourier Transform Infrared Spectroscopy (FTIR). The cell viability test was performed in mesenchymal stem cells of human deciduous dental pulp seeded with the new material. Tissue reaction was tested through subcutaneous implant of NRL-AgNP and compared to Polytetrafluoroethylene (PTFE) at the dorsum of rats. The performance of the NRL-AgNP as an occlusive membrane in Guided Bone Regeneration (GBR) was tested in full thickness critical size bone defects (8 mm) in rat calvaria. Cell viability was 98.8% for NRL-AgNP and did not result in statistically significant differences compared to negative control (p > 0.05 Kruskal–Wallis). All materials presented similar tissue reaction (p > 0.05). In the GBR experiment, the defects covered with NRL-AgNP presented a more advanced stage of bone regeneration in comparison with non-treated defects. The FTIR spectra of NRL-AgNP before and after implantation showed no degradation of NRL-AgNP membranes. These results are in favor of the NRL-AgNP use as an occlusive membrane for GBR.
1. Introduction
Natural rubber latex (or simply latex) extracted from the Hevea brasiliensis tree is a biomaterial that has been studied for regeneration procedures. After polymerization, a membrane is formed with flexibility, elasticity, and mechanical strength, which are desirable characteristics for use as an occlusive membrane for Guided Bone Regeneration (GBR) procedures. Balabanian et al. [1] verified the biocompatibility of this biomaterial through the implant of intra-alveolar bone implant in rats. Ferreira et al. [2] and other studies, including the study by Mendonça et al. [3], suggest the ability of the latex to induce angiogenesis. Ereno et al. [4] and Moura et al. [5] studied latex membrane as an occlusive membrane for GBR procedures to treat critical defects, in animal models, with positive results. Studies have also demonstrated the possibility of adding substances such as proteins and antibiotics to a latex membrane, thus forming an occlusive barrier and drug releasing system simultaneously [6,7,8,9,10,11]. Guidelli et al. [12] incorporated silver nanoparticles (AgNPs) in a NRL membrane (NRL-AgNP) to form a polymeric system that gradually releases this substance.
Several applications of silver nanoparticles mainly due to their antibacterial and antifungal properties were published. Just to name a few, Morones et al. [13] showed the effectiveness of the nanoparticles against Gram-negative Escherichia coli, Vibrio cholerae, Pseudomonas Aeruginosa. Several research works are being conducted to incorporate the nanoparticles in various products for medical/dental care, including surgical instruments, masks, bone substitutes, and dressings. The topical delivery of silver nanoparticles via dressing was effective in healing the skin, and, according to Tian et al. [14], this effect was attributed to the antimicrobial properties of the nanoparticles.
In the same way, in dentistry, silver nanoparticles are being incorporated in materials for dental treatment due to their antibacterial effect [15]. Kassaee et al. [16] showed that 0.5% silver nanoparticles incorporated in dental resin exhibits strong antibacterial effect against E. coli due to the release of silver nanoparticles with little toxicity to humans. Furthermore, the addition of small amounts of silver partially improves the mechanical properties of the acrylic resin. Gomes-Filho et al. [17] evaluated the tissue reaction to silver nanoparticle dispersion as a new alternative irrigating solution for root canal treatment concluding that this dispersion at low concentration is biocompatible. Durner et al. [18] studied the influence of the incorporation of silver nanoparticles in photopolymerizable resins, as silver nanoparticles can assist in reducing dental caries. They concluded that AgNPs may influence the polymerization process and lead to an increase in elutable substances.
Periodontal disease represents an important process of dental contamination, due to the presence of large amounts of microorganisms, Gram-positive, and Gram-negative bacteria such as Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis [19]. The presence of these microorganisms can affect the success of GBR, as the toxins released by the bacteria can cause more intense inflammatory reactions and hinder cell proliferation. Thus, the developments of new biomaterials that can contribute to bone regeneration and are antimicrobial are of great importance. In this work, biological aspects of NRL-AgNP, a new biomaterial that gradually releases silver nanoparticles, is presented aiming toward future applications as an occlusive membrane for GBR. The cell viability, the tissue reaction, and performance of the NRL-AgNP to seal critical defects were tested. In addition, FTIR spectra were performed in the membrane before and after implantation to investigate its possible structural modifications.
2. Materials and Methods
The present study was approved by the Ethical Committee of the Universidade do Sagrado Coração—USC, Bauru, São Paulo State, Brazil and was conducted according to recommendations set forth by the National Institute of Health (NIH) [20].
2.1. NRL and NRL-AgNP Membranes
The NRL membrane was made according to the procedure described in Ereno et al. [4]. Briefly, centrifuged latex colloidal suspension (BDF Comércio de Produtos Agrícolas, São Paulo, Brazil) was placed in a Petri dish and polymerized at 40 °C, forming a membrane of 2 mm thickness.
The NRL-AgNP membrane was prepared according to Guidelli et al. [12]. Briefly, initially silver nanoparticles (AgNP) were synthesized by the chemical reduction of silver nitrate (AgNO3 2 mM) using sodium borohydride (NaBH4 4 mM). The solution was kept under vigorous stirring for 12 h.
A volume of 1 mL of colloidal suspension of latex was dried at 40 °C in a Petri dish for determination of rubber content. Then, a solution with colloidal latex and AgNP was prepared, to reach a proportion of 0.4% of AgNP. This solution was polymerized at 40 °C in a Petri dish forming a membrane with 2 mm thickness.
For morphology and size TEM characterization, NRL-AgNP membrane with 1 cm × 1 cm was immersed in 50 mL of ultrapure water for 2 h for the AgNP to be released. Then, the suspension was dropped onto a copper grid covered with a conductive polymer and dried. TEM images were assessed using JEOL-JEM-100 CXII instrument (JEOL, Tokyo, Japan).
After gamma sterilization using a dose of 25 kGy, these membranes were used in the experiments.
2.2. Cell Viability
Samples of 1 × 1 cm of NRL and NRL-AgNP membranes were placed, separately, in a culture flask of 25 cm2 with approximately 2.8 × 106 cells of human deciduous dental pulp-derived mesenchymal stem cells, CDLH1 line, P5 passage. The cells were cultured on 15 mL of the α-MEM medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 1% glutamine (200 mM) (Gibco), 1% antibiotic–antimycotic (Gibco), maintained at 37 °C in a humidified atmosphere under 5% CO2, by standard methods [21] for 15 days. Another flask without membrane was also prepared as a control. After this period, the culture medium and membranes were removed, the cells were washed with PBS (Merck) solution, and 0.5 mL of TrypLE-Express (Gibco) at 37 °C was added. The flasks were incubated in a humidified incubator at 37 °C with 5% CO2 for 1 to 2 min. The cell detachment was monitored using an inverted microscope. To inactivate TrypLE-Express (Gibco), 10 mL of preheated complete culture medium was added to the medium. The samples were centrifuged at 1000 rpm for 10 min, the supernatant removed, and the cells were resuspended and diluted in 10 mL of culture medium. The suspensions were centrifuged again at 1000 rpm for 10 min and the supernatant removed to perform the cell viability test. A volume of 100 µL of cell suspension and 100 µL of Trypan blue stain (4%) (Dinamica) were placed in a 12 × 75 mm2 polystyrene tube, homogenized, preserved at room temperature for 2 minutes and inoculated in a Neubauer chamber. Cell counting was performed in four quadrants of the external chamber to calculate the cell viability. The stained blue cells were considered dead. The Kruskal–Wallis nonparametric test was used to compare the results and they were considered statistically different when p < 0.05.
2.3. Tissue Reaction Experiment
In this experiment, 18 male rats (Rattus norvergicus albinus, Wistar) with an average weight of 250 g were used. The animals were kept in a plastic cage in an experimental animal room and were fed a standard laboratory diet and water ad libtum.
The animals were submitted to deep sedation with intramuscular administration of Xylazine chlorhydrate (Anasedan–Vetbrands, Brazil) 10 mg/kg body weight, followed by intramuscular administration of general anesthetic ketamine (Dopalen–Vetbrands, Brazil) 100 mg/kg body weight. Trichotomy was performed in the dorsal region, and antisepsis with polyvidone–iodine topic was conducted. Three incisions of approximately 1 cm were performed, followed by dilatation to reach the subcutaneous region to implant the materials. All animals received one membrane of each tested material (NRL-AgNP, Latex, PTFE) in the dorsum, all of them of a circular shape and 5 mm diameter. Subsequently the incisions were sutured with 4.0 silk suture (Figure 1a).
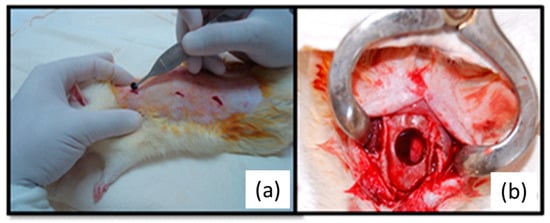
Figure 1.
(a) Tissue reaction experiment showing the implant of NRL-AgNP in the back of a rat. (b) Critical size defect with 8 mm diameter for GBR experiment.
After 7, 15, and 60 d, six animals were euthanized with lethal dose of general anesthetic and samples harvested for microscopic analysis.
The excised samples containing implanted material and adjacent tissues were preserved in 10% neutral buffered formalin for 92 h and prepared for microscopic analysis following routine histology procedures. Sections with 6 µm were stained with Hematoxylin and Eosin and Masson´s Trichrome. A Nikkon H550L photomicroscope was used to acquire the images. Six fields with 40X magnification around each implanted material were used for counting of inflammatory cells: polymorphonuclear, mononuclear, and giant cells. The average of the inflammatory cells for each specimen was ranked using the same parameters used by Yaltric et al. [22] as follows; score 0 for 0 cells (no reaction), 1 for <25 cells (slight reaction), 2 for 25 < cells < 125 (moderate), and score 4 for >125 cells (severe reaction).
2.4. Guided Bone Regeneration Experiment
The performance of NRL-AgNP as an occlusive membrane was studied in critical size bone defects created in rat calvaria.
In this study, 36 male rats (Rattus norvegicus albinus, Wistar) weighing an average of 250 g were used. The animals were kept in a plastic cage in an experimental animal room and were fed a standard laboratory diet and water. A full thickness bone defect with 8 mm diameter was surgically created in the skull (Figure 1b). Preoperatively, deep sedation was intramuscularly induced in animals with xylazine chlorhydrate (10 mg/kg, Anasedan, Vetbrands, Brazil), and ketamine (100 mg/kg, Dopalen, Vetbrands, Brazil) of body weight. The dorsal part of the cranium was shaved and aseptically prepared for surgery. A linear incision of 2 cm was made on the skin at the median sagittal line. The musculature and the periosteum were reflected, exposing the parietal bone, and the defect was created, by means of a trephine bur operating with low rotation under irrigation with sterile physiological solution (0.9% NaCl). Eighteen animals were treated with the NRL-AgNP membrane over the bone defects and the others were not treated (control). The membranes were cut into a circular shape, approximately 1mm beyond the boundaries of defect and fixed with Cyanoacrylate adhesive. The soft tissues and skin incisions were closed with interrupted 4–0 silk sutures.
After 7, 15, and 45 days post-operatively surgery, six animals from each group were euthanized with a lethal dose of anesthetic associated to relaxant and specimens containing the bone defect were collected.
The bone samples were fixed in 10% neutral-buffered formalin for 92 h decalcified using EDTA (Ethylenediaminetetraacetic acid). After decalcification, the pieces were divided into two parts, so that the histological sections in the middle of defects, representing the greatest dimension of defect were prepared. Transverse sections of 6 µm thickness were stained with Hematoxylin and Eosin (HE) and Masson´s Trichrome. Photomicrographs were taken using a Nikon H550L photomicroscope. The morphometric analysis of bone tissue was performed on three regions of defect: in the central part and two edges of defect, by measuring the area of bone tissue and connective tissue through ImageJ software (NIH–National Institute of Health) [23].
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR absorption spectra of the NRL-AgNP and NRL membranes before and after implantation for 60 days on the back of rat back surfaces were recorded on a FTIR spectrometer (Nicolet 380, ThermoNicolet, Whaltam, MA, USA), in the region between 4000 and 800 cm−1, under the Attenuated Total Reflectance (ATR) mode.
3. Results
3.1. AgNP Morphology and Size Distribution
Figure 2a shows TEM image of AgNP released by the NRL-AgNP membrane. Figure 2b shows the size distribution. Most nanoparticles have diameter between 10 and 20 nm.
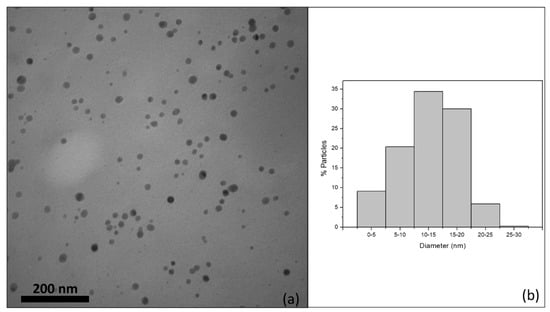
Figure 2.
(a) TEM image of AgNP released by membrane. (b) Histogram of size distribution of particles.
3.2. Cell Viability
Cell viability was determined in triplicate, in cell samples in contact with each material and that of the negative control (cell culture without membrane). The average and standard deviations found were 97.7 ± 1.6%, 97.9 ± 0.3%, and 98.6 ± 0.5% for negative control, NRL, and NRL-AgNP, respectively. There is no statistically significant difference among these results (p > 0.05 Kruskal–Wallis).
3.3. Tissue Reaction
Figure 3 shows a photograph of subcutaneous tissues of the implanted material region 60 days after surgery. No signs of infection were verified in all samples. Figure 4 shows the panoramic photomicrographs 7, 15, and 60 days post-surgery showing the fibrous capsule around all implanted materials.
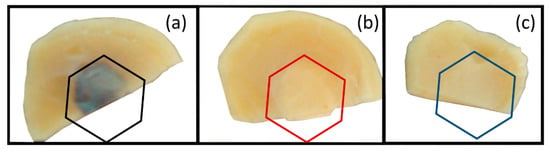
Figure 3.
Photographs of removed samples from the rats. (a) NRL-AgNP membrane. (b) NRL membrane. (c) PTFE membrane 60 d post-surgery. The region delimited by the hexagon shows that the tissues adjacent to the implanted materials does not present aspect that suggests intense inflammation or signs of aggression due to the implanted material.
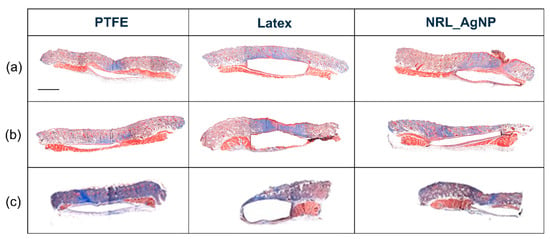
Figure 4.
Photomicrographs of tissue around implanted materials (a) 7 days, (b) 15 days, and (c) 60 days post-surgery. All materials were encapsulated. No signs of infection or necrosis are observed. Masson Trichrome, magnification 2X. Scale bar: 500 μm.
At higher magnifications (Figure 5a,b), granulation tissue is observed in the tissues surrounding the implanted material in the initial periods of 7 and 15 days (Figure 4a,b). This pattern was found for all tested materials. In the granulation tissue it is possible to identify the cells present in the inflammatory infiltrate: mononuclear (blue arrow), polymorphonuclear cells (green arrow), and giant cells (black arrow). Also, collagen matrix disorganization is noted. Figure 5c is from a sample of the 60-day period. Organized tissue with collagen fibers and few cells, mostly fibrocytes (green arrow) and fibroblasts (blue arrow), is observed in the fibrous capsule surrounding the implanted material.
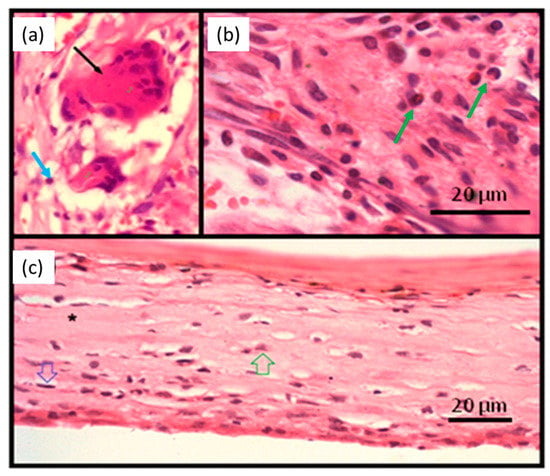
Figure 5.
Photomicrographs of tissue around NRL-AgNP at higher magnificatins: (a) 7 days, (b) 15 days, and (c) 60 days post-surgery showing inflammatory cells and fibrous capsule. See text.
Table 1 shows the score distribution attributed to the average number of inflammatory cells according to the observation period. The value represents the number of slices in the score by total number of slides observed. There are no statistically significant differences among material (p > 0.05, Kruskal Wallis).

Table 1.
Scores obtained for each material and period after conversion of number of inflammatory cells counted around implanted material. The value indicates number of slices in the score by total number of slices evaluated.
3.4. Guided Bone Regeneration Experiment
3.4.1. Radiological Images
Figure 6 shows radiographs of control (a,c) and NRL-AgNP (b,d) samples, 15 and 45 days post-surgery. A higher radiopacity can be observed in the treated group 5d, compared to 5c, related to the period of 45 days, indicating a more advanced stage of bone regeneration.
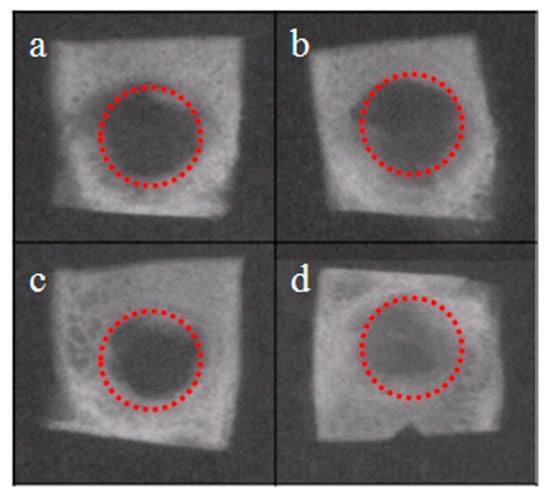
Figure 6.
Radiological images of samples: (a,c) Control and (b,d) NRL-AgNP, 15 (a,b) and 45 days (c,d) post-surgery, respectively. In red is a standardized circle around the defect region for comparison of results.
3.4.2. Microscopic Analysis
The specimens in the control group 7 days post-surgery (Figure 7a) presented a defect filled by highly vascularized connective tissue (CT) and blood clots (BC). It is possible to observe intense inflammation in the central region of the defect. It is observed regions with osteoblastic differentiation (O) on the surface of the bone matrix in edges of defect in all the specimens. In the samples of the NRL-AgNP group, the defect presented filled with fibrous connective tissue (CT) and blood clots (BC) with moderated inflammatory infiltrate and with areas of osteoblastic activity at the edges of the defect. There are areas with osteoblastic differentiation (O) (Figure 7b).
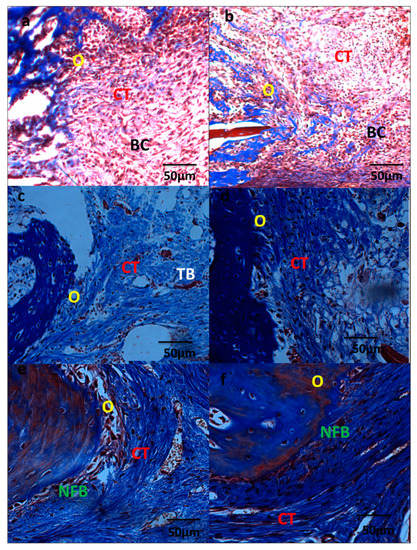
Figure 7.
Photomicrographs of samples from control group (left row) and NRL-AgNP GBR-treated (right row) at 7 days (a,b), 15 days (c,d), and 45 days (e,f) showing the bone regeneration progress. CT: connective tissue, BC: blood clot, O: osteoblastic activity, NFB: newly formed bone. Masson Trichrone stain, magnification 40X.
At 15 days, the defects, in the control group, are filled with a thin layer of organized fibrous connective tissue (CT) (Figure 7c). There is mild and diffuse inflammatory infiltrate. There is osteogenic activity (O) in the edges of the bone defect, and also short primary bone trabeculae located near the wall of the defect. In the samples of NRL-AgNP treatment, the defects are filled by connective tissue (CT) in process of bone neoformation (O) (Figure 7d).
At 45 days, organized and fibrous connective tissue (CT) is filling the region of the surgical defect in the control group (Figure 7e). There are some areas of newly formed bone (NFB) in the edges of the defect. In the NRL-AgNP-treated group (Figure 7f), there is a greater amount of newly formed bone (NFB).
At 15 days in the NRL-AgNP sample, the interaction of connective tissue (CT) with the bone neoformation area (NFB) is observed, demonstrating the osteoblastic activity (O), characterized by cell differentiation and bone matrix formation (Figure 8).
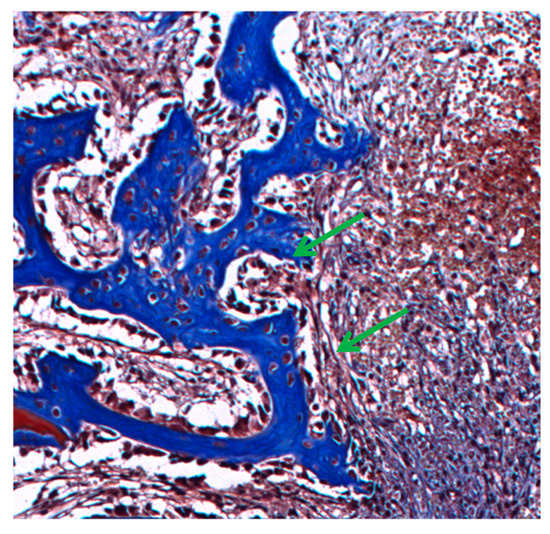
Figure 8.
Interaction of connective tissue with the formation of gaps of bone neoformation in NRL-AgNP sample at 15 days, demonstrating osteoblastic activity (green arrow). Masson Trichrome, magnification 40X.
Figure 9 shows the results of the morphometric measurements of the bone tissue at the edge and center of defect of samples from 15 and 45 days post-surgery. Comparing the amount of newly formed bone tissue in the groups at the same period post-surgery, it is found that there are statistically significant differences due the NRL-AgNP GBR treatment, with higher bone formation (p < 0.05 two sample t-Student test).
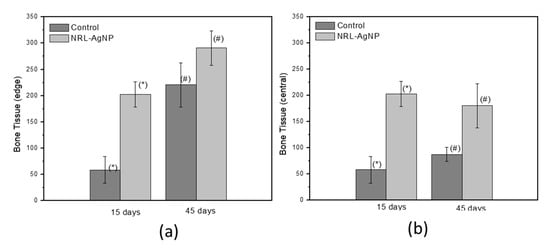
Figure 9.
Morphometric measurements of bone tissue at edge (a) and center (g) of defect of samples from 15 and 45 days post-surgery. There are statistically significant differences in both periods due to NRL-AgNP GBR treatment t-Student test). Same symbol (*#) indicates the groups with statistical difference.
3.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
Figure 10 shows the FTIR spectrum of NR and NRL-AgNP before and after 60 days of implant in the back of rats showing the main peaks attributed to NRL.
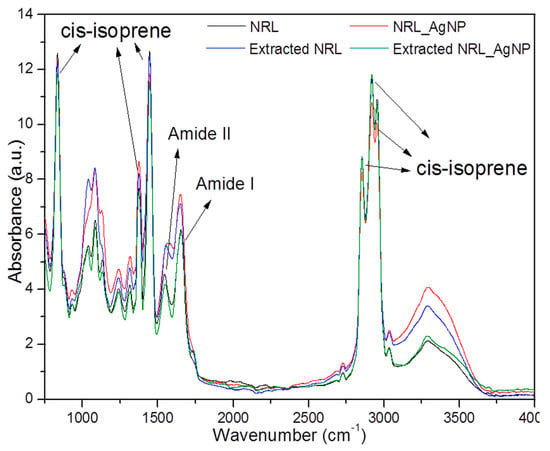
Figure 10.
FTIR spectra of NRL and NRL-AgNP before and after implant in dorsum of rats for 60 days. The peaks at 1855–1960 cm−1 associated to the cs-isoprene rubber molecules were chosen to make comparisons among them and are called fingerprint bands.
4. Discussion
Bacterial infections are one of the biggest complications caused by surgeries with implant biomaterials. The treatment of these infections is often managed surgically with device removal, recession of necrosis, and the administration of broad-spectrum antibiotics. In this sense, silver and, more recently, silver nanoparticles (AgNPs) have been attracting attention because they are able to act strongly against a broad spectrum of bacteria—Gram-positive and Gram-negative—and fungal species including antibiotic resistant strains [24,25,26,27]. The antimicrobial propriety of silver, copper, and other metals has been known for long time [28]. Silver presents bactericidal properties against several bacteria [23,24] including in biofilms [25] with low toxicity to human cells [26,29]. These features lead to the development of several medical and dental devices coated by AgNPs to give them antimicrobial properties, thus reducing the risks of infection [16,25,27,30,31,32]. Tian et al. [14] describes the benefits of topical delivery of AgNps in wound healing, reducing infections, inflammation, and modulation of fibrogenic cytokines.
Particularly in dentistry, as already mentioned, the presence of microorganisms can affect the success of Guided Bone Regeneration, as the toxins released by the bacteria can cause more intense inflammatory reactions and hinder cell proliferation [19]. Therefore, the development of new biomaterials that can contribute to bone regeneration with antimicrobial action simultaneously is of great importance.
NRL-AgNP was developed to act as an occlusive membrane that gradually releases AgNPs. Guidelli et al. [12] demonstrated through UV–Vis spectroscopy monitoring plasmon peak that NRL-AgNP_0.4% gradually releases AgNPs. In aqueous medium, the concentration of AgNPs delivered by the membrane reaches a plateau in the first 24 h. As NRL-AgNP_0.1% does not release AgNPs [12], it can be inferred that part of AgNPs remains bound in the NRL matrix. This feature could be useful in post-surgery, as bacterial colonization may occur at the time of surgery, so AgNPs delivered in the first hours prevent initial infection. The remaining AgNPs can be useful avoiding re-infection of the implant site surroundings. Regarding biocompatibility, our results demonstrate that the viability of DPSC (CDLH1) was not affected by the presence of the biomaterial or by the release of AgNPs from it, which corroborates with previous studies on biocompatibility of latex [1] and AgNPs [33].
Fourier Transform Infrared Spectroscopy (FTIR) spectra were recorded to obtain some insight on the interactions between latex molecules and silver nanoparticles, between NRL membrane and biological tissues, and between the NRL-AgNP system and biological tissues. Figure 10 depicts the FTIR of both NRL membranes and the NRL-AgNP system, before and after extraction from the back of rats 60 days after surgery. Functional groups present in the FTIR spectrum for the pure NRL membrane were also detected in the NRL-AgNP system, and no band shift was observed. The vibration spectra agreed well with the characteristic FTIR spectrum reported in the literature [34,35]. The main attributions correspond to the C–H stretching at 2960 cm−1; the symmetric and asymmetric stretching of the CH3 group at 2920 and 2855 cm−1, respectively; and the symmetric and asymmetric bending of the same group in the region of 1447 and 1376 cm−1, respectively. All these vibration bands are associated to the cis-isoprene molecule, which constitutes the rubber part of the latex, and together they form a fingerprint in the FTIR spectrum of natural rubber latex extracted from Hevea brasiliensis. The broad band in the region of 3100–3600 cm−1 is associated to the overlapping of O–H stretching and to N–H stretching, from water and from amine groups present in the non-rubber constituents of latex (like amino acids and proteins), respectively [36]. The bands in the range from 1200 to 1700 cm−1 are usually associated to the serum fraction from the latex [2]. The bands at 1650 and 1580 cm−1 refer to amide I and amide II, respectively, present in the non-rubber constituents of latex [12].
To make a comparison among the spectra, the three main bands in the region between 1855 and 1960 cm−1 associated to the cis-isoprene rubber molecules were chosen and are called fingerprint bands. The choice of these bands seems to be appropriate because the rubber molecules are not released by the membranes [12], therefore the intensity of these bands should remain constant during the experiments. The fingerprint bands for the sample containing silver nanoparticles is slightly less intense than for the pure latex, NRL membrane. On the other hand, relative to the fingerprint bands, the band in the 3000–3600 cm−1 region is more intense for samples containing silver nanoparticles than for the NRL sample. Bands related to amines I and II are also intensified in the NRL-AgNP sample. Therefore, the increase in the bands related to the non-rubber constituents of latex can indicate an accumulation of non-rubber molecules at the surface of the membranes containing silver nanoparticles. This process probably occurs because some molecules from the latex become attached to the silver nanoparticle surfaces. As silver nanoparticles tend to diffuse to the edge/surface of the membrane, the non-rubber molecules attached to the nanoparticle surfaces may be dragged together. The accumulation of non-rubber molecules at the surface of the NRL-AgNP membranes can also be a reason for the slight decrease in intensity of its fingerprint bands, as the spectra were collected under the Attenuated Total Reflectance (ATR) mode.
Figure 10 also shows the FTIR spectra of the NRL sample after extraction from the back of rats 60 days post-surgery. The fingerprint bands are the same intensity for the NRL membrane after extraction compared to the NRL membrane before implantation, confirming that rubber molecules are not released by the membrane and that the rubber membrane does not degrade. However, bands associated with non-rubber constituents of latex are more intense. One hypothesis for the intensification of these bands is related to the diffusion of non-rubber molecules towards the surface of membranes, as a consequence of the liberation of such molecules to the biological tissue around the membrane. In other words, the accumulation of non-rubber molecules at the surface of the membrane may occur because, while implanted, these molecules diffuse to the membrane surfaces in order to be released. As a consequence, they may accelerate the healing process, as it is known that latex has angiogenic proteins in its composition [2,3].
From Figure 10, it is possible to compare the spectra of NRL-AgNP before and after extraction from the back of rats 60 days post-surgery. It is possible to verify a reduction in bands associated to the non-rubber molecules in the sample removed from animals, suggesting that such molecules are released by the membranes while implanted in the biological tissue. The intensity of the fingerprint bands is slightly lower for the sample before implantation, and this could be explained based on the accumulation of non-rubber molecules at the surface of samples containing silver nanoparticles, and the respective release of these molecules during the healing process.
By comparing the NRL-AgNP sample with the NRL extracted from rats, it is possible to verify that the intensity of the rubber bands keeps the same intensity for both samples, but the bands associated to the non-rubber molecules have their intensities reduced for the NRL-AgNP system. This result suggests that the non-rubber molecules are released more efficiently in the NRL-AgNP system than for the pure NRL. Previously reported work has evidenced that silver nanoparticles are responsible for increasing the release rate of the non-rubber molecules from the latex membranes [4], due to binding of these molecules at the nanoparticle surface. Because these molecules from the serum fraction of latex are responsible for the angiogenic properties of the NRL extracted from Hevea brasiliensis, silver nanoparticles seem to potentialize the angiogenic properties of the pure NRL. De Mel et al. [25] added AgNPs in another polymeric system (POSS-PCU) and found a greater increase in hydrophilicity of the polymer when added to AgNPs. This may be related to the observation in this study, that is, the increased release of non-rubber substances by NRL-AgNP due to easier interaction with water.
Regarding the behavior of NRL-AgNP in biological medium, cell viability or cytotoxicity is of great importance. In this work, mesenchymal stem cells of human dental pulp were seeded with NRL-AgNP to investigate cytotoxicity of AgNPs released and no statistically significant differences were found in relation to the negative control, (cells alone) and with the polymeric matrix (latex), demonstrating that AgNPs released by the NRL-AgNP are not cytotoxic. The AgNPs delivered by NRL-AgNP was estimated by dynamic light scattering (DLS) [12] and present diameter distribution with a peak with ~20 nm. This result agrees with Kim et al. [37] who found that particles less than 10 nm have greater ability to induce apoptosis in comparison with larger ones.
As the in vitro test is limited in scope, the in vivo test was performed. The tissue reaction after implantation of NRL-AgNP in dorsum of rats also does not present differences in inflammatory pattern in relation to NRL or PTFE (Polytetrafluorethylene), with reduction of inflammatory cells with time (Table 1). All materials were encapsulated (Figure 3, Figure 4), typical of foreign body reaction, without necrosis.
The potential for use as an occlusive membrane was tested in critical size bone defects in rat calvaria. The radiological image (Figure 6) shows higher radiopacity in the defect area of the treated group, indicating a more advanced stage of bone regeneration due to the treatment with the NRL-AgNP. Microscopic analysis shows the development of bone regeneration with the defects initially filled with blood clots that evolve into bone tissue. In the samples treated with NRL-AgNP GBR there are bone trabeculae in several regions of the defect site, characterizing a more advanced stage in bone regeneration. This can be seen more clearly in the morphometric results, as the amount of bone tissue was higher in the edge of defect and in the central part of defect (p < 0.05 two samples t-Student test) at 15 and 45 days, evidencing the differences due to GBR with NRL-AgNP membrane treatment. All criteria described by Scantlebury [38]—biocompatibility, the ability to space maintenance, occlusivity, and easy handling—were observed in this paper for NRL-AgNP. Moreover, the release of substances from latex associated to angiogenesis [2,3] and AgNP may contribute to regeneration.
5. Conclusions
An occlusive membrane produced with natural rubber latex and silver nanoparticles, NRL-AgNP, was investigated using biological assays and physical-chemical techniques. In vitro and in vivo tests showed low toxicity, with cell viability of 98% and similar tissue reaction to the membranes in use. Radiographs show that calcification was faster in animals treated with the NRL-AgNP membrane; IR measurements allow inferring no degradation of the membrane, after 60 days in vivo implantation and that non-rubber molecules are released more efficiently in the NRL_AgNP. All the requirements established for GBR occlusive membrane were observed for NRL-AgNP, with the advantage of antimicrobial activity induced by AgNP. In summary, biological aspects of NRL-AgNP, cell viability, tissue reaction, and guided bone regeneration membrane are favorable for use in GBR procedures
Author Contributions
Conceptualization, L.M., G.M., and A.K.; Validation, L.M., G.M., É.G., S.L.M.P., O.B., and A.K., Formal Analysis, A.K.; Investigation, L.M., G.M., É.G., J.T., R.S., S.L.M.P., O.B., and A.K., Resources, A.K., S.L.M.P., and O.B., Data Curation, A.K., Writing—Original Draft Preparation, L.M., G.M., É.G., J.T., and A.K., Writing—Review & Editing, L.M., É.G., J.T., S.L.M.P., O.B., and A.K., Visualization, L.M., J.T., and A.K., Supervision, A.K., Project Administration, A.K., Funding Acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Brazilian agencies Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 03/09505-6), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Acknowledgments
The authors give thanks to Luciano Bachmann for providing the FTIR spectrometer; to Wilson Orcini, Maira Rondina, Carlos Alberto Brunello, and Lourenço Rocha for technical assistance; and to IPEN for sample irradiation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balabanian, C.A.C.A.; Coutinho-Netto, J.; Lamano-Carvalho, T.L.; Lacerda, S.A.; Brentegani, L.G. Biocompatibility of natural latex implanted into dental alveolus of rats. J. Oral Sci. 2006, 48, 201–205. [Google Scholar] [CrossRef]
- Ferreira, M.; Mendonça, R.J.; Coutinho-Netto, J.; Mulato, M. Angiogenic properties of natural rubber latex biomembranes and the serum fraction of Hevea brasiliensis. Braz. J. Phys. 2009, 39, 564–569. [Google Scholar] [CrossRef]
- Mendonça, R.J.; Maurício, V.B.; de Bortolli Teixeira, L.; Lachat, J.J.; Coutinho-Netto, J. Increased vascular permeability, angiogenesis and wound healing induced by the serum of natural latex of the rubber tree Hevea brasiliensis. Phyther. Res. 2010, 24, 764–768. [Google Scholar]
- Ereno, C.; Guimarães, S.A.C.; Pasetto, S.; Herculano, R.D.; Silva, C.P.; Graeff, C.F.O.; Tavano, O.; Baffa, O.; Kinoshita, A. Latex use as an occlusive membrane for guided bone regeneration. J. Biomed. Mater. Res. Part A 2010, 95, 932–939. [Google Scholar] [CrossRef]
- Moura, J.M.L.; Ferreira, J.F.; Marques, L.; Holgado, L.; Graeff, C.F.O.; Kinoshita, A. Comparison of the performance of natural latex membranes prepared with different procedures and PTFE membrane in guided bone regeneration (GBR) in rabbits. J. Mater. Sci. Mater. Med. 2014, 25, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Herculano, R.D.; Alencar de Queiroz, A.A.; Kinoshita, A.; Oliveira, O.N., Jr.; Graeff, C.F.O. On the release of metronidazole from natural rubber latex membranes. Mater. Sci. Eng. C 2011, 31, 272–275. [Google Scholar] [CrossRef]
- Herculano, R.D.; Silva, C.P.; Ereno, C.; Guimaraes, S.A.C.; Kinoshita, A.; Graeff, C.F. Natural rubber latex used as drug delivery system in guided bone regeneration (GBR). Mater. Res. 2009, 12, 253–256. [Google Scholar] [CrossRef]
- Herculano, R.D.; Tzu, L.C.; Silva, C.P.; Brunello, C.A.; de Queiroz, Á.A.A.; Kinoshita, A.; Graeff, C.F. Nitric oxide release using natural rubber latex as matrix. Mater. Res. 2011, 14, 355–359. [Google Scholar] [CrossRef]
- Herculano, R.D.; Guimarães, S.A.C.; Belmonte, G.C.; Duarte, M.A.H.; Oliveira Júnior, O.N.; de Kinoshita, A.; Graeff, C.F. Metronidazole release using natural rubber latex as matrix. Mater. Res. 2010, 13, 57–61. [Google Scholar] [CrossRef]
- Almeida, G.F.B.; Cardoso, M.R.; Zancanela, D.C.; Bernardes, L.L.; Norberto, A.M.Q.; Barros, N.R.; Paulino, C.G.; Chagas, A.L.D.; Herculano, R.D.; Mendonça, C.R. Controlled drug delivery system by fs-laser micromachined biocompatible rubber latex membranes. Appl. Surf. Sci. 2020, 506, 144762. [Google Scholar] [CrossRef]
- Barros, N.R.; de Miranda, M.C.R.; Borges, F.A.; Gemeinder, J.L.P.; Mendonça, R.J.; de Cilli, E.M.; Herculano, R.D. Natural rubber latex: Development and in vitro characterization of a future transdermal patch for enuresis treatment. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 871–876. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Kinoshita, A.; Ramos, A.P.; Baffa, O.; Guidelli, É.J.; Kinoshita, A.; Ramos, A.P.; Baffa, O.; Guidelli, E.J.; Kinoshita, A.; et al. Silver nanoparticles delivery system based on natural rubber latex membranes. J. Nanoparticle Res. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wong, K.K.Y.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K.H. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Duran, G.; Galembeck, A.; Cogo-Mueller, K.; Franz-Montan, M.; Duran, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Kassaee, M.Z.; Akhavan, A.; Sheikh, N.; Sodagar, A. Antibacterial effects of a new dental acrylic resin containing silver nanoparticles. J. Appl. Polym. Sci. 2008, 110, 1699–1703. [Google Scholar] [CrossRef]
- Gomes-Filho, J.E.; de Moraes Costa, M.T.; Cintra, L.T.Â.; Lodi, C.S.; Duarte, P.C.T.; Okamoto, R.; Bernabé, P.F.E.; Nery, M.J.; Cannon, M. Evaluation of alveolar socket response to Angelus MTA and experimental light-cure MTA. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, e93–e97. [Google Scholar] [CrossRef]
- Durner, J.; Stojanovic, M.; Urcan, E.; Hickel, R.; Reichl, F.-X. Influence of silver nano-particles on monomer elution from light-cured composites. Dent. Mater. 2011, 27, 631–636. [Google Scholar] [CrossRef]
- Rosa, O.P.; da Silva, S.M.B.; Costa, B.; Torres, S.A.; Passanezi, E. Periodontopathogens in the saliva and subgingival dental plaque of a group of mothers Periodontopatógenos na saliva e placa subgengival de um grupo de mães. Pesqui. Odontol. Bras. 2002, 16, 313–318. [Google Scholar] [CrossRef]
- Institute of Laboratory Animal Resources (U.S.), Committee on Care and Use of Laboratory Animals; National Institutes of Health (U.S.), Division of Research Resources. Guide for the Care and Use of Laboratory Animals; U.S. Department of Health and Human Services, Public Health Service, National Insititutes of Health: Bethesda, MD, USA, 1985. [Google Scholar]
- Arsham, M.S.; Barch, M.J.; Lawce, H.J. The AGT Cytogenetics Laboratory Manual The AGT Cytogenetics Laboratory Manual Edited by; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 4, ISBN 9781119061175. [Google Scholar]
- Yaltirik, M.; Ozbas, H.; Bilgic, B.; Issever, H. Reactions of Connective Tissue to Mineral Trioxide Aggregate and Amalgam. J. Endod. 2004, 30, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. US National Institutes of Health, Bethesda, MD, USA. Available online: http//imagej.nih.gov/ij/2011 (accessed on 10 January 2020).
- Mortazavi, V.; Nahrkhalaji, M.M.; Fathi, M.H.; Mousavi, S.B.; Esfahani, B.N. Antibacterial effects of sol-gel-derived bioactive glass nanoparticle on aerobic bacteria. J. Biomed. Mater. Res. Part A 2010, 94A, 160–168. [Google Scholar] [CrossRef] [PubMed]
- de Mel, A.; Chaloupka, K.; Malam, Y.; Darbyshire, A.; Cousins, B.; Seifalian, A.M. A silver nanocomposite biomaterial for blood-contacting implants. J. Biomed. Mater. Res. Part A 2012, 100A, 2348–2357. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo-Filho, A.C.; Camargo, E.R.; de Barbosa, D.B. The growing importance of materials that prevent microbial adhesion: Antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents 2009, 34, 103–110. [Google Scholar] [CrossRef]
- Kumar, V.; Jolivalt, C.; Pulpytel, J.; Jafari, R.; Arefi-Khonsari, F. Development of silver nanoparticle loaded antibacterial polymer mesh using plasma polymerization process. J. Biomed. Mater. Res. Part A 2013, 101A, 1121–1132. [Google Scholar] [CrossRef]
- Rameshbabu, N.; Kumar, T.S.S.; Prabhakar, T.G.; Sastry, V.S.; Murty, K.V.G.K.; Rao, K.P. Antibacterial nanosized silver substituted hydroxyapatite: Synthesis and characterization. J. Biomed. Mater. Res. Part A 2006, 79, 963–973. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef]
- Alarcon, E.I.; Udekwu, K.; Skog, M.; Pacioni, N.L.; Stamplecoskie, K.G.; González-Béjar, M.; Polisetti, N.; Wickham, A.; Richter-Dahlfors, A.; Griffith, M.; et al. The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomaterials 2012, 33, 4947–4956. [Google Scholar] [CrossRef]
- Stevanović, M.; Bračko, I.; Milenković, M.; Filipović, N.; Nunić, J.; Filipič, M.; Uskoković, D.P. Multifunctional PLGA particles containing poly(l-glutamic acid)-capped silver nanoparticles and ascorbic acid with simultaneous antioxidative and prolonged antimicrobial activity. Acta Biomater. 2014, 10, 151–162. [Google Scholar] [CrossRef]
- Almeida, L.M.; Magno, L.N.; Pereira, A.C.; Guidelli, É.J.; Baffa Filho, O.; Kinoshita, A.; Gonçalves, P.J. Toxicity of silver nanoparticles released by Hancornia speciosa (Mangabeira) biomembrane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Guidelli, E.J.; Ramos, A.P.; Zaniquelli, M.E.D.; Nicolucci, P.; Baffa, O. Synthesis and characterization of silver/alanine nanocomposites for radiation detection in medical applications: The influence of particle size on the detection properties. Nanoscale 2012, 4, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, C.T.; Nasir, M.; Baharin, A.; Zaman, K. Electron beam irradiation of epoxidized natural rubber. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2000, 171, 455–464. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Ramos, A.P.; Zaniquelli, M.E.D.; Baffa, O. Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 140–145. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100A, 1033–1043. [Google Scholar] [CrossRef]
- Scantlebury, T.V. 1982-1992—A Decade of Technology development for Guided Tissue Regeneration. J. Periodontol. 1993, 64, 1129–1137. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).