Ag Functionalization of Al-Doped ZnO Nanostructured Coatings on PLA Substrate for Antibacterial Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Atomic Force Microscopy (AFM) Analyses
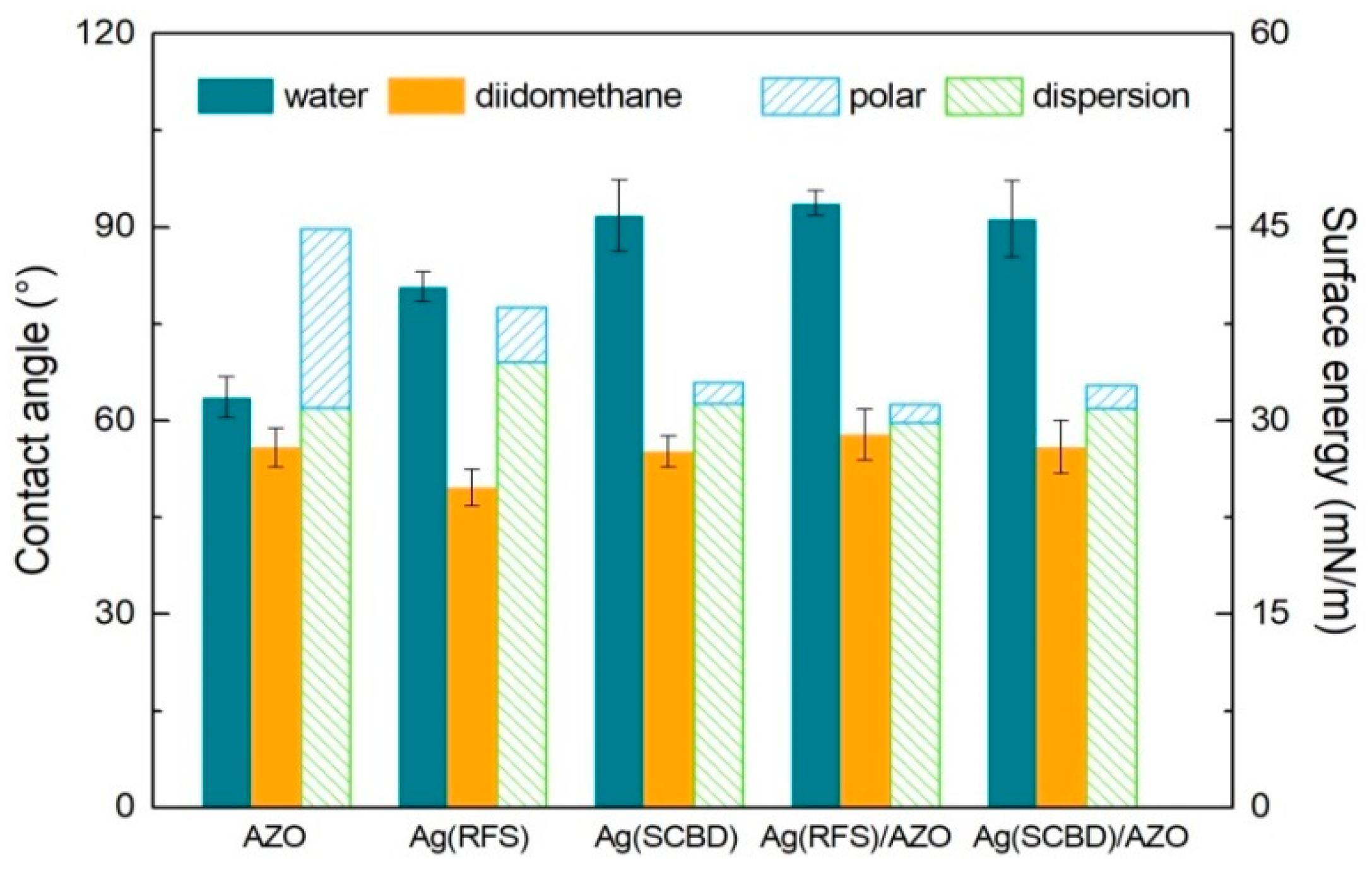
3.2. Wettability Analyses
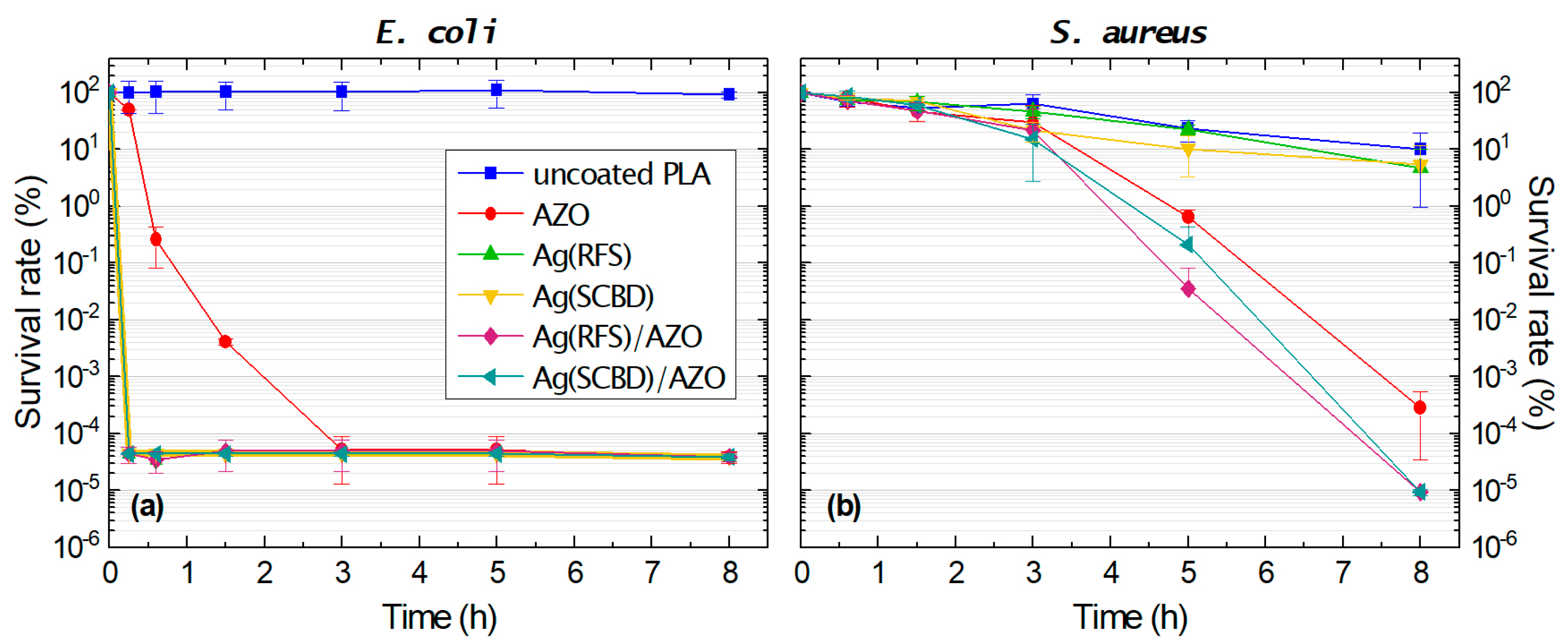
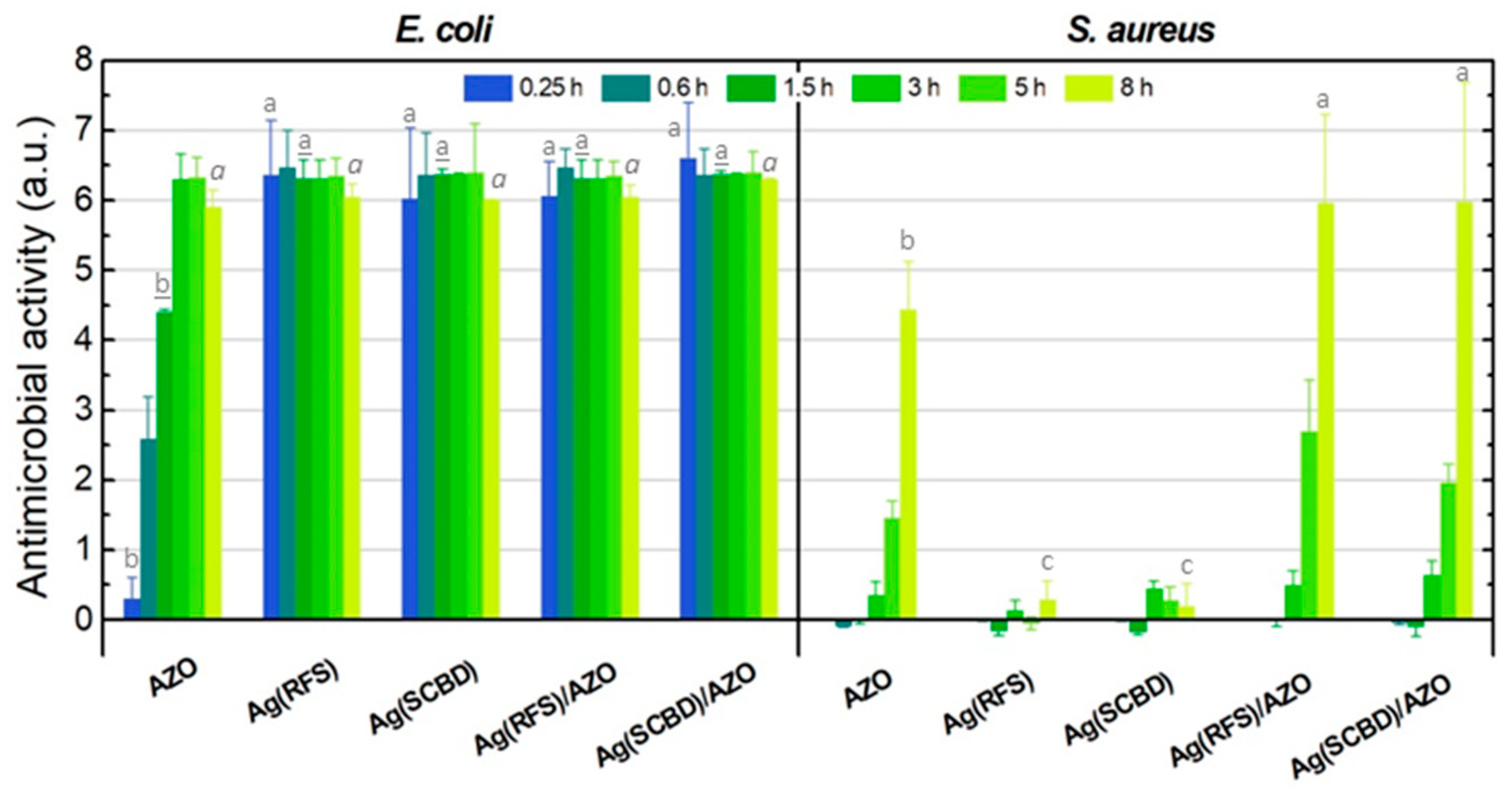
3.3. Antimicrobial Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wille, I.; Mayr, A.; Kreidl, P.; Bruhwasser, C.; Hinterberger, G.; Fritz, A.; Posch, W.; Fuchs, S.; Obwegeser, A.; Orth-Holler, D.; et al. Cross-sectional point prevalence survey to study the environmental contamination of nosocomial pathogens in intensive care units under real-life conditions. J. Hosp. Infect. 2018, 98, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. ACS Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO nanostructures in active antibacterial food packaging: Preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. Food Rev. Int. 2020. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.; Zare, E.; Borzacchiello, A.; Niu, L.; Tay, F. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Di Benedetto, F.; Vigliotta, G.; Capodieci, L.; Terzi, R.; Rizzo, A. Aluminum-doped zinc oxide coatings on polylactic acid films for antimicrobial food packaging. Thin Solid Film. 2018, 645, 187–192. [Google Scholar] [CrossRef]
- Saxena, V.; Chandra, P.; Pandey, L. Design and characterization of novel Al-doped ZnO nanoassembly as an effective nanoantibiotic. Appl. Nanosci. 2018, 8, 1925–1941. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Villani, F.; Rizzo, A.; Caputo, I.; Paolella, G.; Vigliotta, G. Antibacterial Al-doped ZnO coatings on PLA films. J. Mater. Sci. 2020, 55, 4830–4847. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Zhou, W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci. Technol. 2015, 45, 187–199. [Google Scholar] [CrossRef]
- Wegner, K.; Piseri, P.; Tafreshi, H.; Milani, P. Cluster beam deposition: A tool for nanoscale science and technology. J. Phys. D Appl. Phys. 2006, 39, R439–R459. [Google Scholar] [CrossRef]
- Cavaliere, E.; De Cesari, S.; Landini, G.; Riccobono, E.; Pallecchi, L.; Rossolini, G.; Gavioli, L. Highly bactericidal Ag nanoparticle films obtained by cluster beam deposition. Nanomed.-Nanotechnol. Biol. Med. 2015, 11, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, E.; Benetti, G.; Van Bael, M.; Winckelmans, N.; Bals, S.; Gavioli, L. Exploring the optical and morphological properties of Ag and Ag/TiO2 nanocomposites grown by supersonic cluster beam deposition. Nanomaterials 2017, 7, 442. [Google Scholar] [CrossRef] [PubMed]
- Benetti, G.; Cavaliere, E.; Banfi, F.; Gavioli, L. Antimicrobial nanostructured coatings: A gas phase deposition and magnetron sputtering perspective. Materials 2020, 13, 784. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Ström, G.; Fredriksson, M.; Stenius, P. Contact angles, work of adhesion, and interfacial tensions at a dissolving hydrocarbon surface. J. Colloid Interface Sci. 1987, 119, 352–361. [Google Scholar] [CrossRef]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Milani, P.; Iannotta, S. Cluster Beam Synthesis of Nanostructured Materials, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Barborini, E.; Kholmanov, I.; Piseri, P.; Ducati, C.; Bottani, C.; Milani, P. Engineering the nanocrystalline structure of TiO2 films by aerodynamically filtered cluster deposition. Appl. Phys. Lett. 2002, 81, 3052–3054. [Google Scholar] [CrossRef]
- Benetti, G.; Cavaliere, E.; Brescia, R.; Salassi, S.; Ferrando, R.; Vantomme, A.; Pallecchi, L.; Pollini, S.; Boncompagni, S.; Fortuni, B.; et al. Tailored Ag-Cu-Mg multielemental nanoparticles for wide-spectrum antibacterial coating. Nanoscale 2019, 11, 1626–1635. [Google Scholar] [CrossRef]
- Benetti, G.; Cavaliere, E.; Canteri, A.; Landini, G.; Rossolini, G.; Pallecchi, L.; Chiodi, M.; Van Bael, M.; Winckelmans, N.; Bals, S.; et al. Direct synthesis of antimicrobial coatings based on tailored bi-elemental nanoparticles. APL Mater. 2017, 5, 036105. [Google Scholar] [CrossRef]
- Benetti, G.; Caddeo, C.; Melis, C.; Ferrini, G.; Giannetti, C.; Winckelmans, N.; Bals, S.; Van Bael, M.; Cavaliere, E.; Gavioli, L.; et al. Bottom-up mechanical nanometrology of granular ag nanoparticles thin films. J. Phys. Chem. C 2017, 121, 22434–22441. [Google Scholar] [CrossRef]
- Benetti, G.; Gandolfi, M.; Van Bael, M.; Gavioli, L.; Giannetti, C.; Caddeo, C.; Banfi, F. Photoacoustic sensing of trapped fluids in nanoporous thin films: Device engineering and sensing scheme. ACS Appl. Mater. Interfaces 2018, 10, 27947–27954. [Google Scholar] [CrossRef] [PubMed]
- Depla, D.; Mahieu, S.; Greene, J.E. Chapter 5—Sputter Deposition Processes. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 253–296. [Google Scholar]
- Maréchal, N.; Quesnel, E.; Pauleau, Y. Silver thin films deposited by magnetron sputtering. Thin Solid Film. 1994, 241, 34–38. [Google Scholar] [CrossRef]
- Flint, K.P. The long-term survival of Escherichia coli in river water. J. Appl. Bacteriol. 1987, 63, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Clements, M.; Foster, S. Characterization of the starvation-survival response of Staphylococcus aureus. J. Bacteriol. 1998, 180, 1750–1758. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Kim, J.; Kuk, E.; Yu, K.; Kim, J.; Park, S.; Lee, H.; Kim, S.; Park, Y.; Park, Y.; Hwang, C.; et al. Antimicrobial effects of silver nanoparticles. Nanomed.-Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Urzua, L.; Sanchez-Mora, E.; Gonzalez, A.; Romo-Herrera, J.; Arciniega, J.; Morales, L. Damage on Escherichia coli and Staphylococcus aureus using white light photoactivation of Au and Ag nanoparticles. J. Appl. Phys. 2019, 125, 213102. [Google Scholar] [CrossRef]
- Lu, W.; Liu, G.; Gao, S.; Xing, S.; Wang, J. Tyrosine-assisted preparation of Ag/ZnO nanocomposites with enhanced photocatalytic performance and synergistic antibacterial activities. Nanotechnology 2008, 19, 445711. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Zhi, L.; Liu, X.; Jiang, W.; Sun, Y.; Yang, J. The synergetic antibacterial activity of Ag islands on ZnO (Ag/ZnO) heterostructure nanoparticles and its mode of action. J. Inorg. Biochem. 2014, 130, 74–83. [Google Scholar] [CrossRef]
- Agnihotri, S.; Bajaj, G.; Mukherji, S. Arginine-assisted immobilization of silver nanoparticles on ZnO nanorods: An enhanced and reusable antibacterial substrate without human cell cytotoxicity. Nanoscale 2015, 7, 7415–7429. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, L.; Wang, H.; You, Y.; Wang, Y.; Gao, N.; Ren, J.; Qu, X. Defect-rich adhesive nanozymes as efficient antibiotics for enhanced bacterial inhibition. Angew. Chem.-Int. Ed. 2019, 58, 16236–16242. [Google Scholar] [CrossRef] [PubMed]
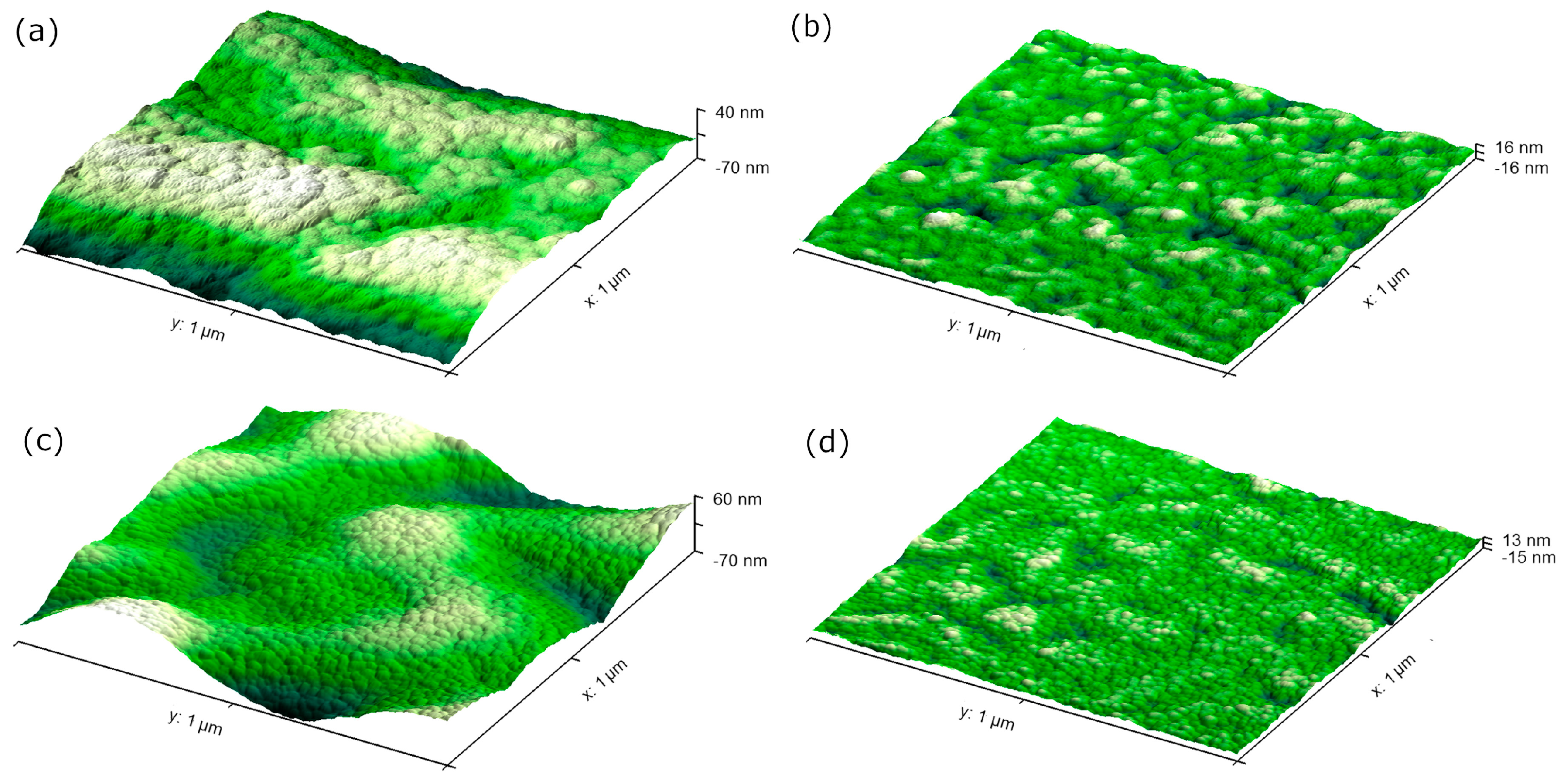
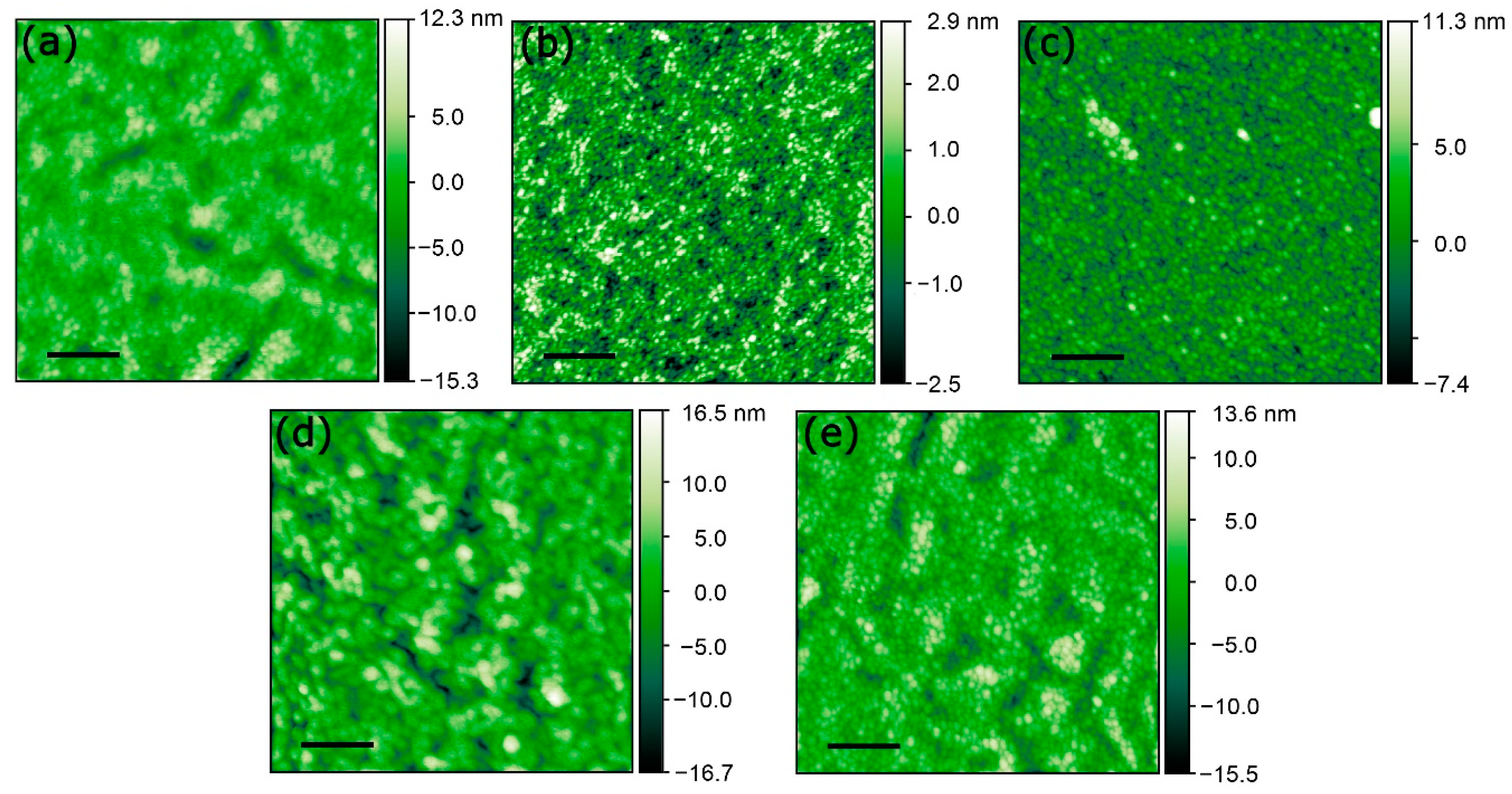
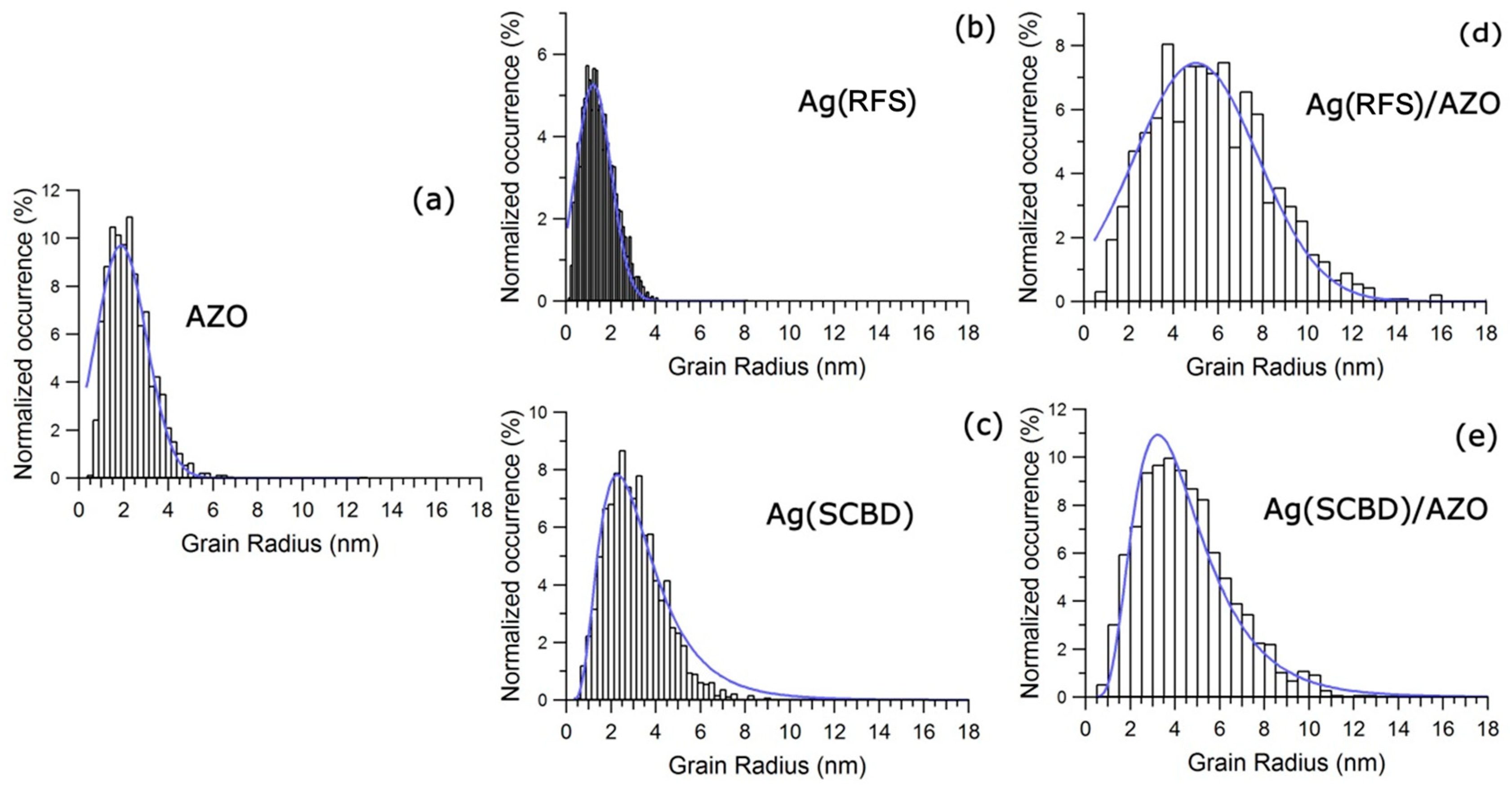
| Sample | Rms Roughness (±0.1 nm) | Height (±0.2 nm) | Distribution Width (±0.1 nm) |
|---|---|---|---|
| AZO | 2.4 | 1.8 | 1.6 |
| Ag(RFS) | 0.9 | 1.2 | 1.1 |
| Ag(SCBD) | 1.9 | 2.2 | 1.9 |
| Ag(RFS)/AZO | 4.2 | 5.0 | 3.9 |
| Ag(SCBD)/AZO | 3.3 | 3.7 | 2.5 |
| Ag(RFS)/Si | 3.6 | 4.3 | 4.4 |
| Ag(SCBD)/Si | 1.1 | 1.2 | 1.4 |
| Sample | Contact Angle (°) | Surface Energy (mJ/m2) | |||
|---|---|---|---|---|---|
| Water | Diiodomethane | Polar Component | Dispersion Component | Total | |
| AZO | 63.6 ± 3.2 | 55.8 ± 3.0 | 13.9 | 31.0 | 44.9 ± 2.7 |
| Ag(RFS) | 80.8 ± 2.3 | 49.6 ± 2.8 | 4.3 | 34.5 | 38.8 ± 1.8 |
| Ag(SCBD) | 91.8 ± 5.5 | 55.2 ± 2.4 | 1.6 | 31.3 | 32.9 ± 1.9 |
| Ag(RFS)/AZO | 93.7 ± 1.9 | 57.8 ± 3.9 | 1.4 | 29.9 | 31.3 ± 2.3 |
| Ag(SCBD)/AZO | 91.3 ± 5.9 | 55.9 ± 4.1 | 1.8 | 30.9 | 32.7 ± 2.8 |
| Bacterial Species | Sample | Antimicrobial Activity (A) | |||||
|---|---|---|---|---|---|---|---|
| Treatment Time (h) | |||||||
| 0.25 * | 0.6 | 1.5 | 3 | 5 | 8 | ||
| E. coli | AZO | 0.30 ± 0.30 | 2.59 ± 0.60 | 4.40 ± 0.04 | 6.30 ± 0.36 | 6.33 ± 0.28 | 5.90 ± 0.25 |
| Ag(RFS) | 6.36 ± 0.78 | 6.47 ± 0.53 | 6.32 ± 0.26 | 6.32 ± 0.26 | 6.40 ± 0.25 | 6.00 ± 0.18 | |
| Ag(SCBD) | 6.03 ± 1.00 | 6.36 ± 0.60 | 6.37 ± 0.08 | 6.37 ± 0.02 | 6.40 ± 0.70 | 6.08 ± 0.01 | |
| Ag(RFS)/AZO | 6.06 ± 0.50 | 6.47 ± 0.27 | 6.32 ± 0.26 | 6.32 ± 0.26 | 6.35 ± 0.20 | 6.05 ± 0.17 | |
| Ag(SCBD)/AZO | 6.60 ± 0.80 | 6.36 ± 0.38 | 6.37 ± 0.05 | 6.37 ± 0.02 | 6.40 ± 0.30 | 6.30 ± 0.01 | |
| S. aureus | AZO | n.d. | −0.08 ± 0.03 | 0.02 ± 0.08 | 0.35 ± 0.19 | 1.45 ± 0.25 | 4.44 ± 0.69 |
| Ag(RFS) | n.d. | 0.00 ± 0.03 | −0.15 ± 0.08 | 0.13 ± 0.15 | −0.05 ± 0.09 | 0.29 ± 0.26 | |
| Ag(SCBD) | n.d. | 0.00 ± 0.02 | −0.17 ± 0.04 | 0.45 ± 0.10 | 0.28 ± 0.19 | 0.19 ± 0.33 | |
| Ag(RFS)/AZO | n.d. | 0.02 ± 0.02 | 0.00 ± 0.10 | 0.49 ± 0.21 | 2.70 ± 0.73 | 5.96 ± 1.27 | |
| Ag(SCBD)/AZO | n.d. | −0.04 ± 0.03 | −0.10 ± 0.14 | 0.64 ± 0.20 | 1.96 ± 0.26 | 5.98 ± 1.70 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerini, D.; Tammaro, L.; Vigliotta, G.; Picariello, E.; Banfi, F.; Cavaliere, E.; Ciambriello, L.; Gavioli, L. Ag Functionalization of Al-Doped ZnO Nanostructured Coatings on PLA Substrate for Antibacterial Applications. Coatings 2020, 10, 1238. https://doi.org/10.3390/coatings10121238
Valerini D, Tammaro L, Vigliotta G, Picariello E, Banfi F, Cavaliere E, Ciambriello L, Gavioli L. Ag Functionalization of Al-Doped ZnO Nanostructured Coatings on PLA Substrate for Antibacterial Applications. Coatings. 2020; 10(12):1238. https://doi.org/10.3390/coatings10121238
Chicago/Turabian StyleValerini, Daniele, Loredana Tammaro, Giovanni Vigliotta, Enrica Picariello, Francesco Banfi, Emanuele Cavaliere, Luca Ciambriello, and Luca Gavioli. 2020. "Ag Functionalization of Al-Doped ZnO Nanostructured Coatings on PLA Substrate for Antibacterial Applications" Coatings 10, no. 12: 1238. https://doi.org/10.3390/coatings10121238
APA StyleValerini, D., Tammaro, L., Vigliotta, G., Picariello, E., Banfi, F., Cavaliere, E., Ciambriello, L., & Gavioli, L. (2020). Ag Functionalization of Al-Doped ZnO Nanostructured Coatings on PLA Substrate for Antibacterial Applications. Coatings, 10(12), 1238. https://doi.org/10.3390/coatings10121238








