Impact of Surface Functionalization by Nanostructured Silver Thin Films on Thermoplastic Central Venous Catheters: Mechanical, Microscopical and Thermal Analyses
Abstract
1. Introduction
2. Materials and Methods
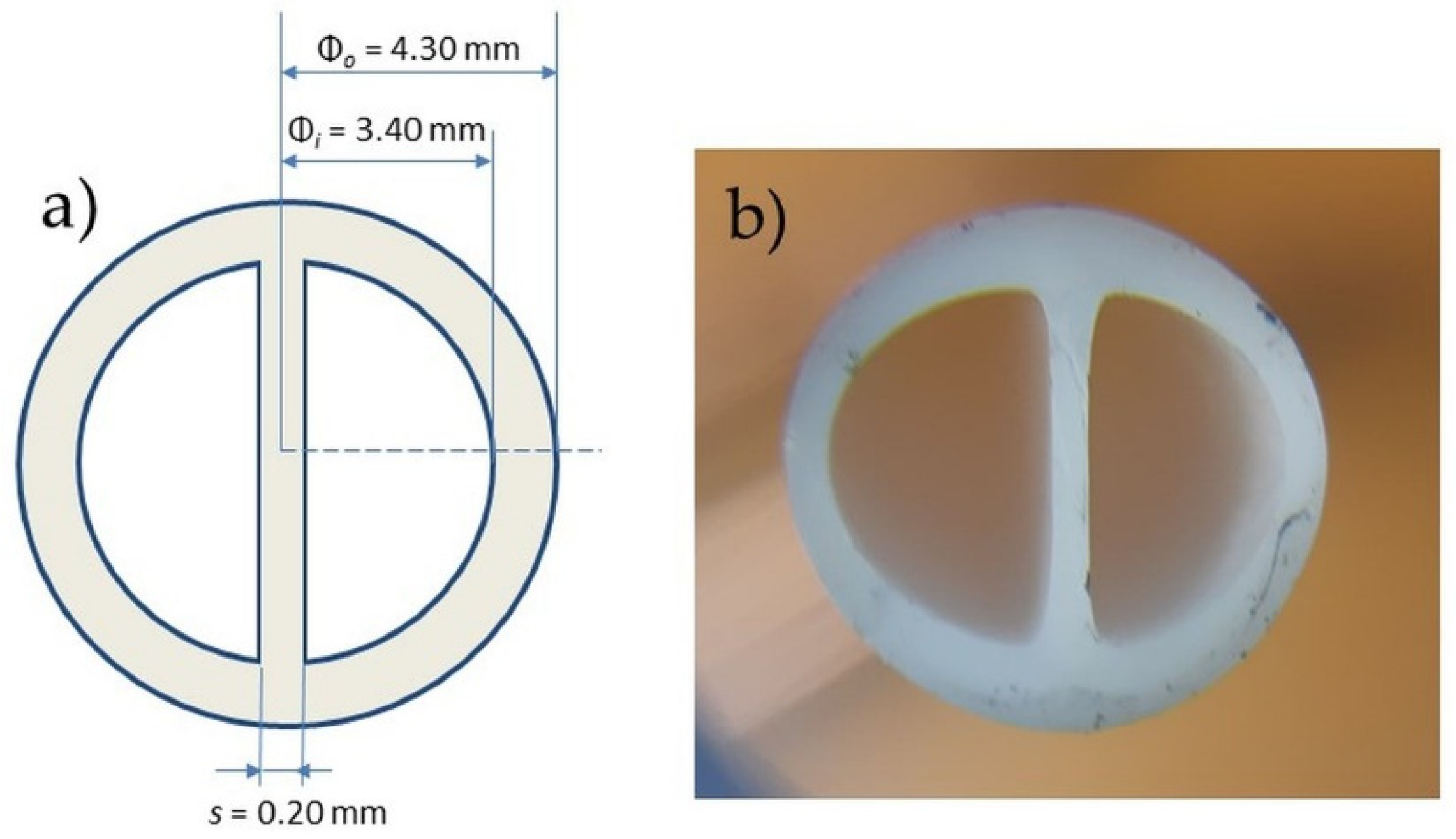
2.1. Central Venous Catheter
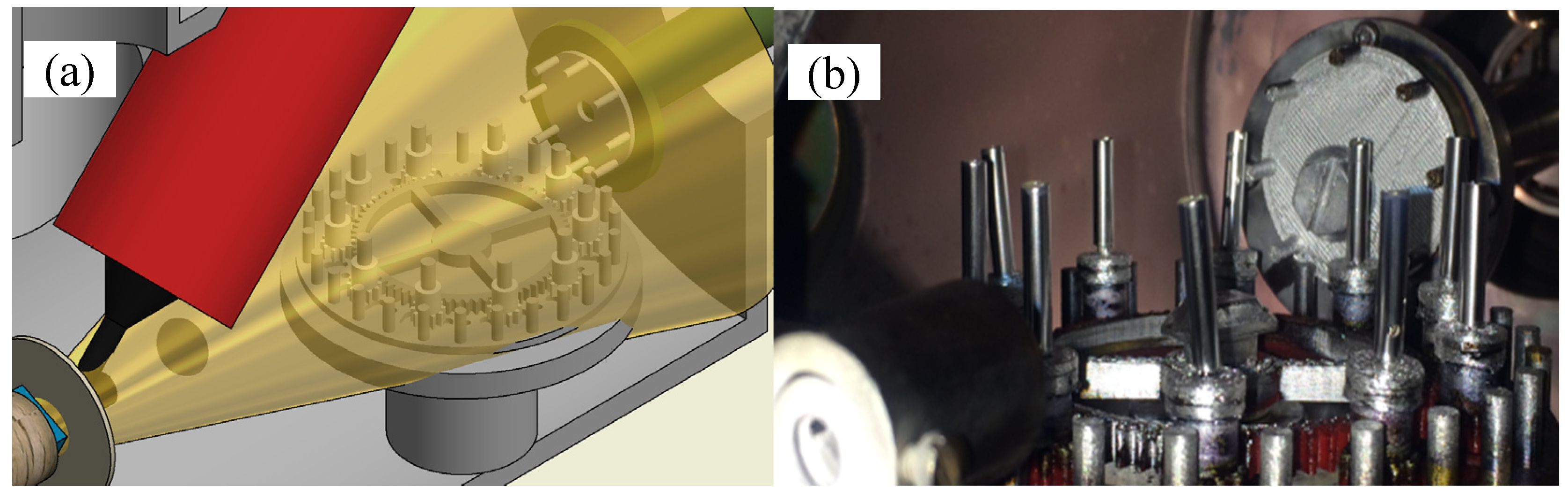
2.2. Silver Deposition
2.3. Uniaxial Tensile Test
2.3.1. Experimental
2.3.2. Finite Element Modeling
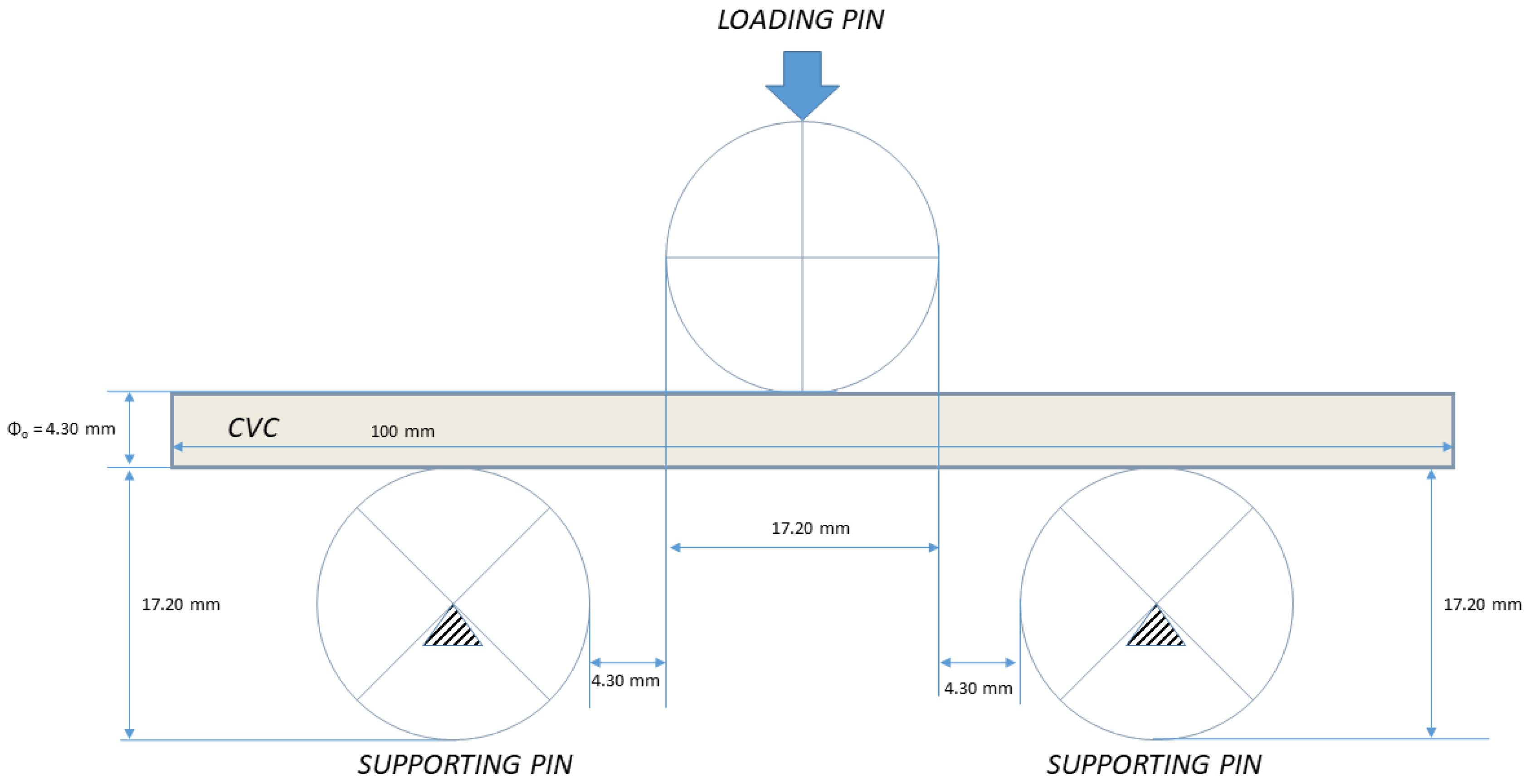
2.4. Bending Test
2.4.1. Experimental
2.4.2. Finite Element Modeling
2.5. Atomic Force Microscopy
2.6. Thermogravimetric and Differential Thermal Analysis
2.7. Notations and Statistical Analysis
3. Results and Discussion
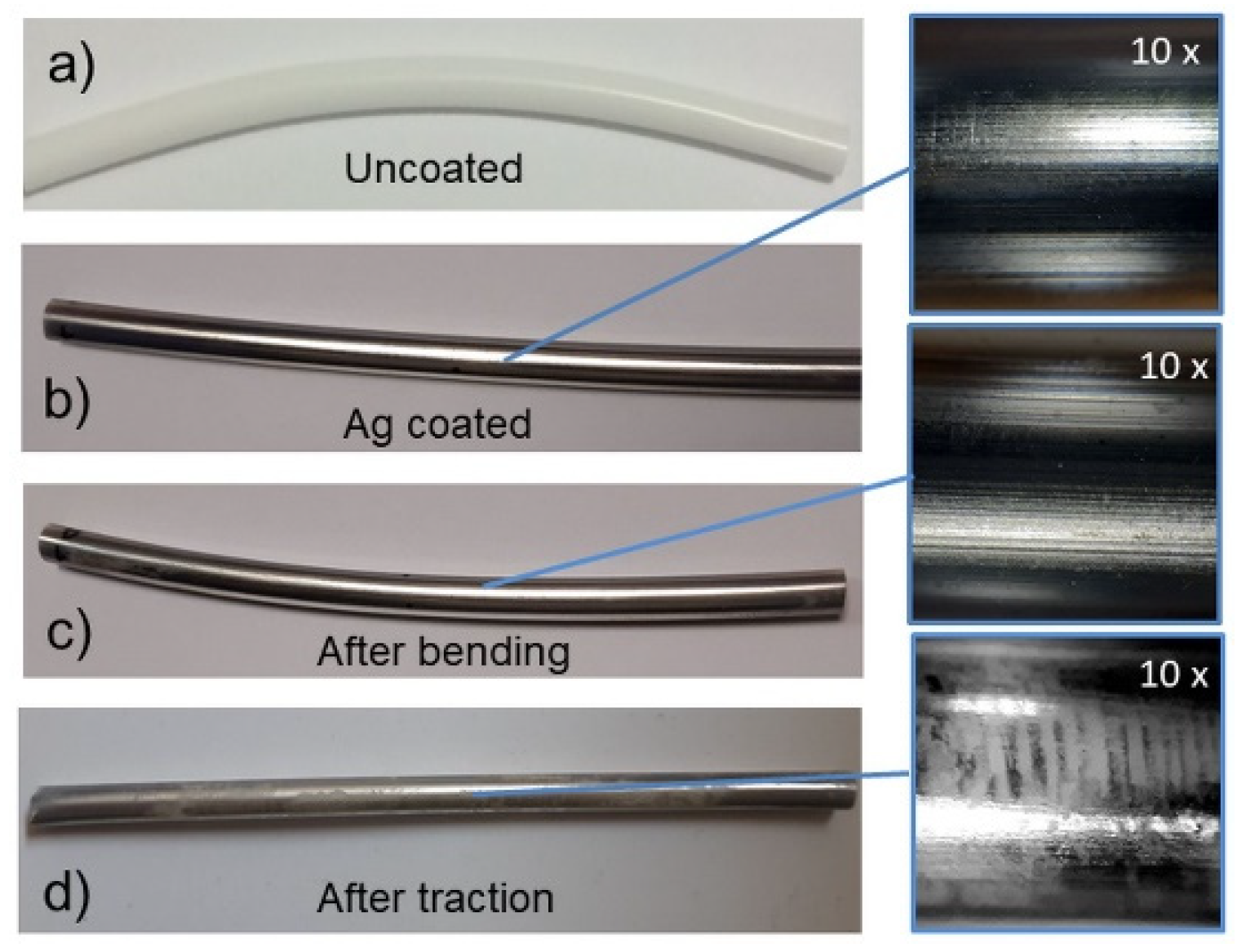
3.1. Effect of the Mechanical Stress on the Coating
3.2. Uniaxial Tensile Tests and FEA
3.3. Bending Tests and FEA
3.4. Atomic Force Microscopy
3.5. Thermogravimetric and Differential Thermal Analysis
3.6. Comparison with Similar Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bosco, R.; Van Den Beucken, J.; Leeuwenburgh, S.; Jansen, J. Surface Engineering for Bone Implants: A Trend from passive to Active Surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Fujibayashi, S.; Yamaguchi, S.; Yamamoto, K.; Otsuki, B.; Takemoto, M.; Tsukanaka, M.; Kizuki, T.; Matsushita, T.; Kokubo, T.; et al. Bioactivity of sol–gel-derived TiO2 coating on polyetheretherketone: In vitro and in vivo studies. Acta Biomater. 2016, 35, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Raua, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-ceramic coated Mg-Ca alloys for biomedical implant applications. Mater. Sci. Eng. C 2016, 64, 362–369. [Google Scholar] [CrossRef]
- Li, P.H.; Chu, P.K. Thin film deposition technologies and processing of biomaterials. In Thin Film Coatings for Biomaterials and Biomedical Applications, 1st ed.; Griesser, H.J., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 3–28. [Google Scholar]
- Chen, Y.H.; Hsu, C.C.; He, J.L. Antibacterial silver coating on poly (ethylene-terephthalate) fabric by using high power impulse magnetron sputtering. Surf. Coat. Technol. 2013, 232, 868–875. [Google Scholar] [CrossRef]
- Romanò, C.L.; Tsuchiya, H.; Morelli, I.; Battaglia, A.G.; Drago, L. Antibacterial coating of implants: Are we missing something? Bone. Jt. Res. 2019, 8, 199–206. [Google Scholar] [CrossRef]
- Chen, Y.M.; Dai, A.P.; Shi, Y.; Liu, Z.J.; Gong, M.F.; Yin, X.B. Effectiveness of silver-impregnated central venous catheters for preventing catheter-related blood stream infections: A meta-analysis. Int. J. Infect. Dis. 2014, 29, 279–286. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Keatch, R.; Corner, G.; Nabi, G.; Murdoch, S.; Davidson, F.; Zhao, Q. In-vitro antibacterial and anti-encrustation performance of silver-polytetrafluoroethylene nanocomposite coated urinary catheters. J. Hosp. Inf. 2019, 103, 55–63. [Google Scholar] [CrossRef]
- Wyatt, M.C.; Foxall-Smith, M.; Roberton, A.; Beswick, A.; Kieser, D.C.; Whitehouse, M.R. The use of silver coating in hip megaprostheses: A systematic review. Hip. Int. 2019, 29, 7–20. [Google Scholar] [CrossRef]
- Donati, F.; Di Giacomo, G.; Ziranu, A.; Spinelli, S.; Perisano, C.; Rosa, M.A.; Maccauro, G. Silver coated prosthesis in oncological limb salvage surgery reduce the infection rate. J. Biol. Regul. Homeost. Agents 2015, 29, 149–155. [Google Scholar]
- Gliga, A.R.; Skoglund, S.; Wllinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Bunshah, R.F. Deposition Technologies: An Overview. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew: Norwich, NY, USA, 2010; pp. 1–31. [Google Scholar]
- Bianchi, M.; Degli Esposti, L.; Ballardini, A.; Liscio, F.; Berni, M.; Gambardella, A.; Leeuwenburgh, S.C.G.; Sprio, S.; Tampieri, A.; Iafisco, M. Strontium doPEA calcium phosphate coatings on poly (etheretherketone) (PEEK) by pulsed electron deposition. Surf. Coat. Technol. 2017, 319, 191–199. [Google Scholar] [CrossRef]
- Lukowski, G.; Weihe, T.; Köhnlein, J.; Schlüter, R.; Schultze, N.; Quade, A.; Wendler, C.; Rackow, K.; Dittrich, A.; Werner, S.; et al. Renewable nano-structured coatings on medical devices prevent the transmission of clinically relevant pathogens. Surf. Coat. Technol. 2019, 366, 227–237. [Google Scholar] [CrossRef]
- Berni, M.; Carrano, I.; Kovtun, A.; Russo, A.; Visani, A.; Dionigi, C.; Liscio, A.; Valle, F.; Gambardella, A. Monitoring morphological and chemical properties during silver solid-state dewetting. Appl. Surf. Sci. 2019, 498, 143890. [Google Scholar] [CrossRef]
- Bianchi, M.; Gambardella, A.; Graziani, G.; Liscio, F.; Maltarello, M.C.; Boi, M.; Berni, M.; Bellucci, D.; Marchiori, G.; Valle, F.; et al. Plasma-assisted deposition of bone apatite-like thin films from natural apatite. Mater. Lett. 2017, 199, 32–36. [Google Scholar] [CrossRef]
- Bellucci, D.; Bianchi, M.; Graziani, G.; Gambardella, A.; Berni, M.; Russo, A.; Cannillo, V. Pulsed Electron Deposition of nanostructured bioactive glass coatings for biomedical applications. Ceram. Int. 2017, 43, 15862–15867. [Google Scholar] [CrossRef]
- Gambardella, G.; Berni, M.; Russo, A.; Bianchi, M. A comparative study of the growth dynamics of zirconia thin films deposited by ionized jet deposition onto different substrates. Surf. Coat. Technol. 2018, 337, 306–312. [Google Scholar] [CrossRef]
- Berni, M.; Marchiori, G.; Gambardella, A.; Boi, M.; Bianchi, M.; Russo, A.; Visani, A.; Marcacci, M.; Pavan, P.G. Lopomo, N.F. Effects of working gas pressure on zirconium dioxide thin film prepared by pulsed plasma deposition: Roughness, wettability, friction and wear characteristics. J. Mech. Behav. Biomed. Mater. 2017, 72, 200–208. [Google Scholar] [CrossRef]
- Berni, M.; Bontempi, M.; Marchiori, G.; Gambardella, A. Roughness conformality during thin films deposition onto rough substrates: A quantitative study. Thin Solid Films 2020, 709, 138258. [Google Scholar] [CrossRef]
- Shimizu, R.N.; Demarquette, N.R. Evaluation of surface energy of solid polymers using different models. J. Appl. Polym. Sci. 2000, 76, 1831–1845. [Google Scholar] [CrossRef]
- Müller, R.; Eidt, A.; Hiller, K.A.; Katzur, V.; Subat, M.; Schweikl, H.; Imazato, S.; Ruhl, S.; Schmalz, G. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials 2009, 30, 4921–4929. [Google Scholar] [CrossRef]
- Hora, J.; Hall, C.; Evans, D.; Charrault, E. Inorganic Thin Film Deposition and Application on Organic Polymer Substrates. Adv. Eng. Mater. 2018, 20, 1700868. [Google Scholar] [CrossRef]
- Hameri, A.P. Technology transfer between basic research and industry. Technovation 1996, 16, 51–92. [Google Scholar] [CrossRef]
- Van Norman, G.A.; Eisenkot, R. Technology Transfer: From the Research Bench to Commercialization: Part 2: The Commercialization Process. JACC Basic Transl. Sci. 2017, 2, 197–208. [Google Scholar] [CrossRef] [PubMed]
- ISO 10555-1. Intravascular Catheters–Sterile and Single-Use Catheters—Part 1: General Requirements, 2013; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- Skocdopole, J.; Kalvoda, L.; Nozar, P.; Netopilik, M. Preparation of polymeric coatings by ionized jet deposition method. Chem. Pap. 2018, 72, 1735–1739. [Google Scholar] [CrossRef]
- Skocdopole, J.; Aversa, L.; Golan, M.; Schenk, A.; Baldi, G.; Kratochvilova, I.; Kalvod, L.; Nozar, P. Preparing of the chameleon coating by the ionized jet deposition method. Acta Polytech. CTU Proc. 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Gambardella, A.; Berni, M.; Graziani, G.; Kovtun, A.; Liscio, A.; Russo, A.; Visani, A.; Bianchi, M. Nanostructured Ag thin films deposited by pulsed electron ablation. App. Surf. Sci. 2019, 475, 917–925. [Google Scholar] [CrossRef]
- Bush, J.D.; Schroder, H.; Sellenschloh, K.; Adam, G.; Ittrich, H.; Huber, G. Test Method for mechanical properties of implantable catheters according to DIN 10555-3. J. Mech. Behav. Biomed. Mater. 2018, 82, 183–186. [Google Scholar] [CrossRef] [PubMed]
- ASTM B571. Standard Practice for Qualitative Adhesion Testing of Metallic Coatings; ASTM International: West Conshohocken, PA, USA, 1997. [Google Scholar]
- Landry, D.L.; Jaber, R.A.; Hanumanthappa, N.; Lipkowitz, G.S.; O’Shea, M.H.; Bermudez, H.; Hathorne, A.P.; Braden, G.L. Effects of prolonged ethanol lock exposure to carbothane- and silicone-based hemodialysis catheters: A 26-week study. J. Vasc. Access 2015, 16, 367–371. [Google Scholar] [CrossRef]
- Mizubayashi, H.; Matsuno, J.; Tanimoto, H. Young’s modulus of silver films. Scr. Mater. 1999, 41, 443–448. [Google Scholar] [CrossRef]
- Aslam, S.; Darouiche, R.O. Mechanical Integrity of Hemodialysis Catheters is Maintained After Exposure to a Novel Catheter Lock Solution. Infect Control Hosp. Epidemiol. 2010, 31, 1124–1129. [Google Scholar] [CrossRef][Green Version]
- Opdahl, A.; Somorjai, G.A. Stretched Polymer Surfaces: Atomic Force Microscopy Measurement of the Surface Deformation and Surface Elastic Properties of Stretched Polyethylene. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2263–2274. [Google Scholar] [CrossRef]
- Volynskii, A.L.; Bazhenov, S.; Lebedeva, O.V.; Bakeev, N.F. Mechanical buckling instability of thin coatings deposited on soft polymer substrates. J. Mater. Sci. 2000, 35, 547–554. [Google Scholar] [CrossRef]
- Lan, Q.; Haugstad, G. Characterization of polymer morphology in polyurethane foams using atomic force microscopy. J. Appl. Polym. Sci. 2011, 121, 2644–2651. [Google Scholar] [CrossRef]
- Kojio, K.; Kugumiya, S.; Uchiba, Y.; Nishino, Y.; Furukawa, M. The Microphase-separated Structure of Polyurethane Bulk and Thin Films. Polym. J. 2009, 41, 118–124. [Google Scholar] [CrossRef]
- Tocha, E.; Janik, H.; Debowski, M.; Vansco, G.J. Morphology of polyurethanes revisited by complementary afm and tem. J. Macromol. Sci. B 2002, 41, 1291–1304. [Google Scholar] [CrossRef]
- Yanguas-Gil, A. Growth and Transport in Nanostructured Materials—Reactive Transport in PVD, CVD, and ALD; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Segala, K.; Dutra, R.L.; de Oliveira, E.N.; Rossi, L.M.; Matos, J.R.; Paula, M.M.S.; Franco, C.V. Characterization of poly-{trans-[RuCl2(vpy)(4)]-styrene-4-vinylpyridine} impregnated with silver nanoparticles in non aqueous medium. J. Braz. Chem. Soc. 2006, 17, 1679–1682. [Google Scholar] [CrossRef]
- Coutinho, F.M.B.; Delpech, M.C. Degradation profile of films cast from aqueous polyurethane dispersions. Polym. Degrad. Stab. 2000, 70, 49–57. [Google Scholar] [CrossRef]
- Mequanint, K.; Sanderson, R.; Pasch, H. Thermogravimetric study of phosphated polyurethane ionomers. Polym. Degrad. Stab. 2002, 77, 121–128. [Google Scholar] [CrossRef]
- Armstrong, G.; Buggy, M. Hydrogen-bonded supramolecular polymers: A literature review. J. Mater. Sci. 2005, 40, 547–559. [Google Scholar] [CrossRef]
- Dressler, M.; Reinsch, S.; Shadrack, R.; Benemann, S. Burnout behavior of ceramic coated open cell polyurethane (PU) sponges. J. Eur. Ceram. Soc. 2009, 29, 3333–3339. [Google Scholar] [CrossRef]
- Herrera, M.; Matuschek, G.; Kettrup, A. Thermal degradation of thermoplastic polyurethane elastomers (TPU) based on MDI. Polym. Degrad. Stab. 2002, 78, 323–331. [Google Scholar] [CrossRef]
- Bjorling, G.; Johansson, D.; Bergstrom, L.; Strekalovsky, A.; Sanchez, J.; Frostell, C.; Kalman, S. Evaluation of central venous catheters coated with a noble metal alloy—A randomized clinical pilot study of coating durability, performance and tolerability. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lim, J.K.; Park, J.J.; Huh, H.; Kim, D.J.; Gong, C.H.; Yoon, S.Z. Chlorhexidine and silver sulfadiazine coating on central venous catheters is not sufficient for protection against catheter-related infection: Simulation-based laboratory research with clinical validation. J. Int. Med. Res. 2017, 45, 1042–1053. [Google Scholar] [CrossRef]
- Heard, S.O.; Wagle, M.; Vijayakumar, E.; McLean, S.; Brueggmann, A.; Napolitano, L.M.; Edwards, L.P.; O’Connell, F.M.; Puyana, J.C.; Doern, G.V. Influence of Triple-Lumen Central Venous Catheters Coated With Chlorhexidine and Silver Sulfadiazine on the Incidence of Catheter-Related Bacteremia. Arch Intern Med. 1998, 158, 81–87. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M.; Deponti, D.; Di Giancamillo, A.; Peretti, G.; Sannino, A. Effect of silver nanocoatings on catheters for haemodialysis in terms of cell viability, proliferation, morphology and antibacterial activity. J. Mater. Sci. Mater. Med. 2013, 24, 1105–1112. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, M.; Catalano, M.; Taurino, A.; Licciulli, A.; Maffezzoli, A.; Sannino, A. Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. J. Mater. Sci. Mater. Med. 2011, 22, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, Y.; Zhang, Y.; Deng, J.; Lin, C. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int. J. Nanomed. 2015, 10, 7241–7252. [Google Scholar] [CrossRef]
- Lupoi, R.; O’Neill, W. Deposition of metallic coatings on polymer surfaces using cold spray. Surf. Coat. Technol. 2010, 205, 2167–2173. [Google Scholar] [CrossRef]
- Chena, C.; Xiea, X.; Xiea, Y.; Yana, X.; Huanga, C.; Denga, S.; Renc, Z.; Liaoa, H. Metallization of polyether ether ketone (PEEK) by copper coating via cold spray. Surf. Coat. Technol. 2018, 342, 209–219. [Google Scholar] [CrossRef]

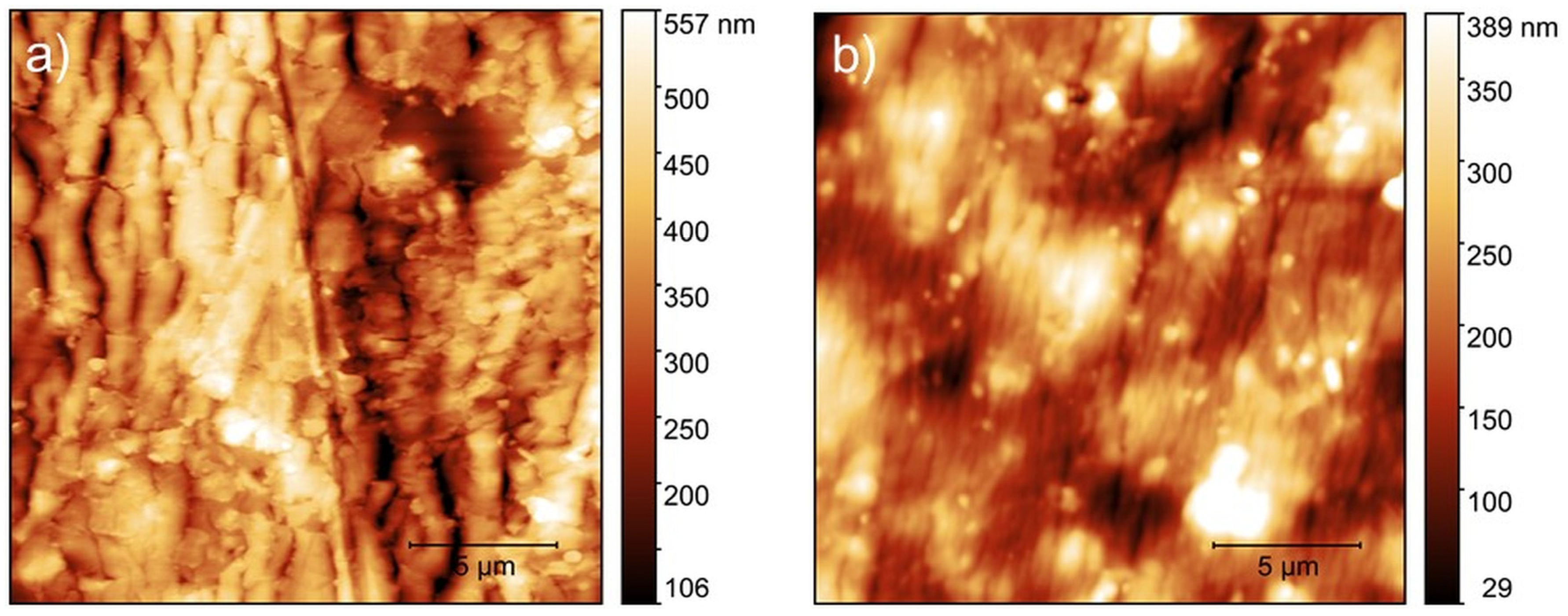
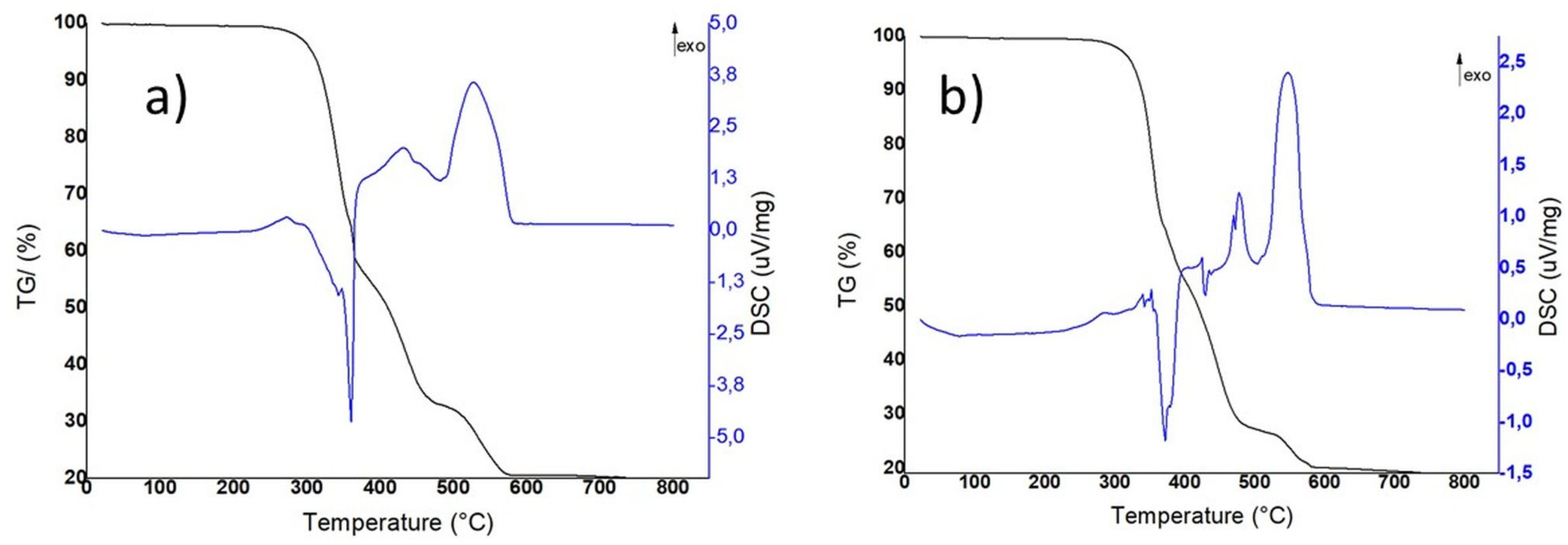
| Group | ST (N/mm) | Fu (N) | du (mm) |
|---|---|---|---|
| Uncoated | 9.47 ± 1.73 (18.3%) | 165.1 ± 8.6 (5.2%) | 184.9 ± 6.6 (3.6%) |
| Ag Coated | 9.92 ± 1.15 (11.6%) | 165 ± 4 (2.5%) | 189.2 ± 6.9 (3.7%) |
| - | Case | ST (N/mm) |
|---|---|---|
| h = 180 nm | −10% h | 9.87 |
| h | 9.92 | |
| +10% h | 9.96 |
| Sample | Before Tensioning | After Tensioning |
|---|---|---|
| Uncoated | 54.6 ± 8.2 | 56.3 ± 6.6 |
| Coated | 94.6 ± 14.7 | 100.5 ± 30.2 |
| Delaminated | - | 75.8 ± 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchiori, G.; Gambardella, A.; Berni, M.; Bellucci, D.; Cassiolas, G.; Cannillo, V. Impact of Surface Functionalization by Nanostructured Silver Thin Films on Thermoplastic Central Venous Catheters: Mechanical, Microscopical and Thermal Analyses. Coatings 2020, 10, 1034. https://doi.org/10.3390/coatings10111034
Marchiori G, Gambardella A, Berni M, Bellucci D, Cassiolas G, Cannillo V. Impact of Surface Functionalization by Nanostructured Silver Thin Films on Thermoplastic Central Venous Catheters: Mechanical, Microscopical and Thermal Analyses. Coatings. 2020; 10(11):1034. https://doi.org/10.3390/coatings10111034
Chicago/Turabian StyleMarchiori, Gregorio, Alessandro Gambardella, Matteo Berni, Devis Bellucci, Giorgio Cassiolas, and Valeria Cannillo. 2020. "Impact of Surface Functionalization by Nanostructured Silver Thin Films on Thermoplastic Central Venous Catheters: Mechanical, Microscopical and Thermal Analyses" Coatings 10, no. 11: 1034. https://doi.org/10.3390/coatings10111034
APA StyleMarchiori, G., Gambardella, A., Berni, M., Bellucci, D., Cassiolas, G., & Cannillo, V. (2020). Impact of Surface Functionalization by Nanostructured Silver Thin Films on Thermoplastic Central Venous Catheters: Mechanical, Microscopical and Thermal Analyses. Coatings, 10(11), 1034. https://doi.org/10.3390/coatings10111034








