Abstract
Paper packaging materials have been widely applied in our daily life. To maintain the quality of packed goods as well as the mechanical property, there is a need to enhance the paper water vapor barrier function. Although long-chain cellulose esters with saturated aliphatic chains have been employed as barrier coatings due to their excellent hydrophobicity as well as film-forming properties, the coated unsaturated cellulose esters would be beneficial to design reactive materials to further enrich their functionalities, e.g., antibacterial performance. Herein, solutions of cellulose undecenoyl esters (CUEs) were bar-coated to base papers. Obvious coating films were formed on the paper surfaces from the coating grammage of 6.25 g m−2. The resulting CUE-coated papers displayed good mechanical performance, hydrophobicity, and water vapor barrier property (the barrier ratio up to 66.35%), and the best coating grammage was 11.62 g m−2. Additionally, the reactivity of the coated paper was evaluated by further immobilization of the antibacterial agent (polyhexamethylene guanidine hydrochloride (PHGH)) using photo-click thiol-ene and condensation reaction. The generated paper exhibited good antibacterial and water vapor barrier performance. The obvious reactivity of our CUE-coated paper indicated the great possibility to design multi-functional paper packaging materials.
1. Introduction
Water vapor barrier property is of great importance for packaging materials. The decrease in the water loss would be beneficial to the quality of packed goods [,]. Although the ubiquitous plastic packaging materials have excellent moisture barrier performance, they result in environmental, energy and health problems [,]. In this case, paper packaging materials have emerged due to their biodegradability, recyclability, renewability, flexibility, low cost, lightweight, good mechanical strength, and printability [,]. However, on one hand, the hygroscopicity is the intrinsic weakness for paper packaging materials due to the abundant hydroxyl groups; on the other hand, the existing porous structure of paper itself also caused its poor barrier performance. Accordingly, the quality of packed goods as well as the mechanical property of paper would be affected [,,]. Therefore, there is a need to enhance the paper water vapor barrier function for packaging applications.
To meet the above-mentioned requirements, paper is usually coated or laminated by hydrophobic polymers, and the commonly used polymers are synthetic polymers, e.g., polyethylene []. Recently, natural polymers have attracted great attention due to the promotion of a sustainable development []. Among them, cellulose has been known as the most abundant natural polymers on the earth, and it would be desirable as coating materials. However, its existing large amount of hydroxyl groups results in its natural hygroscopicity. In this case, cellulose needs to be modified to generate hydrophobic cellulose derivatives [,]. Long-chain cellulose esters, as one of the most studied cellulose derivatives, show excellent hydrophobicity as well as film-forming properties, which have been employed for paper barrier coatings [,]. Furthermore, concerning the coating strategy, dispersion coating method is preferred for barrier application due to the formation of a uniform and dense polymer film on paper surfaces []. In Willberg-Keyriläinen’s research group, they coated cellulose esters onto kraft paper and found that cellulose C16 ester coating with a grammage of 52 g m−2 and a coating thickness of 12.2 μm decreased the WVTR of kraft paper by 84% []. In Havimo’s research, the chloroform or acetone solutions of cellulose hexanoates and cellulose palmitates were leveled to the paperboard surface by a coating grammage of approximately 10 g m−2. The water vapor barrier properties were obviously enhanced by the coating, and the attached chain length in the cellulose structure showed a close relationship with the water vapor transmission performance [].
Although the cellulose esters have been known as excellent barrier coatings for paper and board, there is no reactivity for the coatings which could be further functionalized to broaden the application scope of barrier papers, e.g., antibacterial performance, which is crucial for food packaging to not only inhibit bacteria from being contacted with the paper surface but also prevent the bacterial growth and even kill them [].
In our previous work, we have synthesized insoluble cellulose 10-undecenoyl ester (CUE) []. After spray-coating onto filter paper, the CUE-coated paper displayed superhydrophobicity and reactivity. Morphology analysis showed irregular protuberances with a claw-like bulge of the coated CUE particles on the paper surface resulted in the micro/nanostructure necessary for the superhydrophobic performance. Since the CUE particles could not totally cover the pore of the underneath paper, the coated paper exhibited negligible effect on the moisture barrier property. To endow the CUE with good film-forming performance for barrier application, in this work, we synthesized soluble CUE and coated their solution onto base papers by bar coater. With the thorough investigation of the physical properties, morphology, mechanical property, wettability, and water vapor barrier property of the coated papers with various coating grammages, the optimal coating amount was concluded. Further attachment of PHGH onto the CUE-coated paper endowed its good antibacterial properties, indicating the reactivity of the CUE coating.
2. Materials and Methods
2.1. Materials
Bleached bagasse pulp (Guangxi, China), microcrystalline cellulose (MCC) (50 μm, Sigma-Aldrich, Darmstadt, Germany), 10-undecenoyl chloride (98%, Macklin), 3-mercaptopropionic acid (MPA, 98%, Aladdin, Shanghai, China), 2,2-dimethoxy-2-phenyl-acetophenone (DMPA, 99%, Aladdin), 1-(3-dimethyl-aminopropyl)-3-ethyl-carbodiimide hydrochloride (EDC, 98%, Aladdin), pyridine (AR, Aladdin), ethanol (AR, Aladdin) and chloroform (AR, Aladdin), Polyhexamethyleneguanidine hydrochloride (≥95.0%, 533.03 Da, Guangdong Wengjiang, Shaoguan, China), Nutrient agar (Qingdao Hope Bio-Technology, Qingdao, China) and Escherichia coli (E. coli) (China Center of Industrial Culture Collection, Beijing, China) were used as received.
2.2. Synthesis of Cellulose 10-Undecenoyl Ester (CUE)
CUE was synthesized according to our previous work with minor modification []. Briefly, pre-dried MCC (1.3 g, 8.0 mmol, 1 eq) was suspended in pyridine (40 mL) and heated at 100 °C. 10-Undecenoyl chloride (10.3 mL, 48.0 mmol, 6 eq) was then added into the above suspension. After reaction for 1 h, the raw product was obtained by precipitating the above solution in ethanol (200 mL) and separating by centrifugation. The product was further purified through redissolution in THF and reprecipitation in ethanol for three times. The resulting CUE was finally dried in vacuum to get a white solid (4.5 g).
2.3. Preparation of CUE-Coated Paper
Base papers with a basis weight of 102 ± 2 g m−2 (thickness of 138.6 ± 3.5 µm) were prepared by the conventional Rapid-Koethen hand sheet maker without any additives and fillers by the bagasse pulp (46°SR). Subsequently, the chloroform solutions of CUE with a concentration of 2.5 wt % (viscosity: 5.58 ± 2.81 mPa s) were coated onto the above base papers by bar coater (coating speed: 50 mm s−1, profile rod: 16 μm-#07, ZAA 2300, Zehntner Testing Instruments, Gewerbestrasse, Switzerland). Through the change of the coating times, CUE-coated papers with coating grammages of 0.97, 2.47, 6.25, 11.62 and 21.38 g m−2 were generated. After coating, the resulting papers were dried at room temperature (RT) overnight. Accordingly, the coated papers were named as CUE0.97-coated paper, CUE2.47-coated paper, CUE6.25-coated paper, CUE11.62-coated paper and CUE21.38-coated paper, respectively.
2.4. Characterization
2.4.1. Chemical Structure
The synthesized CUE was characterized by 1H and 13C NMR spectroscopy recorded in CDCl3 at RT using an AVANCE III HD500 spectrometer (Bruker, Ettlingen, Germany). Moreover, the chemical structure of unmodified paper, CUE-coated papers, MPA-attached CUE-coated paper and PHGH-attached CUE-coated paper was characterized by ATR FTIR spectroscopy (TENSOR II, Bruker) with the record range from 4000 to 400 cm−1. Additionally, the CUE-coated papers with various coating grammages were further characterized by an ESCALab 250Xi X-ray photoelectron spectrometer (XPS, ThermoFisher Scientific, Waltham, MA, USA) with an Al X-ray source operated at 150 W.
2.4.2. Physical Properties
The coating thickness of the coated papers was calculated by the average thickness difference between the unmodified paper and CUE-coated papers at the same point by a digital micrometer (Büchel B. V., The TMI Group Company, Veenendaal, The Netherland). The Bekk smoothness of the unmodified paper and coated papers was measured by the automatic Bekk smoothness and porosity tester (K533, Büchel B. V., The TMI Group Company) according to NEN 2014.
2.4.3. Morphology
The scanning electron microscopy (SEM) images of CUE-coated papers with various coating grammages in comparison with unmodified paper were captured by scanning electron microscope (PHENOM F16502, Eindhoven, Netherland) at 10 kV. Samples were gold-sputtered before measurement.
2.4.4. Mechanical Property
The tensile index and elongation at break of unmodified paper and CUE-coated papers with various coating grammages were measured according to ISO1924-3 by a tensile tester (Lorentzen&Wettre, Kista, Sweden). Each sample was tested for five times.
2.4.5. Contact Angle
The contact angle images (after stabilization for 15 s) of unmodified paper and CUE-coated papers with various coating grammages was taken by a contact angle measuring instrument (DSA 100, KRUSS, Hamburg, Germany) at a droplet volume of 4 μL (deionized water), and the according values were automatically calculated.
2.4.6. Water Vapor Transmission Rates
The water vapor transmission rates (WVTRs) of unmodified paper and CUE-coated papers with various coating grammages were measured by a wet cup method at 22 °C and 51% RH (TSY-T1, Labthink, Jinan, China) []. The barrier ratio was calculated to be the ratio of the WVTR value difference between the CUE-coated paper as well as unmodified paper and the WVTR value of the unmodified paper.
2.4.7. Reactivity
The reactivity of CUE-coated paper was identified by the chemical immobilization of MPA through a photoclick thiol-ene reaction followed by the condensation reaction between the attached carboxylic groups and amine groups from polyhexamethylene guanidine hydrochloride (PHGH). In brief, the CUE11.62-coated paper with a size of 3 cm × 3 cm was impregnated into an ethanol solution of MPA (0.058 mol L−1) and DMPA (0.003 mol L−1, catalyst). Upon UV illumination for 10 min, the modified paper was washed by ethanol and dried (named as MPA-attached CUE-coated paper). The above paper was then immersed into an aqueous solution of EDC (0.05 mol L−1) and PHGH (0.037 mol L−1) followed by shaking at RT for 30 min and thoroughly washing by water and drying (named as PHGH-attached CUE-coated paper).
2.4.8. Antibacterial Property
The minimal inhibitory concentration (MIC) of PHGH was measured according to the literature []. Accordingly, the antibacterial property of PHGH-attached CUE-coated paper was then investigated by resisting to the Gram-negative bacteria Escherichia coli (E. coli) through ring diffusion method with CUE-coated paper as reference. In detail, 0.1 mL of E. coli cell suspension of 106 CFU mL−1 was spread on nutrient agar plates with roundish paper (Φ 10 mm) putting on the surface of the agar followed by incubation at 37 °C for 20 h. Images of the according systems were finally captured.
3. Results and Discussion
3.1. Chemical Structure
The 1H and 13C NMR spectra of the synthesized CUE are shown in Figure 1. In the 1H NMR spectrum (Figure 1a), the peaks at 1.00~1.70, 2.00, 2.29, 3.00~5.25, 5.77 ppm were ascribed to the hydrogen from the methylene groups, the methylene groups adjacent to the C=C groups, the methylene groups adjacent to the ester groups, the anhydroglucose units of cellulose including the methylene groups from the C=C group, and the methine groups from the C=C group of the attached undecenoyl groups, respectively []. As for its 13C NMR spectrum (Figure 1b), the peaks at 25.10, 29.14, 34.16, 62.15~101.51, 114.41, 139.25, 171.92~173.26 ppm were attributed to the carbons from the methylene groups adjacent to the ester groups, the methylene groups, the methylene groups adjacent to the C=C group, the cellulose backbone, the methylene groups from the C=C groups, the methine groups from the C=C groups of the undecenoyl groups, and the ester groups, respectively []. These NMR results indicated the successful synthesis of the CUE compound. Furthermore, according to the integral areas of the signals of the cellulose backbone and the C=C groups from the attached undecenoyl groups, the DS value was calculated to be 2.75 according to the literature. Such high DS value of the generated CUE would lead to its good solubility in comparison with our previously synthesized insoluble CUE particle with a low DS value (0.62) [].
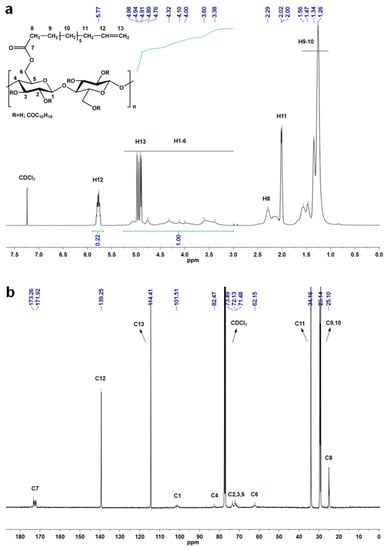
Figure 1.
(a) 1H and (b) 13C NMR spectra of CUE (CDCl3 as solvent) with inserted molecular structure of CUE.
Furthermore, chloroform solutions of the synthesized CUE compounds were coated onto the paper surfaces with various coating grammages, and the FTIR spectrum of the generated CUE-coated paper in comparison with that of the CUE and unmodified paper is demonstrated in Figure 2. The peaks at 3068, 2920, 2854, 1740, 1637, 903, and 722 cm−1 in the CUE were ascribed to the stretching vibration of the =C–H groups, asymmetric stretching vibration and symmetrical stretching vibration of the methylene groups, the stretching vibration of the ester groups and the C=C groups, the bending vibration of the =C-H and methylene groups, respectively [,]. As expected, the CUE-coated paper showed all the signals of the CUE compared with the unmodified paper. Interestingly, the peak at 3307 cm−1 in the unmodified paper that was ascribed to the stretching vibration of the hydroxyl groups blue shifted to 3480 cm−1 and the peak intensity also reduced in the CUE-coated paper. This phenomenon could be explained by the fact that not only the coating hided the signals of paper itself during the ATR FTIR measurement, but also the attachment of the undecenoyl groups to the hydroxyl groups decreased their signals and the formed hydrogen bonds []. However, the existing signals of the hydroxyl groups in CUE and CUE-coated paper indicated the part substitution of the undecenoyl groups onto the cellulose backbone, which was consistent with the calculated DS value from the above 1H NMR result.
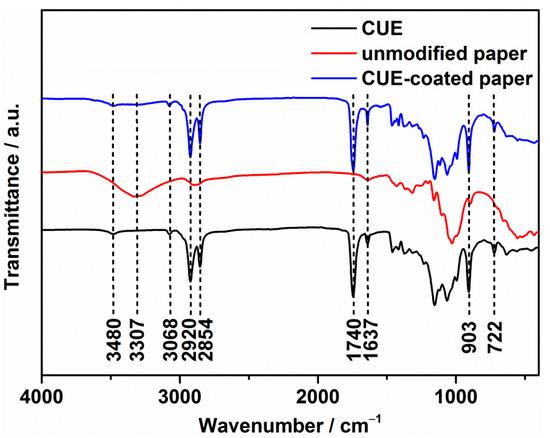
Figure 2.
FTIR spectra of CUE, unmodified paper, and CUE-coated paper (CUE11.62-coated paper).
The CUE-coated paper sheets with various coating grammages were further investigated by XPS spectroscopy. The corresponding high-resolution spectra of C 1s with peak differentiation imitating analysis of the unmodified paper and CUE0.97-coated paper as well as C1s XPS spectra of CUE0.97-coated paper, CUE2.47-coated paper, CUE6.25-coated paper, CUE11.62-coated paper, and CUE21.38-coated paper are displayed in Figure 3a–c, respectively. As seen from Figure 3a, the three peaks at approximately 283.8, 285.5 and 286.8 eV that were attributed to the C–C/H, C–O, and O–C–O structures appeared in the unmodified paper []. Moreover, as for the unmodified paper, the peak area of the C–O structure was bigger than that of the other two structures, which was consistent with the cellulose structure. After coating of CUEs on its surface, a new peak at approximately 287.7 eV emerged (Figure 3b), which was ascribed to the O–C=O structure []. Furthermore, the peak area of the C-C/H structure in the CUE-coated paper increased in comparison with that of the unmodified paper, while the peak area of the C–O and O–C–O structures decreased. All these results suggested that CUEs had been successfully coated on the paper surface. Additionally, Figure 3c demonstrated the peak intensity of C–C/H structures rose with the increase in the coating grammages, which matched well with our preparation procedures.
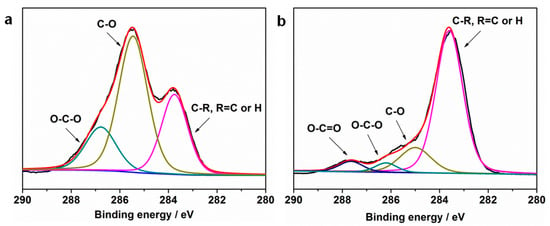
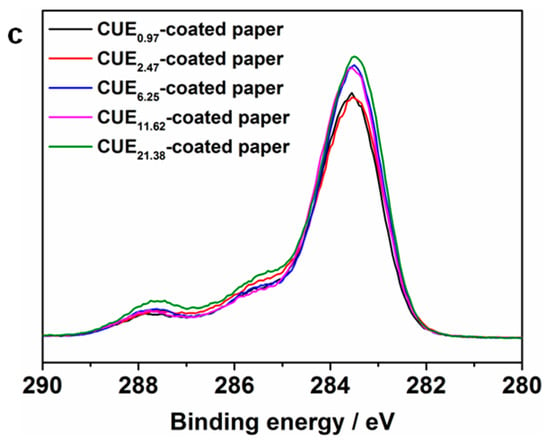
Figure 3.
High-resolution C1s XPS deconvolution spectra of unmodified paper (a) and CUE0.97-coated paper (b); and (c) C1s XPS spectra of CUE0.97-coated paper, CUE2.47-coated paper, CUE6.25-coated paper, CUE11.62-coated paper and CUE21.38-coated paper.
3.2. Physical Properties
The coating thickness and Bekk smoothness of the coated paper was investigated, and the results are shown in Table 1. Please note that the Bekk smoothness was expressed as air penetration time through the paper. The longer the time was used, the higher smoothness of paper was. As for CUE0.97-coated paper, the coating thickness could not be measured and its Bekk smoothness (578 ± 23 s) was similar to that of the unmodified paper (539 ± 16 s). This phenomenon might be due to the relatively low coating grammage. With the further increase in the coating grammage, the coating thickness of the CUE2.47-coated paper, CUE6.25-coated paper, CUE11.62-coated paper, and CUE21.38-coated paper was 1.0 ± 0.4, 3.7 ± 1.7, 8.7 ± 0.9, and 18.3 ± 0.9 μm, respectively. Obviously, the coating thickness increased with the increment in the coating grammage as expected. Additionally, their Bekk smoothness was 639 ± 33, 2689 ± 103, 4197 ± 255, 4182 ± 399 s, respectively. It can be seen that when the coating grammage was 6.25 g m−2, the Bekk smoothness underwent sharp increase, indicating a smoother surface was generated.

Table 1.
Coating thickness and air penetration time of CUE0.97-coated paper, CUE2.47-coated paper, CUE6.25-coated paper, CUE11.62-coated paper and CUE21.38-coated paper.
3.3. Morphology
To further understand the above-mentioned physical properties, the morphology of unmodified paper and CUE-coated paper with various coating grammages was observed by SEM, and the captured pictures are displayed in Figure 4. As shown in Figure 4a,b, cellulose fibers interlaced with each other in the unmodified paper, while the CUE-coated paper with a lower coating grammage (CUE0.97-coated paper) almost exhibited no obvious surface change. Therefore, its coating thickness was difficult to be determined. With the further increase in the coating grammage, the CUEs covered on the cellulose fibers as well as filled in the pores of the paper with the final film formation on the surfaces (Figure 4c–f). This surface morphology was obviously different from that of the superhydrophobic CUE-coated paper in our previous work which exhibited micro/nanostructure particles on the paper surface []. When the coating grammage was 6.25 g m−2, the paper surface almost covered with CUE film. This phenomenon matched well with the Bekk smoothness result. Additionally, the cross-section images of the unmodified paper and CUE11.62-coated paper are shown in Figure 4g,h. Compared with the unmodified paper, an obvious film was observed on the CUE11.62-coated paper surface, which would be desirable for the water vapor barrier.
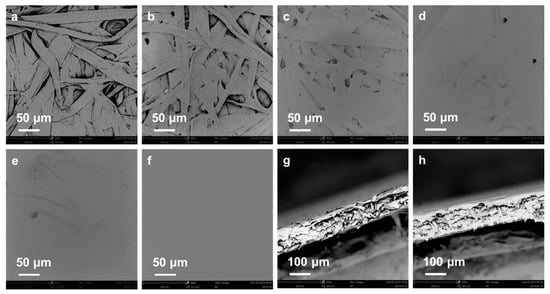
Figure 4.
SEM surface images of unmodified paper (a), CUE0.97-coated paper (b), CUE2.47-coated paper (c), CUE6.25-coated paper (d), CUE11.62-coated paper (e), and CUE21.38-coated paper (f); and SEM cross-section images of unmodified paper (g) and CUE11.62-coated paper (h).
3.4. Mechanical Property
The mechanical properties of packaging materials directly affect their practical application. Herein, the tensile index and elongation at break of the coated papers were measured. According to Figure 5, the unmodified paper showed a tensile index of 41.07 ± 0.80 N m g−1 and elongation at break of 2.44% ± 0.06%. After coating, the tensile index of the resulting paper sheets with various coating grammages almost displayed insignificant changes. This result could be due to the low coating thickness or grammage of the coated papers []. Moreover, the CUE-coated papers displayed a slightly higher elongation at break, which indicated the plasticity of the CUE coatings [].
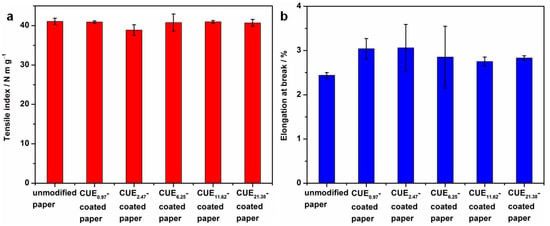
Figure 5.
Tensile index (a) and elongation at break (b) of unmodified paper, CUE0.97-coated paper, CUE2.47-coated paper, CUE6.25-coated paper, CUE11.62-coated paper and CUE21.38-coated paper.
3.5. Wettability
The wettability is expressed as contact angle. Generally, the contact angle depends both on the surface energy and surface roughness. Figure 6 describes the contact angle of unmodified paper, CUE0.97-coated paper, CUE2.47-coated paper, CUE6.25-coated paper, CUE11.62-coated paper and CUE21.38-coated paper with inserted contact angle images taken after 15 s. It could be seen that the unmodified paper was hydrophilic, while the CUE-coated paper sheets were hydrophobic with the contact angle ranging from 117° ± 1° to 101° ± 1°. In comparison with that of unmodified paper which had the similar level of surface smoothness (shown in Table 1), the obviously increased contact angle of CUE0.97-coated paper and CUE2.47-coated paper could be due to the lower surface energy of the CUE coatings resulted from the attached hydrophobic undecenoyl groups. Furthermore, from the coating grammage of 6.25 g m−2, the contact angle of the coated papers slightly decreased, which could be attributed to their sharply increased surface smoothness. Additionally, we found that the CUE coating prepared by soluble CUEs displayed a lower surface contact angle compared with that made of insoluble CUEs as demonstrated in our previous work []. This phenomenon could be due to the formed CUE film with a lower surface roughness in this work.
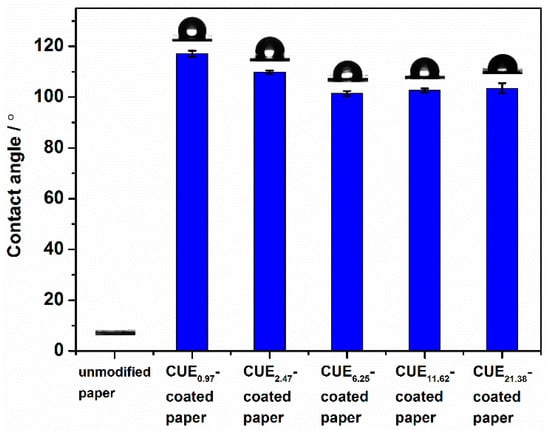
Figure 6.
Contact angle of unmodified paper, CUE0.97-coated paper, CUE2.47-coated paper, CUE6.25-coated paper, CUE11.62-coated paper, and CUE21.38-coated paper with inserted contact angle images (taken after 15 s).
3.6. Water Vapor Barrier Property
The water vapor barrier property was expressed as the measured WVTR value. As shown in Figure 7, the WVTR value of the unmodified paper, CUE0.97-coated paper, CUE2.47-coated paper, CUE6.25-coated paper, CUE11.62-coated paper and CUE21.38-coated paper was 622.4 ± 21.4, 441.2 ± 30.5, 369.7 ± 9.7, 273.1 ± 13.2, 209.4 ± 11.2 and 192.0 ± 8.1 g m−2 d−1, respectively. The according barrier ratio was calculated to be 29.12, 40.60, 56.12, 66.35 and 69.15%, respectively, indicating the good moisture barrier performance of our designed CUE-coated papers. In comparison with the barrier properties of CUE particle coated paper in our previous work [], the obviously enhanced water vapor barrier function indicated the surface morphology played an important role in the barrier performance of the coated paper. Accordingly, the barrier mechanism could be the hydrophobicity of the CUE coatings as well as the film formation on the paper surfaces that decreased the paper porosity []. Furthermore, considering the coating amount and barrier performance, the best coating grammage of 11.62 g m−2, which would be a good guideline for its practical application.
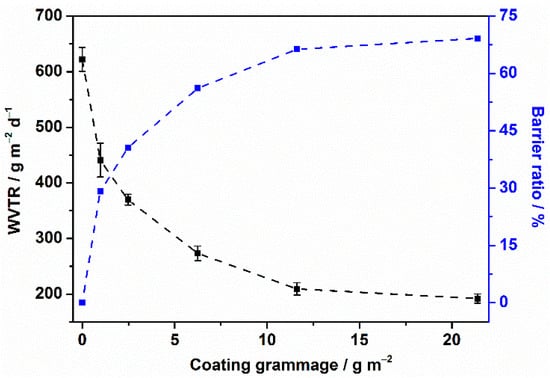
Figure 7.
WVTR value and barrier ratio of the CUE-coated papers as a function of the coating grammage.
3.7. Reactivity
As is known to us, the C=C groups can undergo thiol-ene reaction after UV irradiation []. Therefore, the reactivity of the CUE-coated paper was identified by the first photoclick thiol-ene reaction to immobilize MPA and further condensation reaction with the PHGH agent. The resulting papers were named as MPA-attached CUE-coated paper and PHGH-attached CUE-coated paper, respectively. The corresponding schematic illustration of the preparation was demonstrated in Figure 8a. In the following, their chemical structure was analyzed by ATR FTIR and XPS spectroscopy. As shown in Figure 8b, in comparison with the CUE-coated paper, the MPA-attached CUE-coated paper showed lower intensity at peaks of 3068, 1637 and 903 cm−1, which were attributed to the signals of CH=CH2 groups. This phenomenon suggested the successful reaction between CH=CH2 groups and MPA under UV illumination. Moreover, the peak at 1740 cm−1 that was ascribed to the ester group slightly red shifted in the MPA-attached CUE-coated paper due to the appearance of the carboxylic groups []. With the further attachment of PHGH onto the MPA-attached CUE-coated paper, the peak at 3480 cm−1 red shifted to 3311 cm−1 with enhanced intensity as well as broad range, which could be due to the appearance of the amine groups from PHGH as well as the hydrogen bonding between amine groups and the hydroxyl groups from cellulose itself []. Additionally, the enhanced signals at 1637 and 1546 cm−1 could be resulted from the formed carbonyl amide groups and N-H groups, respectively []. Interestingly, the signal for carboxylic groups decreased without disappearance, suggesting the part substitution of PHGH onto MPA-attached CUE-coated paper. Furthermore, the XPS spectra of CUE-coated paper, MPA-attached CUE-coated paper and PHGH-attached CUE-coated paper are demonstrated in Figure 8c. The CUE-coated paper exhibited obvious peaks at 532 and 284 eV, which were assigned to O1s and C1s, respectively []. New peaks at 227 and 163 eV that were attributed to S2s and S2p appeared in MPA-attached CUE-coated paper. Further attachment of PHGH agents leaded to the appearance of N1s signal at 399 eV []. The XPS results also confirmed the successful fabrication of PHGH-attached CUE-coated paper. In the next, the effect of the immobilization of PHGH agents onto the coated paper on the water vapor barrier performance was studied by WVTR measurement. The according WVTR results are shown in Figure 8d. The WVTR value of PHGH-attached CUE-coated paper was 202.4 ± 6.8 g m−2 d−1, which was slightly lower than that of the CUE-coated paper (209.4 ± 11.2 g m−2 d−1 for CUE11.62-coated paper). This phenomenon could be due to the hydrophilic property of the PHGH agents [], leading to the contact angle of the attached CUE-coated paper with a value of 67° ± 1° (inserted in Figure 8d). This phenomenon also indicated the bottom CUE coatings had a good moisture barrier function. Finally, the antibacterial property of CUE-coated paper and PHGH-attached CUE-coated paper was studied by the inhibition zone method. Please note that the MIC of PHGH against E. coli was 11.95 ppm. As shown in Figure 8e, there was an obvious inhibition zone with a diameter of 1.6 cm in the plate of the PHGH-attached CUE-coated paper compared with that in the CUE-coated paper, indicating that E. coli was deactivated by the attached PHGH moieties. According to the literature, the antibacterial mechanism of PHGH was based on the destruction of the bacteria cell membrane, leading to the leakage of intracellular components from bacterial cells []. Therefore, it can be concluded that the PHGH-attached CUE-coated paper showed both water vapor barrier as well as antibacterial property. This preparation strategy could be employed for the design of other multi-functional barrier materials by such reactive CUE-coated paper.
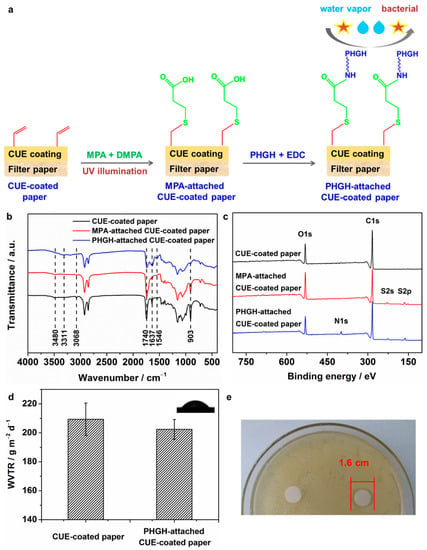
Figure 8.
(a) Schematic illustration of the preparation of PHGH-attched CUE-coated paper; (b) FTIR spectra of CUE-coated paper (CUE11.62-coated paper), MPA-attached CUE-coated paper, and PHGH-attached CUE-coated paper; (c) XPS spectra of CUE-coated paper, MPA-attached CUE-coated paper, and PHGH-attached CUE-coated paper; (d) WVTR value of CUE-coated paper (CUE11.62-coated paper) and PHGH-attached CUE-coated paper with inserted contact angle image of PHGH-attached CUE-coated paper; and (e) Antibacterial property of CUE11.62-coated paper and PHGH-attached CUE-coated paper.
4. Conclusions
In summary, the solutions of synthesized CUEs with various coating grammages from 0.97 to 21.38 g m−2 were coated onto lab-engineered paper sheets by bar coater. The obvious CUE signals in the FTIR and XPS spectra of the coated papers, indicating the successful coating of CUEs on the papers. When the coating grammage was 0.97 g m−2, the surface morphology of the coated paper displayed no significant change in comparison with that of the unmodified paper, leading to the difficulty in the measurement of the coating thickness. With the further increase in the coating grammage, the Bekk smoothness of the coated paper increased, and fibrous structure on the coated papers gradually became invisible with obvious film formation as shown in their SEM images. Specifically, the coating thickness ranged from 1.0 to 18.3 μm and the Bekk smoothness was from 639 to 4182 s corresponding to the coating grammage from 2.47 to 21.38 g m−2. Furthermore, the coated papers exhibited good mechanical strength. Due to the hydrophobic property of CUEs, the contact angle of the coated paper ranged from 117° ± 1° to 101° ± 1°. Accordingly, the WVTR values of the coated papers were much lower than that of the unmodified paper with the barrier ratio from 29.12% to 69.15% during the coating grammage range. Considering the coating amount as well as the barrier ratio, the best coating grammage was 11.62 g m−2. This result would be a good guideline for industrial manufacture. Finally, the reactivity of the CUE-coated paper was investigated by the photo-click thiol-ene reaction. With the further attachment of PHGH onto the above reacted CUE-coated paper, the generated paper showed desirable antibacterial property and water vapor barrier performance. Therefore, multi-functional packaging materials could be designed by our CUE-coated paper.
Author Contributions
Conceptualization and funding acquisition, W.L.; methodology, formal analysis, and writing—original draft preparation, W.W.; resources and software, S.Z.; writing—review and editing, W.L.; supervision and project administration, W.L. and C.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Guangxi Province [2018GXNSFBA138027]; Scientific Research Foundation of Guangxi University [XGZ170232]; the Dean Project of Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control [ZR201804-7].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aloui, H.; Khwaldia, K. Effect of coating weight and nanoclay content on functional and physical properties of bionanocomposite-coated paper. Cellulose 2017, 24, 4493–4507. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xiao, H. Dual-functional beeswaxes on enhancing antimicrobial activity and water vapor barrier property of paper. ACS Appl. Mater. Interfaces 2013, 5, 3464–3468. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Bäumler, M.; Stäbler, A. Inter-correlation among the hydrophilic-lipophilic balance, surfactant system, viscosity, particle size, and stability of candelilla wax-based dispersions. Coatings 2018, 8, 469. [Google Scholar] [CrossRef]
- Nechita, P.; Roman, M. Review on polysaccharides used in coatings for food packaging papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Sängerlaub, S.; Brüggemann, M.; Rodler, N.; Jost, V.; Bauer, K.D. Extrusion coating of paper with poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)-packaging related functional properties. Coatings 2019, 9, 457. [Google Scholar] [CrossRef]
- Kopacic, S.; Walzl, A.; Zankel, A.; Leitner, E.; Bauer, W. Alginate and chitosan as a functional barrier for paper-based packaging materials. Coatings 2018, 8, 235. [Google Scholar] [CrossRef]
- Lin, D.; Kuang, Y.; Chen, G.; Kuang, Q.; Wang, C.; Zhu, P.; Peng, C.; Fang, Z. Enhancing moisture resistance of starch-coated paper by improving the film forming capability of starch film. Ind. Crops Prod. 2017, 100, 12–18. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Bio-based coatings for paper applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P. Bilayer biocomposites based on coated cellulose paperboard with films of polyhydroxybutyrate/cellulose nanocrystals. Cellulose 2018, 25, 2419–2434. [Google Scholar] [CrossRef]
- Shen, J.; Fatehi, P.; Ni, Y. Biopolymers for surface engineering of paper-based products. Cellulose 2014, 21, 3145–3160. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional nanomaterials through esterification of cellulose: A review of chemistry and application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef]
- De Campos, A.; Claro, P.C.; Luchesi, B.R.; Miranda, M.; Souza, F.V.D.; Ferreira, M.D.; Marconcini, J.M. Curaua cellulose sheets dip coated with micro and nano carnauba wax emulsions. Cellulose 2019, 26, 7983–7993. [Google Scholar] [CrossRef]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Willberg-Keyriläinen, P.; Vartiainen, J.; Harlin, A.; Ropponen, J. The effect of side-chain length of cellulose fatty acid esters on their thermal, barrier and mechanical properties. Cellulose 2017, 24, 505–517. [Google Scholar] [CrossRef]
- Shen, Z.; Kwon, S.; Oh, K.; Abhari, A.R.; Lee, H.L. Facile fabrication of hydrophobic cellulosic paper with good barrier properties via PVA/AKD dispersion coating. Nord. Pulp Pap. Res. J. 2019, 34, 516–524. [Google Scholar] [CrossRef]
- Willberg-Keyriläinen, P.; Ropponen, J.; Alakomi, H.L.; Vartiainen, J. Cellulose fatty acid ester coated papers for stand-up pouch applications. J. Appl. Polym. Sci. 2018, 135, 46936. [Google Scholar] [CrossRef]
- Havimo, M.; Jalomäki, J.; Granström, M.; Rissanen, A.; Iivanainen, T.; Kemell, M.; Heikkilä, M.; Sipi, M.; Kilpeläinen, I. Mechanical strength and water resistance of paperboard coated with long chain cellulose esters. Packag. Technol. Sci. 2011, 24, 249–258. [Google Scholar] [CrossRef]
- Šumiga, B.; Šumiga, B.; Ravnjak, D.; Podgornik, B.B. Antimicrobial paper coatings containing microencapsulated cymbopogon citratus oil. Coatings 2019, 9, 470. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Wang, W.; Wang, S.; Qin, C. Reactive superhydrophobic paper from one-step spray-coating of cellulose-based derivative. Appl. Surf. Sci. 2019, 497, 143816. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Wang, W.; Qin, C.; Wu, M. Facile preparation of reactive hydrophobic cellulose nanofibril film for reducing water vapor permeability (WVP) in packaging applications. Cellulose 2019, 26, 3271–3284. [Google Scholar] [CrossRef]
- Wei, D.F.; Li, Z.L.; Wang, H.; Liu, J.; Xiao, H.N.; Zheng, A.N.; Guan, Y. Antimicrobial paper obtained by dip-coating with modified guanidine-based particle aqueous dispersion. Cellulose 2017, 24, 3901–3910. [Google Scholar] [CrossRef]
- Wang, Y.G.; Heinze, T.; Zhang, K. Stimuli-responsive nanoparticles from ionic cellulose derivatives. Nanoscale 2016, 8, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gellerich, A.; Zauner, M.; Wang, X.; Zhang, K. Differential anti-fungal effects from hydrophobic and superhydrophobic wood based on cellulose and glycerol stearoyl esters. Cellulose 2018, 25, 1329–1338. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dupont, C.; Martin, V.; Jeguirim, M.; Dufresne, A. Utilization of torrefied coffee grounds as reinforcing agent to produce high-quality biodegradable PBAT composites for food packaging applications. ACS Sustain. Chem. Eng. 2017, 5, 1906–1916. [Google Scholar] [CrossRef]
- Böhm, A.; Gattermayer, M.; Trieb, C.; Schabel, S.; Fiedler, D.; Miletzky, F.; Biesalski, M. Photo-attaching functional polymers to cellulose fibers for the design of chemically modified paper. Cellulose 2013, 20, 467–483. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Yang, J.; Wang, X.; Lan, T.; Peng, L. Fabrication of food-safe superhydrophobic cellulose paper with improved moisture and air barrier properties. Carbohyd. Polym. 2019, 211, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Pandey, A.; Yagci, B.; Ozguz, V.; Qureshi, A. Graphene nano-mesh-Ag-ZnO hybrid paper for sensitive SERS sensing and self-cleaning of organic pollutants. Chem. Eng. J. 2018, 336, 445–455. [Google Scholar] [CrossRef]
- Battisti, R.; Fronza, N.; Júnior, Á.V.; da Silveira, S.M.; Damas, M.S.P.; Quadri, M.G.N. Gelatin-coated paper with antimicrobial and antioxidant effect for beef packaging. Food Packag. Shelf Life 2017, 11, 115–124. [Google Scholar] [CrossRef]
- Sothornvit, R. Effect of hydroxypropyl methylcellulose and lipid on mechanical properties and water vapor permeability of coated paper. Food Res. Int. 2009, 42, 307–311. [Google Scholar] [CrossRef]
- Bedane, A.H.; Eić, M.; Farmahini-Farahani, M.; Xiao, H. Theoretical modeling of water vapor transport in cellulose-based materials. Cellulose 2016, 23, 1537–1552. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Du, X.; Feng, W.; Welle, A.; Trapp, O.; Grunze, M.; Hirtz, M.; Levkin, P.A. Reactive superhydrophobic surface and its photoinduced disulfide-ene and thiol-ene (bio)functionalization. Nano Lett. 2014, 15, 675–681. [Google Scholar] [CrossRef]
- Liu, K.; Xu, Y.; Lin, X.; Chen, L.; Huang, L.; Cao, S.; Li, J. Synergistic effects of guanidine-grafted CMC on enhancing antimicrobial activity and dry strength of paper. Carbohyd. Polym. 2014, 110, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; An, Q.; Li, X.; Qian, L.; He, B.; Xiao, H. Synergistic effects of chitosan-guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresour. Technol. 2010, 101, 5693–5700. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ji, J.; Zhang, W.; Wang, G.; Zhen, Z.; Wang, P. Guanidine-based polymeric microspheres with a nonleaching, antibacterial performance. J. Appl. Polym. Sci. 2017, 134, 44821. [Google Scholar] [CrossRef]
- Ashraf, K.M.; Roy, K.; Higgins, D.A.; Collinson, M.M. On the importance of silane infusion order on the microscopic and macroscopic properties of multifunctional charge gradients. ACS Omega 2020, 5, 21897–21905. [Google Scholar] [CrossRef]
- Pan, Y.; Xiao, H.; Cai, P.; Colpitts, M. Cellulose fibers modified with nano-sized antimicrobial polymer latex for pathogen deactivation. Carbohyd. Polym. 2016, 135, 94–100. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).