1. Introduction
The production of metallic powders has traditionally been of interest to classical powder metallurgists and research and development engineers alike. Powder metallurgy techniques include hot isostatic pressing, injection molding, and powder forging, among others [
1,
2,
3]. However, the advent and realization of metal additive manufacturing’s potential has enabled a renaissance within the powder production, powder metallurgy, and alloyed powder design communities. Current methods for producing such powders consist of water, gas, and plasma atomization, plasma spheroidization, granulation, sintering–deoxygenation, plasma rotating electrode processing, and more [
4]. While each of these methods has their own set of benefits and drawbacks, an almost universal drawback across all processes is that the compositions are not optimized for specific processes [
5]. Extensive work in the early days of powder metallurgy showed that rapidly solidified powder-based versions of wrought and/or cast counterparts, with equivalent compositions and designations, have refined secondary and primary dendrite arm spacing, extended solid solubility, and a superior combination of strength, toughness, and resistance to corrosion and fatigue upon undergoing powder metallurgy processing [
5]. Still, in recent years, the powder-based additive manufacturing community and computational materials scientists alike have come to assume a consistency between bulk material properties and their powder counterparts [
6,
7,
8,
9,
10].
The solid-state powder-based metal additive manufacturing and materials consolidation technology known as cold gas-dynamic spray, or more simply cold spray, has been used in the aerospace industry and defense sector for repair and reclamation applications, saving time and money over the traditional method of decommissioned part replacement [
11]. Cold spray metal additive manufacturing is of growing industrial interest because of the lack of melting associated with the technology, which results in the retainment and refinement of feedstock material properties during processing, among other benefits, such as tuneability as a function of application-specific performance requirements [
12]. The feedstock material for these processes is frequently gas-atomized powders [
13]. During the gas-atomization process, molten metal is atomized using a gas stream, where the metal rapidly solidifies and is subsequently collected. Rapid solidification results in a generally equiaxed, dendritic, and/or cellular, microstructure that can contain the segregation of alloying elements depending upon the composition of the powder in relation to the gas-atomization parameters utilized. The size and shape of the powder particles are influenced by the alloy composition and the processing parameters utilized during gas atomization as well. For aluminum alloys, the powder particles tend to be primarily spherical in shape, yielding a wide size distribution when produced via gas atomization.
Computational tools can greatly decrease the time required to fully understand cold-spray material consolidation processing and manufacturing, thus decreasing the number of experiments needed to better comprehend the influence of parameters and properties of interest. Accordingly, models have been developed to (1) utilize commercially available thermodynamic and kinetic software, such as Thermo-Calc and Thermo-Calc’s DICTRA module (Thermo-Calc Software, Stockholm, Sweden), which uses the chemical composition of the feedstock material, and then (2) couples the outputs associated with (1) with the cold spray consolidation process parameters, such that (3) the output material properties of a consolidated part/component may be derived [
14]. The process of integrating feedstock composition with processing parameters and the resultant component performance, by way of taking an in silico approach, has previously been described within the literature as a through-process modeling approach to cold spray materials consolidation and cold spray metal additive manufacturing.
If properties such as strength and toughness are desired outputs of the cold spray through-process model, for example, then similar properties must be input to the model because of the retentive nature of the microstructures and properties associated with cold spray processing. These properties can be obtained from data generated experimentally and/or data gathered from published literature. It is already common practice to gather data on cast/wrought materials through both sources of data; handbooks and decades of experiments provide a significant comparison between literature values and those generated experimentally [
15]. Currently, the research community must apply the same approach at a more rapid pace for the accurate modeling of solid-state metal additive manufacturing, especially cold spray, and methods that utilize powders as the feedstock. However, when literature is consulted, there exists a discrepancy between the types of properties reported for alloyed powders and those reported for the same alloys in their respective cast/wrought conditions. Properties such as hardness, strength, melting temperature, and microstructure are reported for cast alloys [
16,
17,
18], for instance, while properties such as flowability, particle size distribution (PSD), melting temperature, and combustion temperature are more commonly reported and accessible for metallic powders [
19,
20,
21].
To demonstrate the fundamental differences between a material in its bulk condition, as opposed to its gas-atomized state, this paper compares three common aluminum aerospace alloys. More specifically, gas-atomized Al 2024, Al 6061, and Al 7075 powders are contrasted against their respective cast counterparts through an analysis of differences in grain size and microstructure, secondary phases, and hardness using optical microscopy, scanning electron microscopy (SEM), dynamic nanoindentation, and microhardness testing. Additionally, differential scanning calorimetry (DSC) analysis was employed for the gas-atomized powders and cast counterparts in their as-manufactured conditions, which enabled secondary phase formation and dissolution kinetics to be explored as a function of material condition. Thereafter, discussion surrounding the implications and immediate consequences of the uniqueness of material properties in the gas-atomized condition are considered in relation to cold spray materials consolidation given the significance of the aforementioned penalties associated with assuming consistency between bulk and atomized properties during particle impact modeling and process parameter optimization, for example.
2. Methodology
Gas atomization is known to achieve cooling rates as high as 10
7 °C·s
−1 [
14]. Since the rate of cooling during gas atomization is orders of magnitude greater than those reported for castings, the classical models relating the microstructure to cooling rates cannot be directly applied to rapidly solidified powders. As such, a relatively simple Newtonian heat transfer model, which was reported by Shiwen et al. [
22], was found to be adequate in capturing the mechanisms behind single particle solidification during gas atomization. The simplified heat transfer model for rapidly solidified powders is given in Equation (1), such that
where
is the molten metal droplet temperature in °C,
is the time in seconds,
is the gas atomization temperature in °C,
is the droplet density in kg·m
−3,
is the specific heat of the metal droplet in J/(kg·°C),
is the thermal conductivity of the gaseous species utilized during gas atomization in W/(m·°C), and
is the droplet diameter in meters.
Inspection of Equation (1) reveals the fact that the cooling rate is inversely proportional to the droplet diameter, as shown in Equation (2), such that
Table 1 tabulates
,
, and
for each of the aluminum alloys studied herein. Since the powders provided by Valimet, Inc. (Stockton, CA, USA), were gas-atomized in N
2 gas, the
was 0.024 W/(m·°C), while the
was 26.85 °C for cooling rate calculations.
In casting or solidification metallurgy, there is a proven relationship between secondary dendrite arm spacing (
) and solidification cooling rate, as given in Equation (3), such that
wherein
and
n are alloy-dependent constants,
n is a unitless exponent, and
is expressed in units of length. The materials property simulation software JMatPro (Sente Software, Guildford, UK; Version 9.1.1) was used to determine the value of
for Al 2024, Al 6061, and Al 7075. Accordingly,
was found to be 86.741, while
was found to be 99.853, and
was found to be 90.049. A value of 0.33 was used for
n for all of the aluminum alloys.
To relate the powder particle cooling rates with their microstructural sub-grain size, which behaves as an effective grain size for rapidly solidified polycrystalline aluminum powders, Equations (1) and (3) were combined, resulting in the relationship given in Equation (4), such that
With respect to the alloys in their cast conditions, thermocouples were used to experimentally determine the cooling rates experienced upon solidification during the casting process. According to Ghoncheh et al. [
23], the relationship between dendrite arm spacing (DAS) and cooling rate for cast Al 2024 is given in Equation (5), such that
and DAS is in μm. In the case of Al 6061 and Al 7075, the DAS relation given in Equation (6) is a reasonable approximation [
23], such that
For the cast samples, Al 2024, Al 6061, and Al 7075 were purchased as sheets, subsequently melted, and cast in-house into cylinders with 28 mm diameters. The cooling rates were measured in the center of the cylinder. Optical emission spectrometry (OES) was used to measure the compositions of the three as-cast alloys. The OES device was made by SPECTRO Analytical Instruments (Kleve, Germany).
As previously mentioned, each of the alloyed aluminum powders was gas-atomized in nitrogen by Valimet, Inc. The cooling rate during this process was in the order of 10
4–10
5 °C·s
−1. The as-atomized powders were mechanically sieved from the initial PSD to the studied ranges, with D
10′s, D
50′s, and D
90′s shown
Table 2. In statistical terms, D
10 signifies that 10% of the particles are smaller than this value and so on for D
50 and D
90. The compositions of the powders were evaluated using direct current plasma emission spectroscopy as performed by Luvak Laboratories, Inc. (Boylston, MA, USA).
Thermal processing was performed on one set of samples at a time in the DSC, since the DSC maintains highly accurate temperature control. One set of samples for each alloy was left untreated in their as-solidified conditions as controls. Cast samples were cut to fit into DSC crucibles, with two parallel flat sides. Heat treatments were performed using a heating rate of 50 °C·min−1 and a cooling rate of 120 °C·min−1. Samples were brought to the thermal treatment temperature determined for each alloy (490 °C for Al 2024, 530 °C for Al 6061, and 480 °C for Al 7075) and held for 1 h before being quenched. Nitrogen was used as the purge gas. Specifically, a TA Instruments (New Castle, DE, USA) Discovery DSC with an LN2P cooler was used. DSC thermograms of the gas-atomized and as-cast samples were generated at a heating rate of 5 °C·min−1 in a nitrogen environment. As will be discussed hereafter, the composition of the cast Al 2024 specimens, which was obtained via OES analysis, was outside of the specified limits for the alloy.
The grain/sub-grain size for each of the samples was characterized via etching and subsequent optical microscopy. For etching, samples were compression mounted in a phenolic resin and then ground and polished with a final 0.05 μm colloidal silica suspension step. Once polished to a mirror finish, the samples were etched using the reagents and times described in
Table 3.
Then, microstructural sizes were measured using optical micrographs and Olympus Stream’s software package for grains and intercepts (Olympus Corporation, Shinjuku City, Tokyo, Japan). To reduce the distortion due to the local curvature on the edge of the polished powder particle cross-sections, only features near the center of the front face of the powders were included in the measurements. Due to the contribution of the in relation to the measurements, grains were measured in a comparable method: from the center of the grain boundaries to the center of the grain.
Samples were prepared for SEM the same way they were prepared for etching. SEM micrographs were taken using a tungsten-filament-source SEM (EVO MA-10, Carl Zeiss Microscopy, Thornwood, NY, USA). Both secondary electron and backscattered electron micrographs were taken. The backscattered electron micrographs were used to evaluate the number of secondary phases present in each condition. This was accomplished by way of contrast thresholding using image analysis software. Previous work was referenced to correlate the appearance of secondary phases to their stoichiometries [
24,
25,
26]. The diffraction pattern obtained from scanning transmission electron microscopy analysis of a dark phase within an Al 6061 gas-atomized particle was performed to validate the prior works referenced as well.
Nanoindentation testing was performed using an iMicro Pro system from Nanomechanics, Inc. (Oakridge, TN, USA), which is now part of KLA Instruments (KLA Corporation, Milpitas, CA, USA), on the metallographically prepared powder samples. The InForce 50 mN actuator was employed with a diamond Berkovich tip from Micro Star Technologies Inc. (Huntsville, TX, USA). In total, 25 particles were selected for nanoindentation testing per sample. Nanoindentation hardness measurements were reported at depths of 250 nm using the advanced dynamic hardness and modulus method provided by Nanomechanics, Inc. Using standard methods, the tip was cleaned, and the contact area function was analytically determined via analysis of the load–depth data from a fused silica sample. Thermal drift, pile-up, and creep-related phenomena were corrected for during testing. For additional information regarding the application of nanoindentation testing to particulate feedstock for cold spray, one may consider the work of Sousa et al. in [
27].
Microhardness testing of the cast samples was performed with a DiaMet Hardness Tester from Buehler (Lake Bluff, IL, USA). The microhardness data were collected using a Vickers indenter tip and an applied force of 0.1 kilogram-force (kgf). Since the Vickers and Berkovich tip geometries have essentially the same contact area and also result in similar strains during indentation, the nanoindentation hardness (
HNI), in units of MPa, was readily converted to a Vickers Hardness Number (VHN) via Equation (7), such that
Therefore, the nanoindentation hardness’s were directly converted to a VHN for direct comparison with the microhardness responses associated with the cast counterparts. For the purpose of comparison, note that the load required to achieve an indentation depth of 250 nm during nanoindentation of the gas-atomized alloyed powders studied herein was only 0.000254929 kgf versus the 0.1 kgf applied during microindentation testing.
3. Results
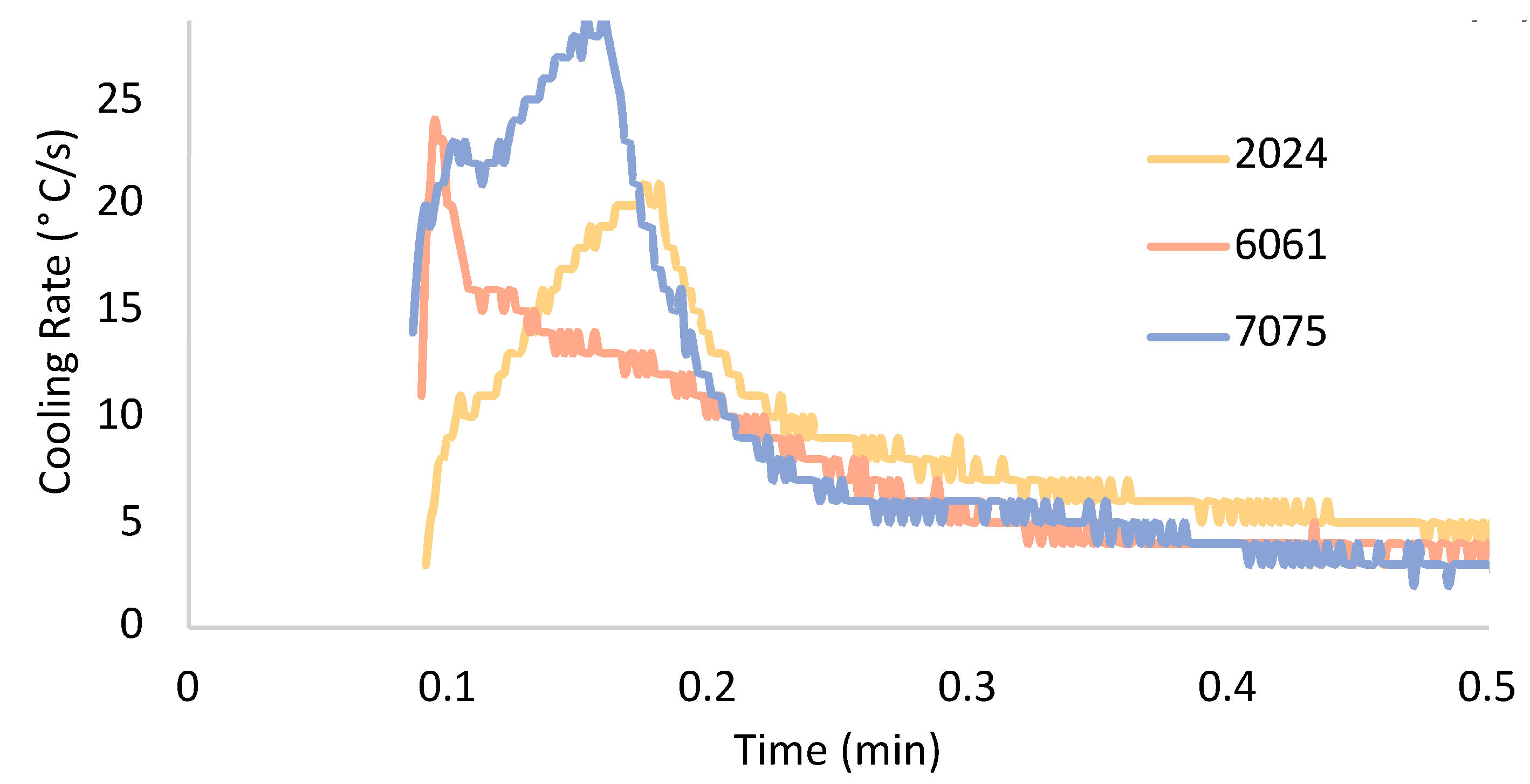
The temperature of the three conventionally cast alloyed aluminum counterparts as a function of time was determined as shown in
Figure 1. Interestingly,
Figure 1 captures various intersectional points along the three temperature–time curves, wherein alloy-specific curves are found to change course relative to one another. Said otherwise, Al 6061 and Al 7075 initially appeared to experience a more rapid rate of change in temperature as function of time than that of Al 2024. However, inspection of the intersections between two curves along the two-dimensional plot suggests that Al 2024 eventually achieves and retains a greater cooling rate as a function of time than that of Al 6061 and Al 7075, for example.
Considering the depiction of temperature as a function of time for the three conventionally cast alloyed aluminum counterparts, as shown in
Figure 1, the associated cooling rates as a function of time between 6 and 30 s are given next. Accordingly, the experimentally measured cooling rates for cast Al 2024, Al 6061, and Al 7075, which were obtained by way of the use of the thermocouples described in the Methodology section, are shown in
Figure 2. The aforementioned inspection of the temperature–time curves presented in
Figure 1 and
Figure 2 enabled the actual cooling rates as a function of time to be evaluated. The Al 6061 and Al 7075 castings initially achieved greater cooling rates as a function of time. As expected, Al 2024 eventually surpasses the discrete cooling rate values at equivalent points in time that follow Al 2024’s measured cooling rate versus time curve intersections with Al 6061 and Al 7075.
Figure 2 also revealed the fact that Al 2024’s curve intersects Al 6061’s curve before intersecting Al 7075’s curve. The curves associated with Al 6061 and Al 7075 also experienced at least two points at which the curves intersected one another. However, within the range of time associated with
Figure 2, as can be found along the horizontal axis of the cooling rate versus time plot, Al 2024 only intersected each of the two other curves one time. As for the peak cooling rates achieved for each of the three curves given in
Figure 2, Al 7075 achieved the greatest cooling rate during solidification upon casting, which was followed by Al 6061 and then Al 2024. Still, Al 6061 reached its maximal casting cooling rate before Al 7075, while both Al 6061 and Al 7075 reached their peak cooling rates before Al 2024. Finally, the difference between the maximum and minimum cooling rates as a function of time for each alloy designation considered herein could be organized from greatest to lowest as follows: Al 7075, Al 6061, followed by Al 2024.
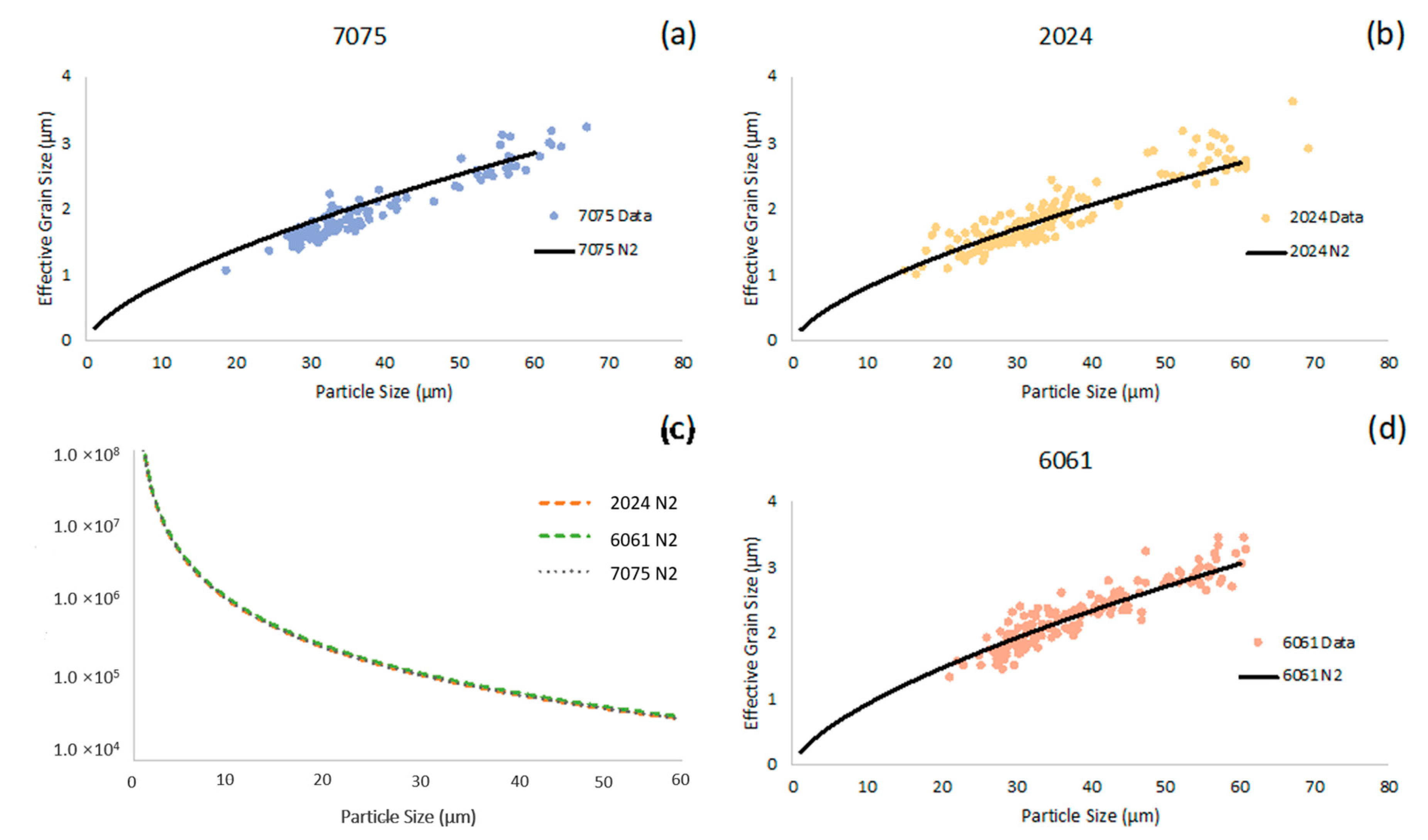
Having presented temperature–time curves (see
Figure 1) as well as cooling rate versus time curves (see
Figure 2) for the conventionally cast Al 2024, Al 6061, and Al 7075 bulk material counterparts, the cooling rates associated with the gas-atomized powders were ascertained as a function of particle diameter for each of the gas-atomized alloys considered, as shown in
Figure 3c. At the same time,
Figure 3a,b,d presents the experimentally measured as well as calculated effective grain sizes as a function of particle size for Al 2024, Al 6061, and Al 7075, respectively. The effective cooling rate versus particle diameter function (recall Equation (4)) was used to calculate the effective grain size for each alloy. Appreciable agreement between the grain size calculations (solid line in
Figure 3a,b,d) and the experimentally measured grain sizes (data points in
Figure 3a,b,d) was observed.
From
Figure 3c, one may note that the calculated cooling rates as a function of particle diameter for each of the gas-atomized systems considered during the course of this work were similar to one another.
Figure 3c also enabled the visualization of consequences associated with the inverse proportionality between molten metal droplet diameter and cooling rate, which was mathematically expressed in Equation (2) upon inspection of the simplified heat transfer model for the rapidly solidified powders presented in Equation (1). A detailed consideration of
Figure 3c also identified gas-atomized Al 6061 as the rapidly solidified alloy with the greatest cooling rate for a given particle size, followed by Al 7075 and Al 2024, in that order.
Given the fact that the curves presented within
Figure 3c followed from the use of Equation (1) as well as the fact that secondary dendrite arm spacing was formulated as a function of the solidification cooling rate in Equation (3), the substitution of Equation (1) into Equation (3) enabled powder particle cooling rates to be expressed in relation to their microstructural effective grain size, as shown in Equation (4);
Figure 3c can be linked with
Figure 3a,b,d. Mathematical manipulation of the computed cooling rates and calculated microstructural effective grain sizes enabled the effective grain size as a function of powder particle size to be calculated for Al 7075, Al 2024, and Al 6061, as shown in
Figure 3a,b,d, respectively, in the form of continuous black curves. Then, the experimentally measured effective grain sizes as a function of particle size for the three rapidly solidified aluminum alloys in the as-atomized state were able to be directly captured by way of plotting the calculated curves alongside the measured data points in
Figure 3a,b,d.
Analysis of
Figure 3a,b,d appears to suggest that Al 6061’s calculated effective grain size as a function of particle size was the most consistent with experimentally measured findings and observations, followed by Al 2024 and Al 7075 in that order. As shown in
Figure 3a, the Al 7075 calculations over-estimated the effective grain size as a function of particle size when compared with the corresponding experimental data. On the other hand, the Al 2024 computed curve trajectory was reasonably consistent with the experimentally measured values until surpassing a particle diameter of approximately 45 μm.
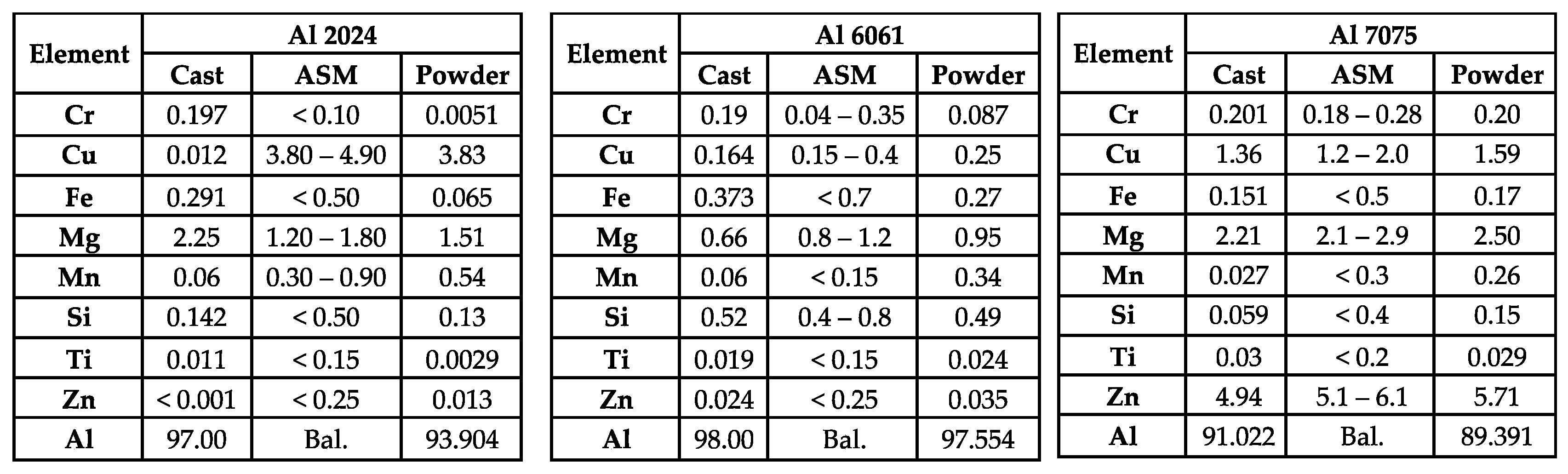
Transitioning from cooling rate calculations and analysis toward kinetic behavior and compositions, the experimentally derived chemistries of the gas-atomized powders as well as the as-cast samples are compared to the standard compositional ranges defined by ASM International in
Figure 4 [
24,
28]. As will be discussed, the copper, chromium, manganese, and magnesium weight percentages obtained from the cast Al 2024 sample nullifies its ability to be defined as non-nominal Al 2024. As for Al 6061, the magnesium weight percent associated with the cast counterpart was less than the lower limit associated with the alloys’ compositional range. As for gas-atomized Al 6061, the manganese content was shown to be greater than the upper limit provided by ASM International. Regarding Al 7075, the zinc weight percent for the cast counterpart was found to be shy of the lower limit prescribed by ASM International, too.
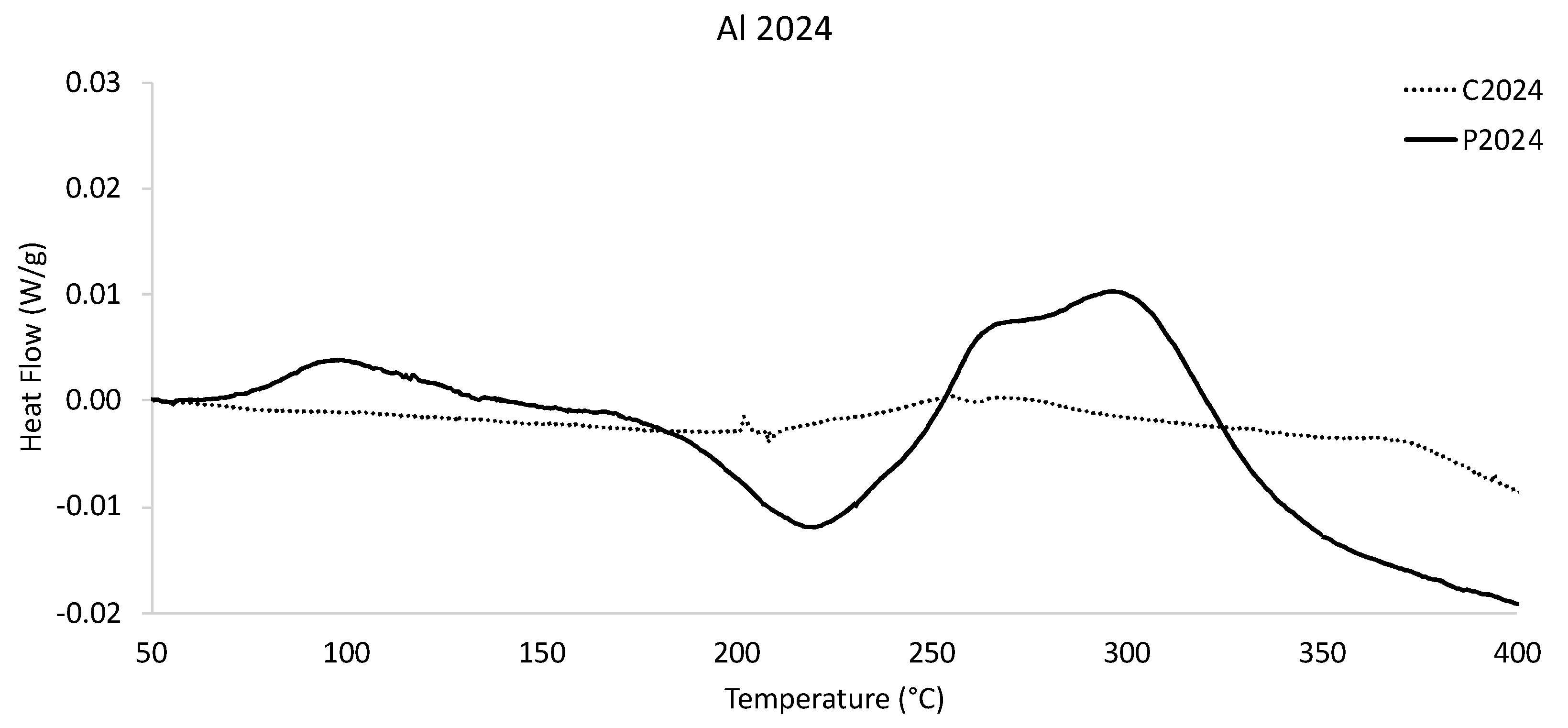
Normalized DSC-based heat flow versus temperature thermograms were obtained for both the nominally cast Al 2024 counterpart as well as the gas-atomized Al 2024 powder as shown in
Figure 5. Considering the fact that
Figure 4 demonstrated the fact that so many of the alloying elements associated with Al 2024 in the cast condition were either above or below the ASM International specified thresholds, the lack of secondary phase activity captured for the as-cast condition was not surprising. While the as-cast heat flow versus temperature curve revealed little to no activity between 50 and 200 °C, the gas-atomized Al 2024 DSC scan revealed a peak local maximum at a temperature of about 100 °C. The non-negative nature of the local maxima suggests secondary phase formation activity in the gas-atomized Al 2024. Clearly, such activity was absent from the data associated with the cast counterpart.
Following the initial local maximum identified in
Figure 5, another local minimum was observed around a temperature of approximately 220 °C for the gas-atomized Al 2024 powder. While discontinuities were also observed in the as-cast Al 2024 counterpart that were between approximately 210 and 240 °C, the magnitude of the blip prevents immediate and definitive conclusions from being drawn in terms of secondary phase formation and/or dissolution. That being said, the bimodal local maxima and activity in the cast Al 2024 specimen DSC data between 250 and 275 °C warrants prospective consideration. Whilst the dual local maxima between 250 and 275 °C in the cast Al 2024 were virtually equal in magnitude with one another, the two locally convoluted peaks in the gas-atomized Al 2024 condition were clearly differentiable from one another, with the peak associated with a temperature of at least 260 °C being slightly less than that of its subsequent peak around a temperature of 300 °C or so.
Considering the temperatures associated with said bimodal local peaks, the peaks associated with the gas-atomized powder were shifted to the right relative to the casting’s temperature-based location within the curve. While the DSC curve for the cast condition presented in
Figure 5 between 275 and 400 °C appeared to partially plateau before decreasing after a temperature of 360 °C was crossed, the gas-atomized Al 2024 DSC curve continuously decreased immediately following the onset of the bimodal local maxima.
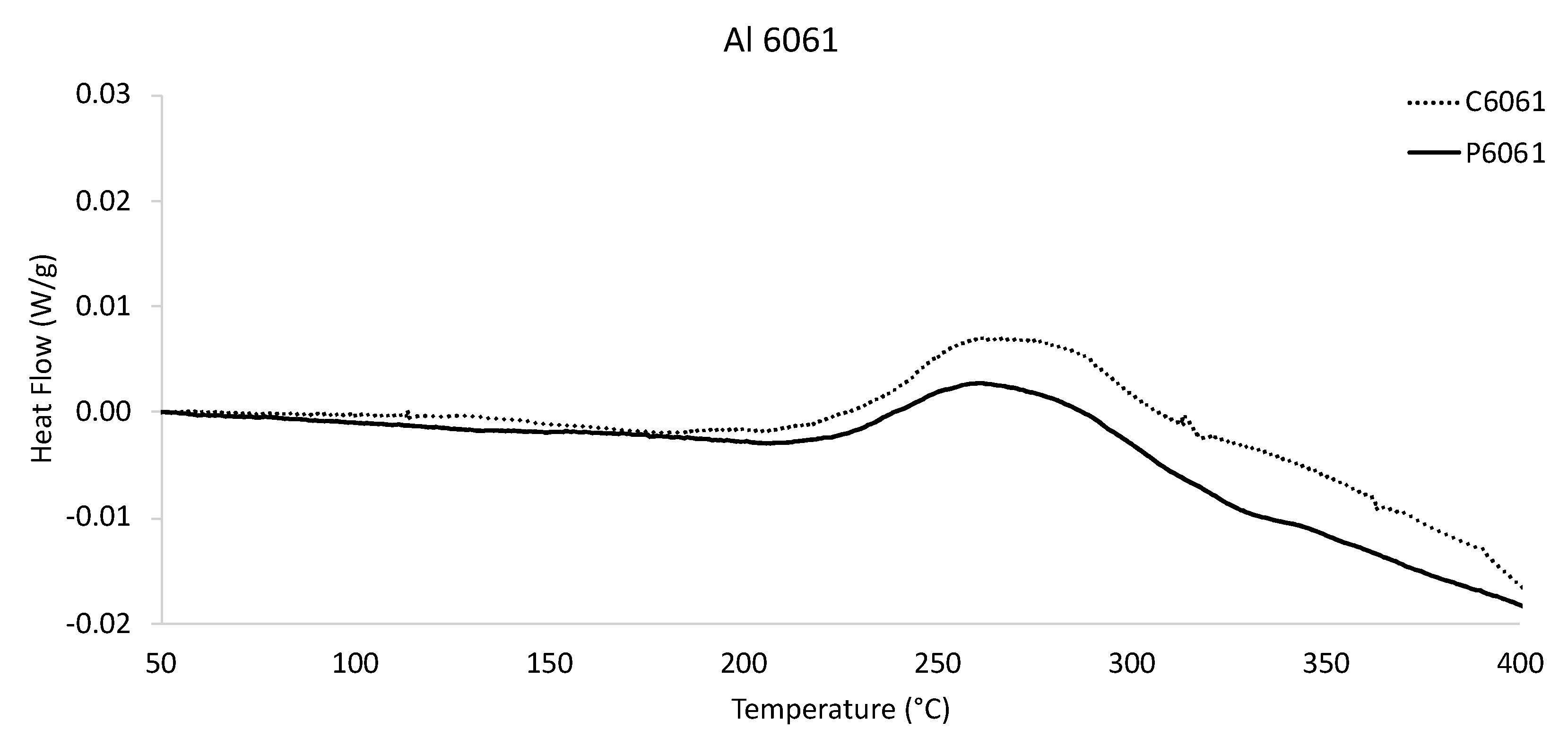
Figure 6 presents another pair of DSC-derived heat flow versus temperature curves, which were obtained by way of considering the gas-atomized and conventionally cast Al 6061 specimens. The thermogram presenting the heat flow versus temperature behavior of the gas-atomized Al 6061 as well as the as-cast Al 6061 in
Figure 6 were normalized prior to being presented graphically. Unlike the thermogram and data associated with the rapidly solidified Al 2024 and Al 2024 casting, which were both presented in
Figure 5, the DSC curves associated with both the gas-atomized Al 6061 and traditionally solidified Al 6061 specimens were remarkably similar with each other. While the two Al 6061 heat flow versus temperature curves take on similar shapes relative to each other, the data begin to no longer share near one-to-one coordinates after 200 °C or so. Nevertheless, the widely distributed or broad peaks associated with both Al 6061 curves between 250 and 300 °C with respect to the
x-axis were self-similar. After a temperature of 300 °C or more was surpassed, the gas-atomized Al 6061 and conventionally cast Al 6061 DSC curves steadily decreased in a comparable manner until a temperature of 400 °C was reached during testing.
In the case of the as-cast Al 2024 versus the gas-atomized Al 2024 DSC thermogram (shown in
Figure 5), the kinetics associated with secondary phase formation and/or dissolution, as a function of temperature, was virtually absent from the as-cast Al 2024 DSC curve. Furthermore, the cast DSC-based behavior for Al 2024 was clearly discernable from that of the gas-atomized data for Al 2024, which contained multiple, obvious exothermic and endothermic responses. Alternatively, the Al 6061 heat flow versus temperature curves affiliated with the conventionally cast specimen and rapidly solidified powder were uniquely similar to one another (shown in
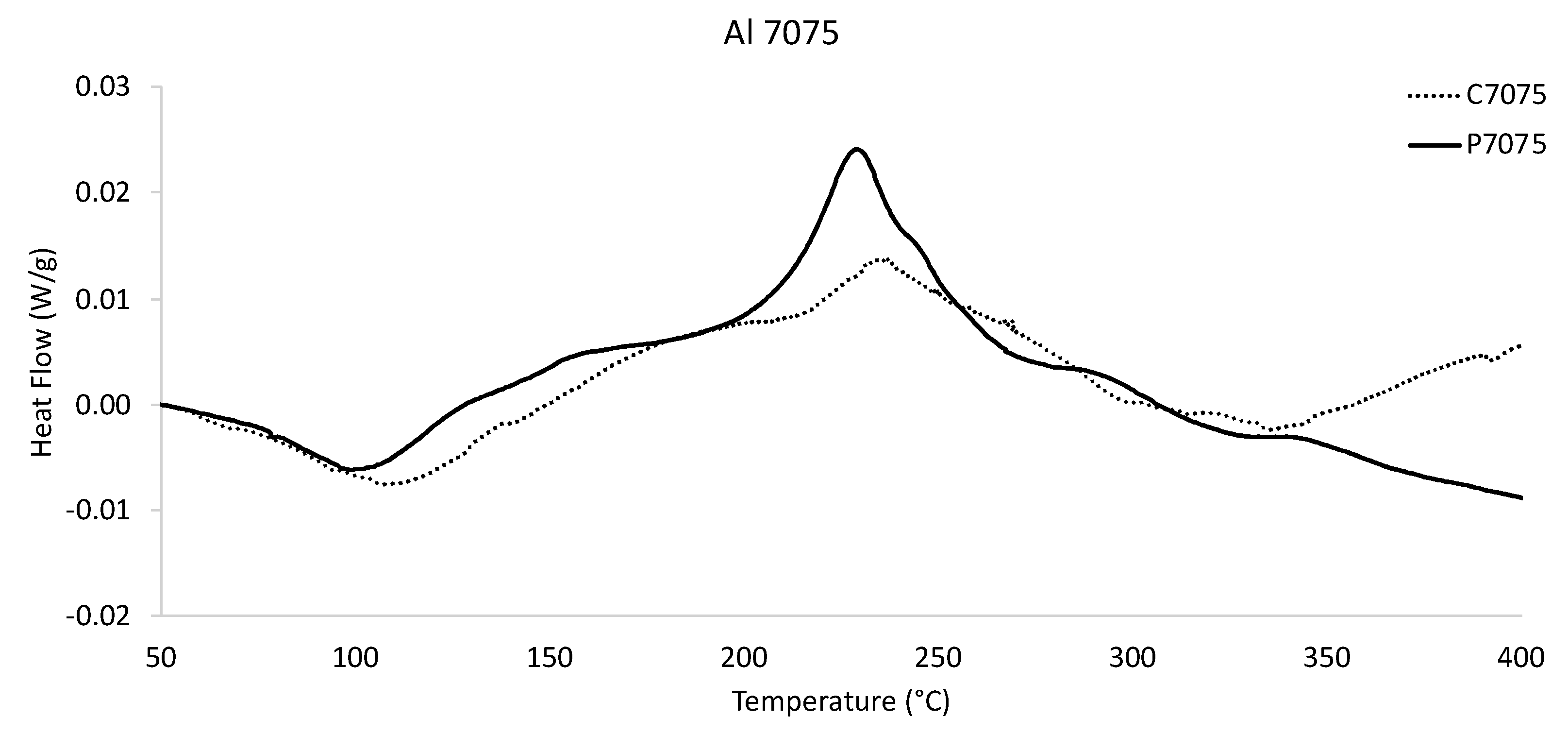
Figure 6). More to the point, the gas-atomized Al 6061 DSC curve contained confidently identifiable secondary phase formation peaks, whereas the gas-atomized Al 2024 DSC curve housed phase formation local maxima and phase dissolution minima. Given the appreciable differences between the Al 2024 thermogram and the Al 6061 thermogram, the defining phase formation/dissolution curve characteristics of the cast Al 7075 and rapidly solidified Al 7075 systems are shown in
Figure 7.
Although the similarity of the Al 7075 DSC curves was also self-evident via comparative analysis, the casting DSC data was shifted to the right. That is to say, similar secondary phase formation/dissolution peaks were present in the bulk specimen and the microparticles; however, the temperature about which such instances achieve regional maxima/minima was greater in the castings than that of the gas-atomized Al 7075. Specifically, secondary phase dissolution appears in both the cast and gas-atomized specimens at approximately 100 and 110 °C, respectively. Secondary phase formation peaks are also found between 200 and 250 °C for both material conditions. Intriguingly, the maximum heat flow value at the peak just identified for the gas-atomized Al 7075 powder was greater than that of the magnitude of the peak in the counterpart’s data.
Between 250 and 350 °C, both of the Al 7075 DSC curves continuously decrease in a largely consistent manner. That said, fine-scale inspection of the way in which the Al 7075 heat flow values defined within 250 and 350 °C revealed a local quasi-sinusoidal oscillation of the heat flow values for each condition while also steadily decreasing on average as the temperature increases until reaching a temperature of 350 °C. Lastly, a clear divergence between the as-cast Al 7075 and gas-atomized Al 7075 DSC responses was attained. Stated otherwise, the Al 7075 casting appeared to approach another occurrence of secondary phase formation. Yet, the gas-atomized Al 7075 DSC response was consistent with the onset of dissolution.
Figure 8 shows example optical micrographs for Al 7075 after etching. Specifically, the micrograph on the left-hand side of
Figure 8 shows the as-cast condition, and the micrograph on the right-hand side of
Figure 8 shows the as-atomized powder condition. These micrographs were used to calculate the grain size and qualitatively show the drastic size difference between the polycrystalline microstructure in powders versus their cast counterparts. As will be discussed hereafter, this contributes to the difference in hardness (Hall–Petch relationship) and a difference in secondary phases (diffusion distance to grain boundaries).
Figure 9 shows electron micrographs comparing the microstructures of the as-cast, as-atomized, thermally treated cast, and thermally treated gas-atomized powder via SEM for Al 7075. The difference in granular structure seen in
Figure 8 can be seen here again. Qualitatively, the powder and cast in the thermally treated condition (
Figure 9b,d) are similar in that there are discrete phases, both light and dark contrasting. However, the as-atomized and as-cast structures are quite different. The as-cast structure has discrete phases, whereas the as-atomized powder structure has an interconnected network of phases at the boundaries. This is likely due to the solidification times; the cast condition cooled much slower than the powder (rapid solidification via gas atomization), allowing more secondary phases to form.
The secondary phase area percent of each alloy is presented in relation to each of the conditions studied herein. More specifically, the phase area percentages for the as-cast, as-atomized, thermally treated casts, and thermally treated gas-atomized powders are shown in
Table 4.
Table 4 is further refined in terms of the Mg or Fe, or equivalent substitutional elements for a given phase, and the richness of each of the phases was analyzed.
Figure 10 shows scanning transmission electron micrographs of the processed Al 6061 powder, highlighting the secondary phases. Additionally, diffraction-based analysis was performed on the dark phase, revealing it to be Mg
2Si. This phase was also found in the cast Al 6061, although the amounts present differ.
The microstructural feature sizes for the three alloys are given in
Table 5. Similar to
Table 4,
Table 5 presents the grain sizes (
, or secondary dendrite arm spacing (SDAS), and DAS sizes) for each of the conditions, which includes the as-cast, as-atomized, thermally treated casts, and thermally treated and powders. Lastly, the VHNs for each of the alloys in all four conditions are given in
Table 6.
4. Discussion
4.1. On Comparing Indentation Hardnesses
The indentation size effect (ISE) has been widely studied since Nix and Gao formally presented a strain gradient plasticity model to conceptualize the observed decrease in hardness as a function of indentation depth [
29,
30]. As such, an analytical framework was introduced that enabled hardness (
) versus indentation depth (
) data to be fit to Equation (8), such that
where
is the hardness in the infinite depth limit, in other words, the true hardness of the material, and
is a characteristic length that depends on the shape of the indenter, the shear modulus, and
[
30]. Accordingly, the direct application of curve-fitting procedures enables one to directly obtain
and
from experimentally measured nanoindentation data. The underlying physical and mechanical relations associated with
and
are mathematically formulated in Equation (9), such that
and Equation (10), such that
In Equations (9) and (10),
is a constant, 0.5,
is the Burgers vector,
is the shear modulus,
is the geometric angle separating the surface of the indenter tip and the material surface orthogonal to the direction of indentation, and
is the density of statistically stored dislocations introduced by the process of indenting to a given depth [
30]. Although the aforementioned plasticity parameters (
and
) are identifiable by way of direct analysis of the nanoindentation data according to Equation (8),
can also be obtained by surpassing or achieving an indentation depth of 2000 nm [
31]. Since the reported hardness’s of the gas-atomized powders were reported at a depth of 250 nm, it is reasonable to assume that the ISE caused the recorded hardness to be higher than that of
and therefore questionably comparable with the VHNs obtained for the cast material systems without prior transformation to an equivalent
value.
Considering the fact that
, additional testing of Al 7075 gas-atomized powder using dynamic, that is, the continuous stiffness measurement (CSM) method, and an MTS Nanoindenter XP enabled the
nanoindentation hardness to be compared with that of
and
to identify a proportionality that allowed the
data to be converted to an approximately lower limit value for
given the influence of the mounting material upon particle indentation at such depths, too [
27]. Proper conversion of the
data to an equivalent
value enables direct comparison with the HVN obtained for the cast samples. As illustrated through the consideration of
Figure 11, it was shown that the
is approximately equivalent to
, which is approximately equal to (
.
Therefore, Equation (11) may be expressed as follows:
The described approach to converting the
to a virtually equivalent
value, which can be directly compared with the VHNs measured for the bulk materials, is worth critical assessment. Such a critical assessment need not follow from the approximate equivalence relation between
,
,
,
, and VHNs; rather, an important question arises surrounding the choice to measure powder particle properties at a depth of 250 nm instead of 2000 nm or through the use of a nanoindenter instead of a conventional Vickers or micro-indentation tester. The choice of a nanoindentation depth of 250 nm, as well as the use of a nanoindenter over a microhardness tester for powder, follows from the fact that powder particle mounting material matrix has been shown to affect the recorded nanoindentation data if care is not taken when performing such characterization [
27].
The influence of mounting materials upon the nanoindentation of metallic powder particles embedded in a more compliant medium was initially brought to light by Shives et al. at the microindentation scale [
32]. The work by Shives et al. has unfortunately gone relatively unnoticed as evidenced by the limited number of times their published findings have been cited (six citations at the time this article was written, according to Google Scholar). In any case, since the maturation of nanoindentation over the course of the previous two to three decades, researchers have even observed a mounting material effect at the nanoindentation scale [
33].
From the late 1990s and early 2000s, nanoindentation researchers have been raising concerns regarding the need for the continued development of models for evaluating plastic and elastic properties of hard particles embedded in relatively softer mounting material [
33,
34,
35,
36]. Researchers have applied a “thin film” correction to the protocol for indentation testing of powder in a mounting resin. The underlying intuition behind this adaptation of indentation testing is certainly commendable [
8,
37]. Unfortunately, the geometrical variation behind a planer thin film on a compliant substrate is not similar enough to an embedded hemispherical particle surrounded by a compliant mounting material, which is otherwise analogously similar to a complaint substrate. Careful consideration of the previously reported findings within the literature has found that the thin film protocol cannot be extended to particle/matrix systems [
36,
38].
Notably, even when experimentally considering a number of models developed for correctly extracting particle hardness given a softer mounting material effect, the data collected during the course of our research speaks to the need for continued refinement of the currently available models. For example, Cao et al. proposed that the hardness of a stiffer particle embedded in a soft mounting material can be collected with less than 10% error if the following relation, given in Equation (12), was adhered to [
36], such that
where
is equivalent to the maximum indentation depth of a particle before the softer mounting material affects the measured hardness, and
is the radius of a given particle.
By way of solving for
for a particle with a radius of approximately 19 μm, derived from the average
reported in
Table 2 for each of the three Al powders, one calculates
of 2565 μm. Fortunately, previous work by Chen et al. reported indentation properties for gas-atomized Al 6061 at depths near 2565 μm in a soft epoxy and measured hardness values between 0.15 and 0.51 GPa [
39], which is absolutely far too low for Al 6061 gas-atomized powder and therefore speaks to the limitation of Cao et al.’s model [
8,
37,
40]. Regardless of the model proposed by [
36] that aims to quantify an indentation depth limit, one could alternatively consider the plastic zone radius for a given indentation load and can be defined according to continuum mechanics [
41], such that Equation (13) is given as
where
is the plastic zone radius,
is the applied load, and
is the yield strength of the powder particle. Alternatively, the plastic zone radius has been defined by Chen et al. in [
42], given in Equation (14) as follows,
The magnitude of the plastic zone radius was calculated by way of utilizing the SDAS, and therefore the effective grain size data presented in
Figure 3 and listed in
Table 5 to predict the yield strength. Keeping with Al 6061, the yield strength for the powder was determined via the Hall–Petch relation, which is given in Equation (15) as follows,
where
is the yield strength of the powder particle,
is the average grain size,
is rationalized as a frictional stress resisting the motion of gliding dislocations, and
is a measure of the resistance of the grain boundary against slip transfer [
43]. For alloyed aluminum gas-atomized powder,
MPa and
MPa m
1/2.
By that rational, and by way of considering the application of a microindenter with a lower load limit of 0.01 kgf, it follows that for an aluminum alloy, the plastic zone radius is between 11 and 14 μm, assuming the two models above. While we could argue that for a particle of 38 μm diameter, the predicted plastic zone radius from continuum mechanics alone is less than the particle radius and therefore feasible, work by others began to incorporate the occurrence of dislocation accumulation into the prediction of a more refined plastic zone radius. Therefore, when dislocations are considered as well for the 0.01 kgf applied to said powder particle, the plastic zone radius is actually between 55 and 75 μm, which exceeds the powder particle radius by two to three times [
44], thus including the effect of the mounting material on the measured hardness.
For the sake of completion, if we assume an applied nanoindentation load of 0.005 N for a similar Al 6061 powder particle with a yield strength of 273 N·mm−2, then the plastic zone radius would be between 2.33 and 2.94 μm from continuum mechanics alone. From a cautionary standpoint, if the accumulation of dislocations is considered once more, then the combined plastic zone radius range is between 11.66 and 14.71 μm, where both of which are less than the radius of the D50 powder particle, therefore ensuring that the nanoindentation depth is effectively free of a mounting material influence.
4.2. Al 7075 Powder as a Case Study
Although this paper addresses the lack of scientific literature explicitly differentiating the nature of GA (rapidly solidified) alloyed Al powders with their cast (slowly solidified) counterparts, previous work has investigated the microstructure-processing-properties-performance paradigm for cold spray by focusing upon powder pre-processing effects. Focusing upon Al 7xxx, Sabard et al. studied the consolidation of solution heat treated, naturally aged, and artificially aged Al 7075 in relation to cold spray [
45]. In order to thermally pre-process their 7075 powder, Sabard et al. elected to secure powder within a tube of quartz and perform heat treatments using a commercially available box furnace. Sintering of powders was not noted by this work, nor was cooling rate during quenching measured [
46]. Beyond the limitations identified by Liu et al. surrounding Sabard et al.’s work, differences also exist between the methods reported herein and those deployed during Sabard et al.’s work. Specifically, a DSC was utilized to perform thermal processing in order to ensure that the processing conditions were near ideal and the cooling rates were maximized to ensure that the thermally processed microstructure was retained upon cooling. Consideration of the kinetics associated with the formation and modification of Al alloyed powders has demonstrated much more rapid rates of phase transformation versus that of bulk material [
14,
24,
25,
37,
47].
Similarly, noteworthy differences between Sabard et al.’s research methodology and our own follows from the fact that 480 °C was used during the course of this work for the purpose of solutionization instead of the conventional standard heat-treating temperature prescribed for bulk Al 7075 alloys. This temperature was selected according to the equilibrium phase diagram obtained using the measured chemical make-up of the Al 7075 powder reported herein by way of the computational materials software Thermo-Calc. The respective phase diagram has previously been reported upon in [
26]. One may suspect that a 5 °C difference in the processing temperature would have limited effects.
However, the slightly greater difference of 15 °C relative to 465 °C was shown to form the S-phase (Al
2CuMg) alongside the T-phase (Al
2Mg
3Zn
3), whereas the 480 °C treatment did not form the S-phase and achieved greater dissolution of the T-phase at 1 h. Interestingly, Sabard et al. did not discuss the implications of phases such as Al
2CuMg or Al
2Mg
3Zn
3 and only considered Guinier–Preston (GP) zones and
h/
h’ (Mg(Zn,Al,Cu)
2) in [
45]. From the associated phase diagram in [
26], 480 °C is also more optimal in terms of avoiding any unexpected or non-predicted local equilibria phenomena within the powder, since the highly non-equilibrium nature of rapid solidification is not completely captured through the thermodynamic approach taken in computationally formulating a phase diagram with Thermo-Calc, as illustrated through the unexpected formation of Al
2CuMg at 465 °C, for example.
Three additional differences between the findings reported by Sabard et al. and the research presented herein concerns the DSC analysis approach to characterizing the hardness of the powders and the supplier/chemistry/manufacturing of the Al 7075 powders used. With respect to the supplier of the powder, our research was performed upon Al 7075 powder purchased from Valimet, Inc (Stockton, CA, USA). On the other hand, Sabard et al. acquired their feedstock material from TLS Technik and found the D10, D50, and D90 to be 31.5, 48.5, and 73.1 μm, respectively. Compared with the PSD characteristics associated with the Al 7075 powder presented during the course of this research, Sabard et al.’s D10 was 20.69% larger, while their D50 and D90 were 30.73% and 38.19% greater, respectively.
Of more immediate relevance is the chemical discrepancy between the TLS Technik spherical gas-atomized Al 7075 powder and the Al 7075 powder from Valimet, which is based on the difference in the alloying element content between the two batches of powder. Although the powder used by Sabard et al. is also within the ASM specification standard, certain alloying elements were significantly varied in contrast with one another. While Cr, Zn, Cu, and Mg were found to be virtually equivalent in weight percent, the TLS Technik powder had 33.33% more Si and 17.65% more Fe.
Alternatively, there was 80.77% less Mn in the TLS powder as well as 65.52% less Ti. Slight variations in alloying composition, even within tolerable ranges, have been shown to vary the tendency to form specific secondary phases, precipitates, and dispersoids [
14]. Such variation in alloying elemental content can also serve to shift the temperature required for phase dissolution, thus speaking to the need for Sabard et al. to refine future work through the use of chemistry-specific thermodynamic phase diagrams using platforms such as Thermo-Calc, FactSage (Thermfact/CRCT, Montreal, Canada and GTT-Technologies, Aachen, Germany), or Pandat (CompuTherm LLC, Middleton, WI, USA), among others, when designing thermal processing parameters for powder.
Even though prior and forthcoming work has demonstrated the fact that alloying elements that are not contained within a given phase can influence the tendency of such a phase to nucleate within a material [
14], the specific elemental constituents within a given phase are still the main driving force behind the likelihood of a particular phase’s formation. Accordingly, since Zn, Mg, and Cu were effectively equivalent between the two batches of powder, it was surprising that
h/
h’ was assumed. As such, Sabard et al. would benefit from continued refinement of their future work by way of applying TEM-based energy dispersive X-ray spectroscopy analysis of the nano-scale precipitates formed within as-atomized and thermally processed Al 7075 powders instead of assuming a conventional strengthening sequence of GP zones to
h/
h’.
Due to the fact that the strengthening sequence depends upon the ratio of Mg/Zn and the fact that both Mg and Zn were virtually equivalent between the Valimet and TLS powders, it stands to reason that the actual strengthening phase sought after by Sabard et al. is Al2Mg3Zn3. More to the point, the focus upon h/h’ by Sabard et al. is also inconsistent when compared with their earlier work, which attempted to analyze elemental mapping within Al 7075 powder that was sourced from Valimet via SEM-EDS analysis. Given the lack of confidence in phase specification that they articulated in an earlier manuscript, the more recent focus upon h/h’ three years after their original proclamation is surprising.
Beyond the work of Sabard et al., Rokni et al. previously characterized the microstructure of Al 7075 gas-atomized powder [
6]. Rokni et al. also purchased their powder from a different supplier than the one selected during the course of this research, which was also different from the manufacturer selected by Sabard et al. [
45]. Rokni et al. reportedly sourced their Al 7075 powder from CenterLine (Windsor) Limited; however, a current survey of their presently offered powder products seems to no longer consist of 7xxx series powder as a consumer option. Therefore, a limited analysis of processing parameters can be made. Regardless of the varied supplier in this case, it is important to note that Rokni et al. elected to favor the formation of ή in the form of MgZn
2 precipitates, even though they conceded that the possible primary precipitates in this alloy are ή (MgZn
2), η (MgZn
2), Mg(Zn,Cu,Al)
2, and T phase ((Al,Zn)
49Mg
32) [
6]. Although preliminary EDS analysis was interpreted as being potentially suggestive of ή, Rokni et al. admits that a detailed study of the precipitate composition and structure was lacking in their study.
The observation of the T-phase in the as-atomized 7075 powder reported upon herein [
26] is also consistent with powder X-ray diffraction (PXRD) performed by Molnárová et al. and substantiated by [
48], wherein Molnárová identified <1 wt.% content of Mg
32(Zn,Al,Cu)
49 as the predominant precipitate [
49]. In [
49], the authors claim that microhardness measurements enable strength comparisons between powders. They measured Vickers microhardness of atomized powder particles as 95 ± 18 HV; the large standard deviation is attributed to variation in powder particle microstructures due to solidification rates. As discussed in the aforementioned sections, the use of a microhardness tester for measuring powder and recording such low values for Al 7075 powder speaks to the limitation of the reasoning underlying the quoted passage provided.
4.3. Additional Analysis of the Reported Results
Figure 1 shows the temperature of the three casts as a function of time, which speaks to the cooling rates experienced by the cast materials in contrast to the rapidly solidified powders.
Figure 2 contrasts the cast cooling rates with time. The cooling rates expected in both cases are consistent with those presented in earlier works [
50,
51]. In fact, the peak Al 6061 cooling rate measured and presented in
Figure 2 was found to be consistent with the peak cooling rate recorded by Xu et al. in a study concerned with the effect of cooling rates upon twin-rolled cast Al 6061 when Xu et al.’s casting was allowed to cool and solidify in air [
52].
Considering the D
50 reported for gas-atomized Al 6061, for example, which was presented in
Table 2, we may capture the order of magnitude associated with a gas-atomized Al 6061 powder particle with a diameter of approximately 40 μm. With the peak cooling rate associated with the conventionally cast counterpart in mind, by way of comparison, one readily appreciates the fact that the cooling rate for such a powder particle was multiple orders of magnitude greater than that associated with the casting (10
5 °C s
−1 versus 10
0 °C s
−1). Considering the fact that slight variations in the average cooling rates affiliated with cast Al 6061 have previously been found to have significant effects on the mechanical properties and resultant microstructures of bulk counterparts, one can readily appreciate the consequences of the vastly different cooling rates for the atomized and cast systems studied herein, too. The same holds true when the D
50 droplet cooling rates are compared with the cast cooling rates for Al 2024 and Al 7075 as well.
The relationship between grain size and particle diameter for Al 2024, Al 6061, and Al 7075 ought to be noted herein, too. Experimental validation of the cooling rate calculations and computed effective grain sizes as a function of particle size also revealed the fact that an increase in particle diameter from 20 to 60 μm increases the grain size by nearly three to four times for each aluminum alloy considered. Through the lens of the Hall–Petch relation between grain size and strength, one may also consider the implications for cold spray, as will be discussed in even greater detail hereafter. In the meantime, such variations between the particle strength as a function of particle diameter will vary the critical particle impact velocities required for particle–substrate bonding, wherein particles with a greater diameter would require lower critical impact velocities than that of smaller particles. Such an immediate observation is consistent with research by Dowding et al., which found that the critical velocity was approximately 33% greater for an aluminum particle of approximately 10 μm in diameter compared with a particle of approximately 40 μm in diameter [
53].
In light of the compositional wt.%’s measured by OES for the Al 2024 casting, limited comparative analysis can truly be expressed herein. In fact, the OES-based Cu wt.% was not only out of ASM International specifications for Al 2024, it was also much lower than the general copper content range prescribed to Al 2xxx series, which typically resides within a lower bound of 2 wt.% and an upper bound of 10 wt.% copper. More troubling is the fact that the measured OES-based Al 2024 cast Cu wt.% was only 0.012 wt.%, thus potentially acting as a trace element in a cast material that was supposed to be primarily alloyed with copper as the main alloying element. That being said, Cu was not the only alloying element in the cast Al 2024 specimen found to be below or above the prescribed tolerances. Specifically, the Cr content was measured as 0.197 wt.%, which is nearly double that of the allowable upper limit according to ASM International. On the other hand, the Mn content was only 0.06 wt.%, and the Mg content exceeded the standardized upper bound at a value of 2.25 wt.%.
Then, an attempt was made to rationalize and deduce what was potentially responsible for the measured out-of-range alloying elements. Understanding the source of the varied chemical composition within bulk Al 2024 not only follows from the need to consider the limitations surrounding a direct comparison of the gas-atomized Al 2024 properties with the powders’ cast counterpart. Rather, the need for a reasonable explanation also stems from the desire to assess whether or not the Al 6061 and Al 7075 cast and gas-atomized conditions can be confidently compared with one another, too, given the fact that the cast Al 6061 and Al 7075 compositions were also obtained using the same OES system. Potential sources of error or influence with respect to the present issue at hand are formulated as follows: (1) OES device contamination, (2) elemental loss from inclusion formation during casting, (3) deterioration of the notably dated OES system available, (4) elemental loss from volatilization or evaporation during liquid processing, (5) errors in performing the casting process, and/or (6) out-of-specification wrought aluminum alloy casting feedstock. Given the number of potential sources that must be considered, continued discussion is provided next.
If OES-device contamination was responsible for the recorded Cu, Cr, Mg, and Mn contents, one would expect to find errors throughout the Al 6061 and Al 7075 castings, too. To test the feasibility of the notion that (1) was responsible, one would expect the Mg content within the Al 6061 and Al 7075 castings to be overestimated, too. However, the fact that the wt.% of Mg within the cast Al 6061 sample was slightly lower than the specified lower limit of 0.8 wt.%, at 0.66 wt.%, detracts from the feasibility of explanation (1). The fact that the wt.% of Mg within the cast Al 7075 sample was within the defined range at 2.21 wt.% also detracts from (1) listed above as well. It is also worth noting that Cr, Cu, and Mn were all within specification for cast Al 6061 and Al 7075, too, which once again nullifies (1).
As for (2), one may test the veracity of the prospective explanation by way of focusing on the amount of Cu present in Al 6061 and Al 7075 casts, since one would expect the content to be substantially lower than the lower bound prescribed by ASM International. This is due to the fact that if copper was lost during casting by way of forming inclusions and slag at the molten or liquid Al 2024 surface, then one would reasonably expect alternative aluminum alloy systems to also lose a proportional amount of the element, too. From
Figure 4, this was clearly not the case, since the Cu wt.% in the cast Al 7075 material was within range, at a value of 1.36 wt.%, and two orders of magnitude greater than the trace amount measured in the Al 2024 casting via OES. Just as the Al 7075 did not appear to support (2), the resultant Cu content within the cast Al 6061 was also within an accepted range at 0.164 wt.%. Therefore, claims (3) through (6) must endure continued consideration, analysis, and discussion herein.
Unlike claims (1) and (2), which required greater detail and explanation be presented to remove them from further consideration, (3) can more readily be nullified. If (3) was responsible for the out-of-specification alloying element contents measured via OES, then the Al 6061 and Al 7075 would have been deleteriously influenced in a comparable manner. Yet, as clearly shown in
Figure 4, all of the elements out of range in the Al 2024 specimen were found to be in accordance with specified tolerances for the Al 7075 cast counterpart. Similarly, the Cu, Cr, and Mn wt.%’s within the cast Al 6061 were also well within specification, with only Mg being slightly lower than standard limits. Hence, the claim that instrument deterioration was responsible for the resultant Al 2024 casting composition does not hold either. Claim (4) can also be dismissed following a relatively straightforward comparison of the out-of-specification alloying elements in the Al 2024 casting with the way in which they were found to be out of range (i.e., greater than the upper limits or less than the lower limits, respectively) and their particular boiling points.
Therefore, the most likely culprits are claims (5) and (6). In light of the fact that the casting process was successful for Al 6061 and Al 7075 castings, it stands to reason that errors in performing the casting process are less likely to be the primary source of Al 2024 casting nullification than that of out-of-specification wrought aluminum alloy casting feedstock, as expressed in claim (6). Given the careful inspection of the prospective sources of error, one can conclude that Al 6061 and Al 7075 comparisons between the bulk and atomized conditions hold, since (6) was responsible for the out-of-specification alloying element contents measured via OES and was therefore isolated to Al 2024.
Returning to Al 2024,
Figure 5 presented the DSC curves for the nominally cast Al 2024 counterpart as well as the gas-atomized Al 2024 powder. While limited knowledge can be ascertained by way of directly comparing the two Al 2024 conditions with one another, given the compositional discrepancies just discussed in great detail, insights can still be derived from consideration of the DSC data for gas-atomized Al 2024. Compared with earlier works of scholarship that incorporated DSC-based analysis of Al 2024 materials into their research, the exothermic and endothermic gas-atomized Al 2024’s peaks present in
Figure 5 can be classified in accordance with the formation or dissolution of specific secondary phases. The initial local maxima about a temperature of 100 °C or so for the gas-atomized Al 2024 likely follows from the formation of GP zones.
The first local minima recorded during DSC analysis of rapidly solidified Al 2024 powder was found to be consistent with the dissolution of the GP zones. The second bimodal set of local maxima presented within the thermogram between 250 °C and more than 300 °C were consistent with the formation of coherent
phases followed by semi-coherent
and S’ phase formation as well as
-phase and S-phase formation, too. At the same time, the unique solidification conditions associated with gas-atomized Al 2024 processing has been shown to contain S-phase precipitates and Al
2Cu precipitates in the as-atomized condition [
25]. Such findings by Walde et al. are consistent with the partial attribution of S-phase secondary phase formation in
Figure 5, too.
4.4. Relation to and Implications for Cold Spray
Within the cold spray community, powder-specific material properties have been sought after for the purpose of performing single-particle and multi-particle impact simulations and computational analysis via finite element analysis (FEA). Whether the Preston–Tonks–Wallace (PTW) approach is taken, which is partly presented in Equation (17), or the Johnson–Cook (JC) formulation [
54], given in Equation (16), is entertained, material-dependent input parameters are required. The JC and PTW relations are expressed as follows,
and
Traditionally, these values have been borrowed from bulk alloy data rather than measured powder properties, as discussed in detail within the Introduction section. However, the assumed ability to utilize input parameters from literature data for a bulk material counterpart when determined using the Eulerian algorithm in Abaqus using bulk parameters was definitively shown to underestimate the temperature distribution during impact [
55]. As pointed out by Assadi et al., it is possible that this difference could be relevant when considering the bonding mechanism in cold spray. Plastic strain has typically been considered a main factor for bonding in cold spray, and it is similar for the two cases [
55]. Given the fact that
,
, and
are related to the flow stress, it stands to reason that the measured and calculated yield strengths obtained herein could be analyzed further for the purpose of determining JC parameters from load–depth nanoindentation data.
In addition to the plastic constitutive properties that are dependent upon powder properties, rather than bulk, as shown elsewhere as well as herein via microstructural evaluation and mechanical characterization of the gas-atomized alloy condition versus the cast alloy condition, particle impact simulations could also be enhanced via microstructurally accurate particle rendering within FEA. For example, instead of simply etching and measuring or calculating the given size within a powder particle, electron backscatter diffraction analysis of the cross-sections of a set of particles for a given particle could be utilized for 3D reconstruction, and Abaqus importable synthetically reconstructed volumetric microstructures could be rendered from Dream.3D, for example. Then, such renderings could be confirmed according to sectioning protocols and utilized in FEA of cold spray impact phenomena. As a result, impact modeling would not only capture the unique mechanical behavior of a given powder but also the microstructure, making the simulation that much more proximal to reality. In the meantime, an example set of FEA simulations using microstructure-informed renderings of particulate feedstocks exposed to varied thermal processing parameters are shown in
Figure 12 and
Figure 13.
Beyond the realm of enhancing particle impact modeling, this provides unequivocal confirmation that gas-atomized alloyed aluminum powders may not be conceptualized, in terms of material properties, as a similar enough material system to their bulk counterparts to determine cold spray processing parameters. Specifically, the essential parameter known as the critical impact velocity (
) has historically been formulated as being dependent upon the ultimate tensile strength (
) of a particle in [
56], as shown in, as follows,
such that
Previous research has also established a link between
and particle diameter. The established link found that the
increases as the particle diameter decreases. Since our work presented herein demonstrated a direct link between particle diameter and grain size followed by grain size and strength, the above direct relation between
and
in terms of particle diameter as well was found to also be consistent with the previously established connection. The consistency between the
and
and
and particle diameter dependency corresponds with the fact that smaller particles will have greater
, since their grain sizes are consequently smaller. Being able to use rapid mechanical and microstructural assessment methods for powder particle evaluation will enable more robust and rapid processing parameter optimization and cold spray performance with minimal trial and error. Thus, one may relate
to
in accordance with
where
is either obtained from the Tabor relation using the
nanoindentation-derived value or computed from the Hall–Petch relation.
In addition to the ability to refine the parameters for particle impact modeling as well as the capacity to predict from the mechanical properties and microstructure of a given powder, the kinetics and thermal processing response unique to powders versus their bulk counterpart can assist in the pursuit of achieving strength–toughness synergy in cold spray. This can be done by way of utilizing the thermal processing knowledge obtained herein and building upon it to achieve a sprayable powder that has greater deposition efficiency, enhanced particle–particle bonding, better particle–substrate adhesion, and greater elongation-to-failure. Furthermore, this can be achieved without postprocessing the consolidated material through powder pre-processing prior to cold spray deposition. By way of assessing the transformation kinetics highlighted herein, one may more easily formulate a heat-treatment protocol that maximizes intermetallic precipitate and compound dissolution while minimizing the deleterious coarsening of secondary phases and dispersoids.
The slight grain growth from thermal processing and residual strain relief will certainly enhance the degree of deformation associated with particle impact as well. Nevertheless, the prospective implications put forth within this subsection of the manuscript called upon less recent mechanistic frameworks for particle bonding during cold spray impact phenomena. A more modern and experimentally substantiated and observed
framework has since been declared in [
57,
58] in terms of shockwave-mediated hydrodynamics, spall strength, the elastic limit, and the speed of sound as a function of the bulk modulus (which can be expressed as a function of the modulus of elasticity) of a material and the materials density, such that
and