Novel Tunable Green-Red Luminescence in Mn2+ Doped Ca9Tb(PO4)7 Phosphors Based on the Mn2+ Regulation and Energy Transfer
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Synthesis
2.2. Characterization
3. Results and Discussion
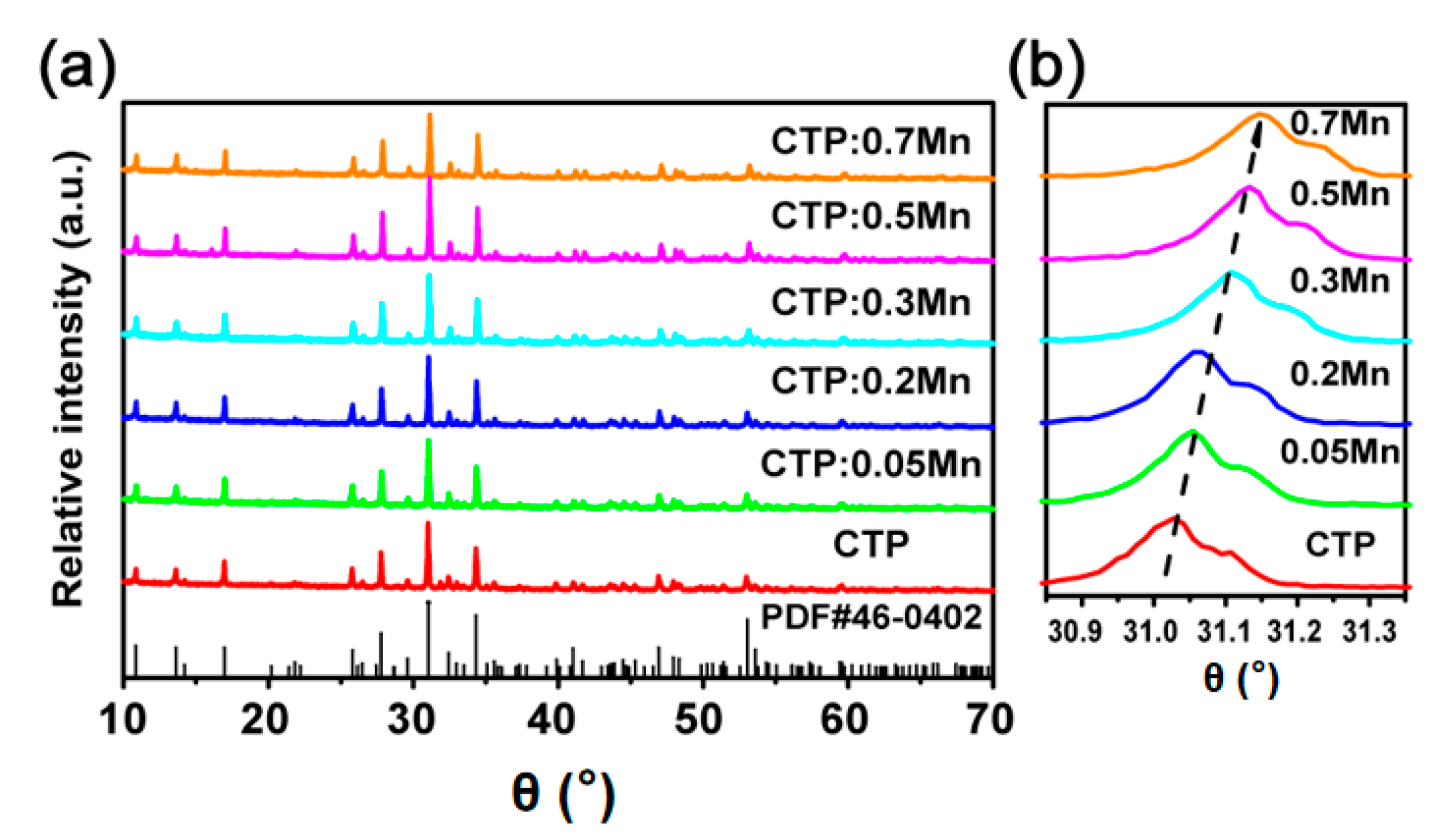
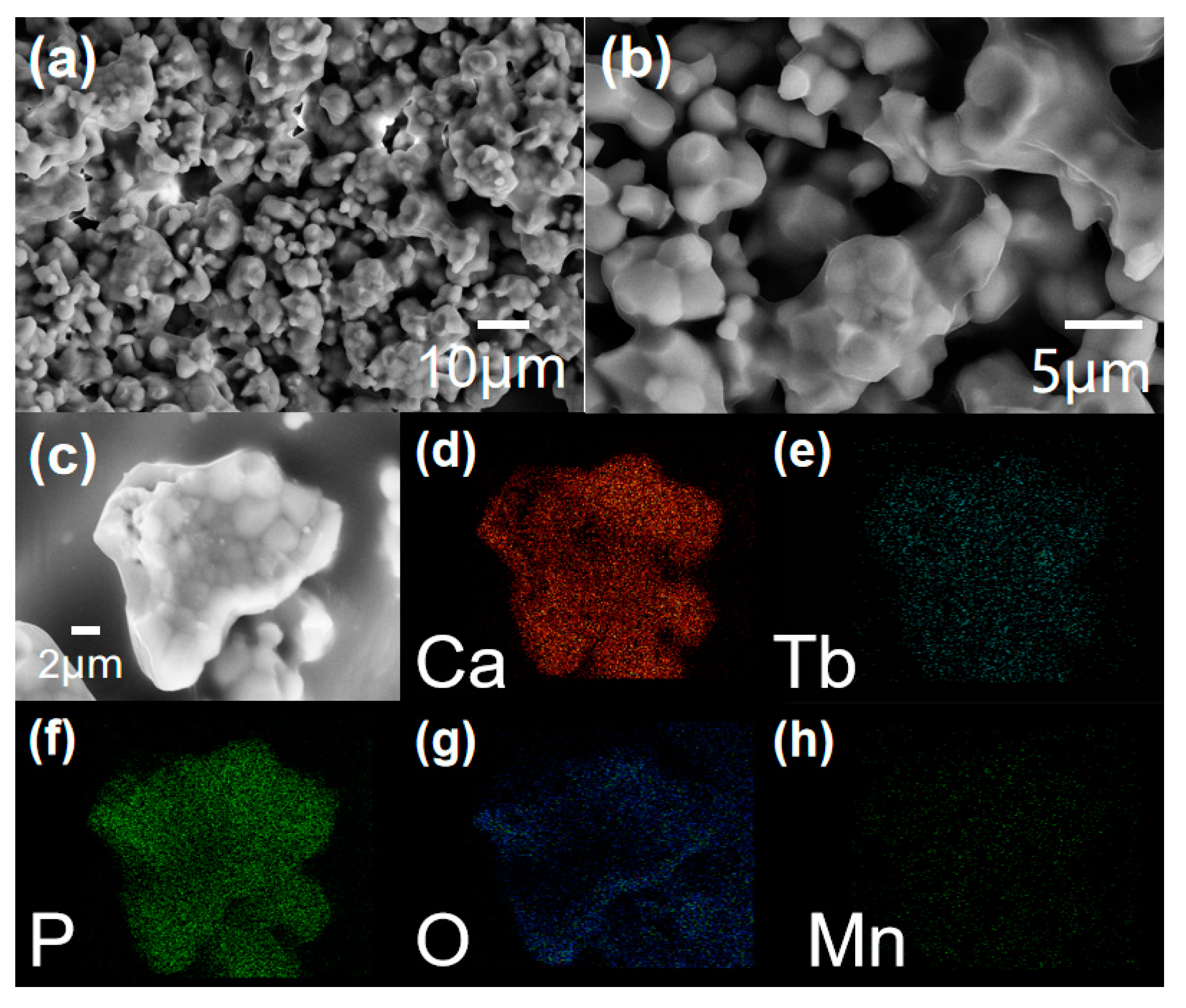
3.1. Phase Characterization and SEM Analysis
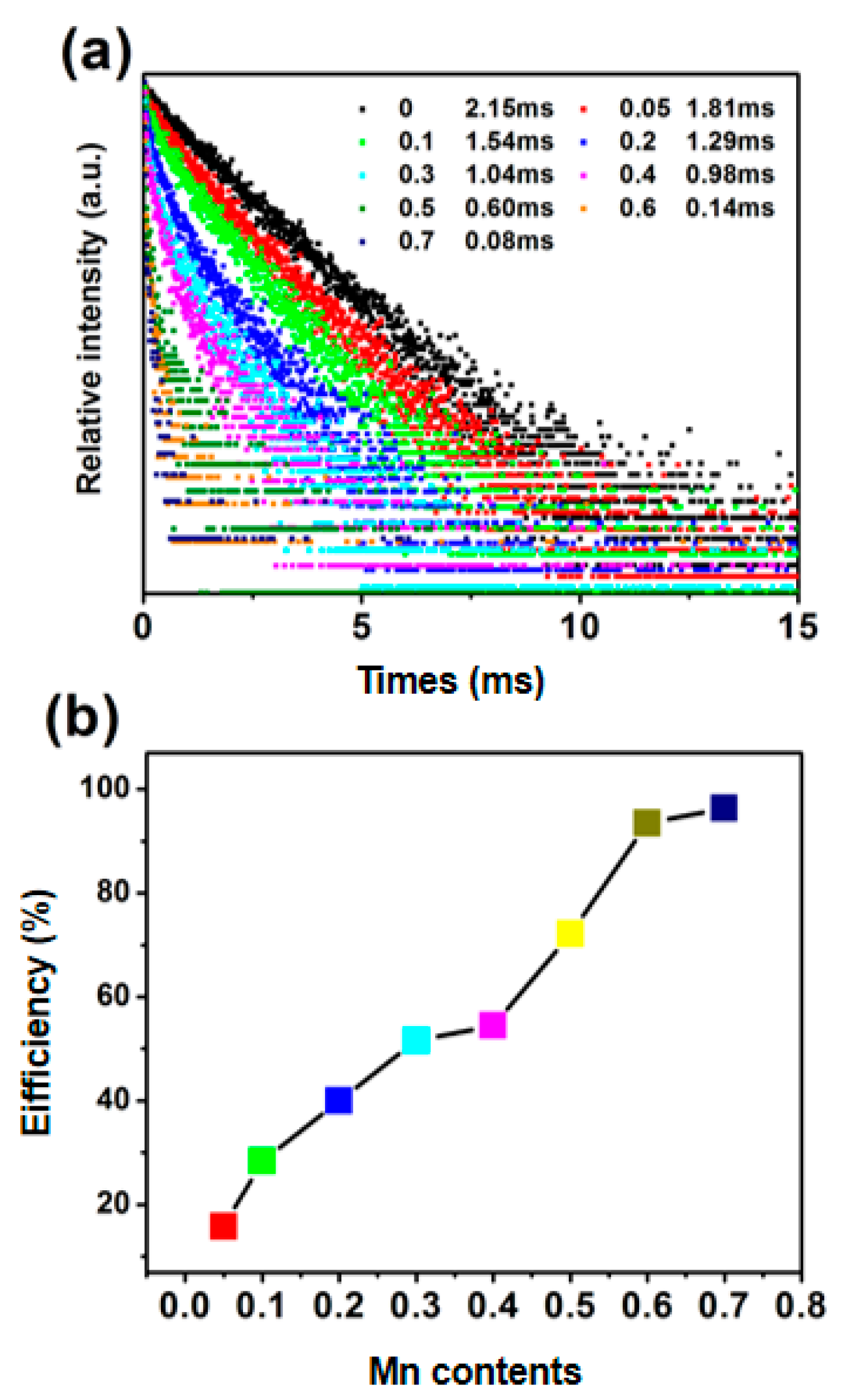
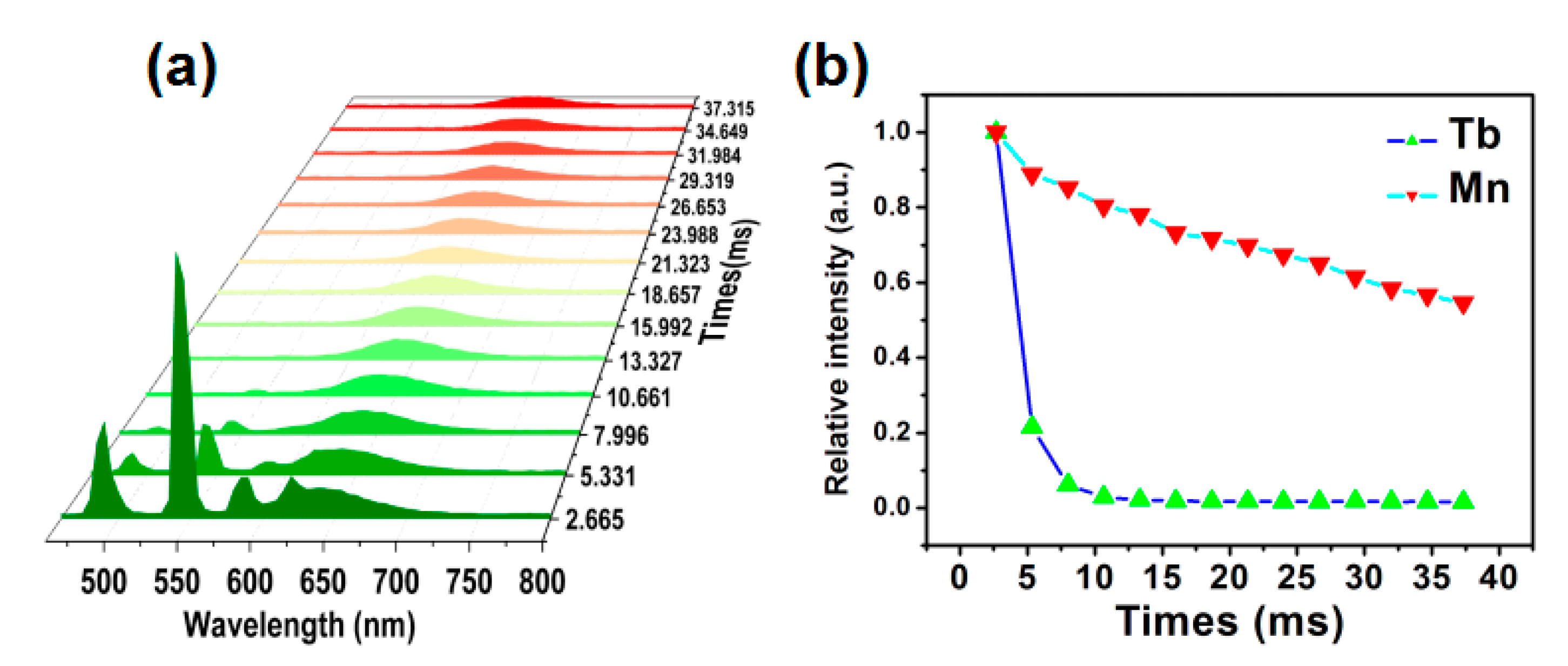
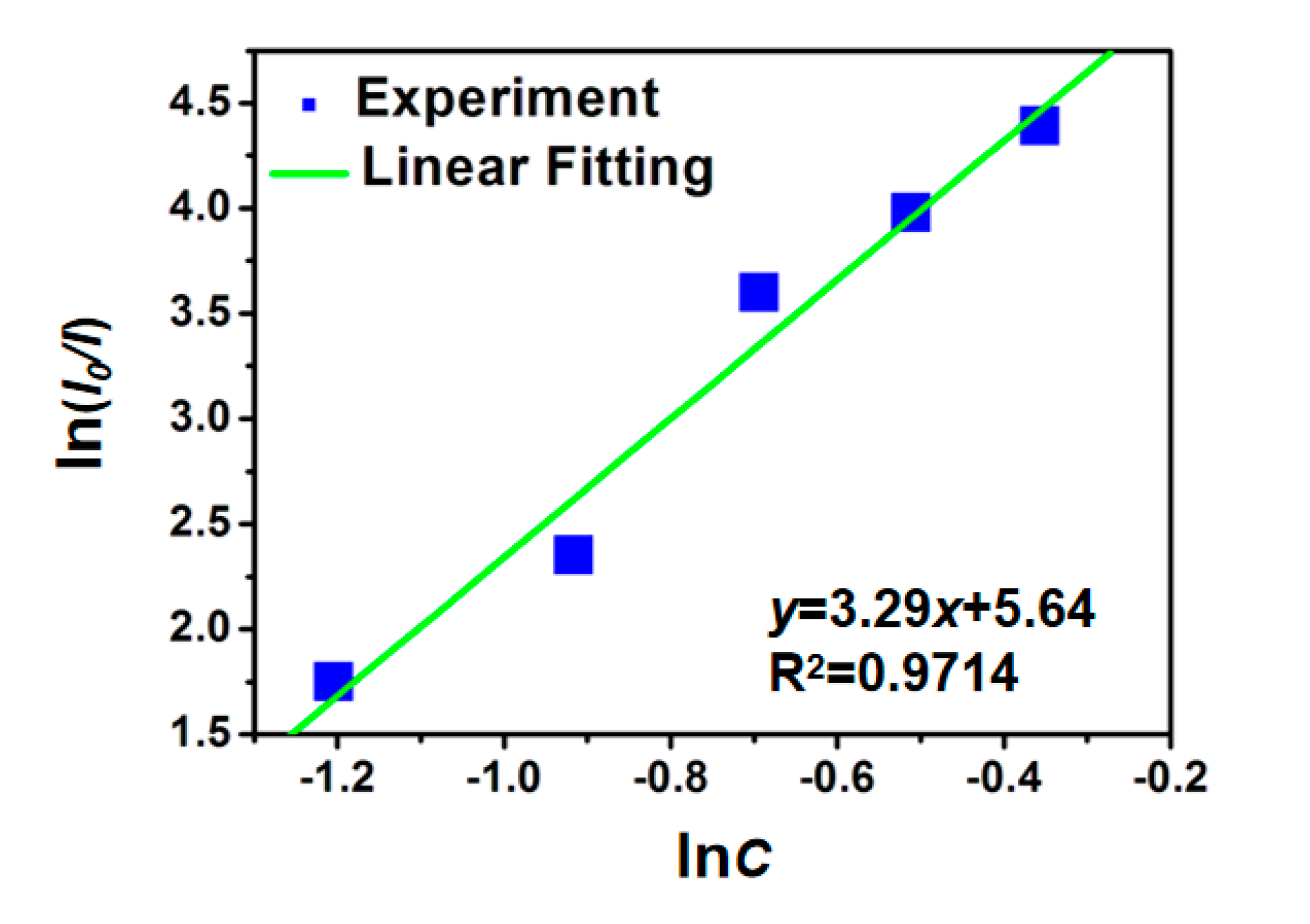
3.2. Luminescence Performance and Energy Transfer of CTP:Mn2+ Phosphor
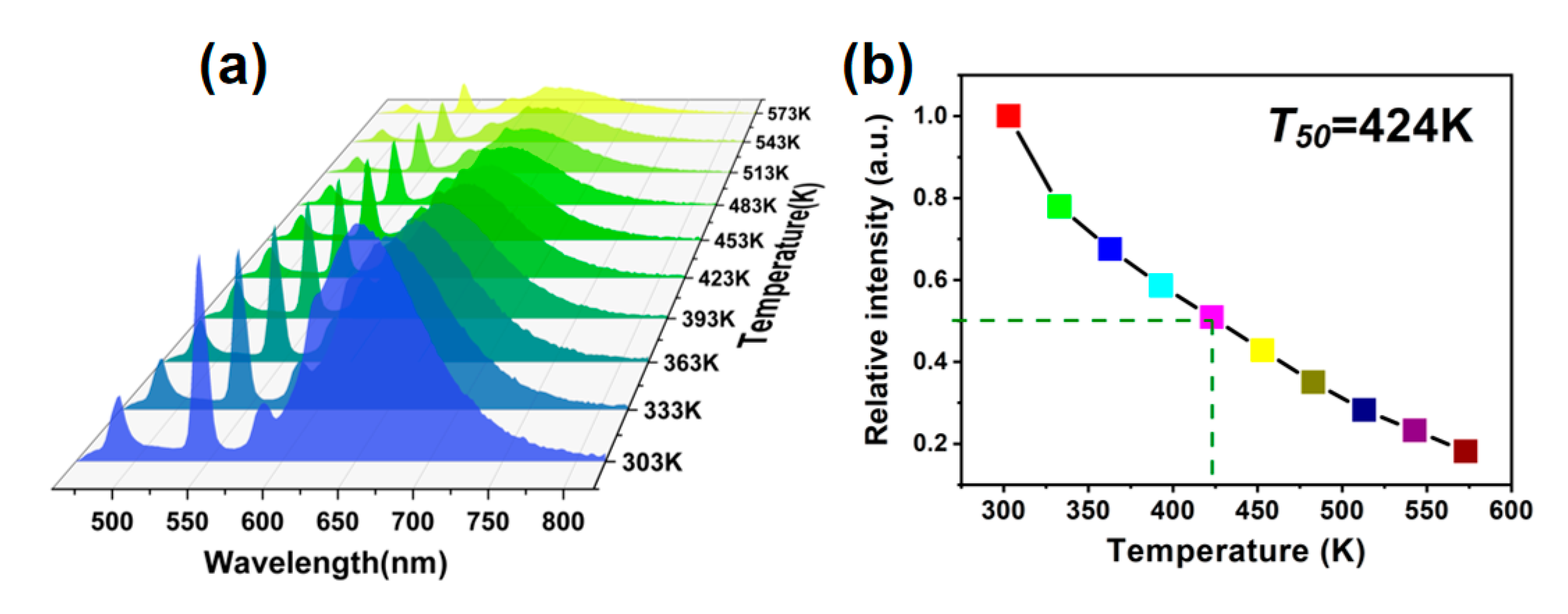
3.3. Temperature-Dependent PL Performance of CTP:Mn2+ Phosphor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, H.J.; Yim, D.K.; Cho, I.-S.; Roh, H.-S.; Kim, J.S.; Kim, D.-W.; Hong, K.S. Luminescent properties of phosphor converted LED using an orange-emitting Rb2CaP2O7:Eu2+ phosphor. Mater. Res. Bull. 2012, 47, 4522–4526. [Google Scholar] [CrossRef]
- Sun, W.; Jia, Y.; Pang, R.; Li, H.; Ma, T.; Li, D.; Fu, J.; Zhang, S.; Jiang, L.; Li, C. Sr9Mg1.5(PO4)7:Eu2+: A novel broadband orange-yellow-emitting phosphor for blue light-excited warm white LEDs. ACS Appl. Mater. Interfaces 2015, 7, 25219–25226. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Sun, J.; Song, D.; Shang, M.; Zhou, P.; Li, K.; Lin, J. Tunable luminescence and energy transfer properties in Ca8MgLu(PO4)7:Ce3+, Tb3+, Mn2+ phosphors. J. Mater. Chem. C 2015, 3, 4471–4481. [Google Scholar] [CrossRef]
- Li, K.; Shang, M.; Zhang, Y.; Fan, J.; Lian, H.; Lin, J. Photoluminescence properties of single-component white-emitting Ca9Bi(PO4)7:Ce3+, Tb3+, Mn2+ phosphors for UV LEDs. J. Mater. Chem. C 2015, 3, 7096–7104. [Google Scholar] [CrossRef]
- Liang, S.; Dang, P.; Li, G.; Molokeev, M.S.; Wei, Y.; Lian, H.; Shang, M.; Al-Kheraif, A.A.; Lin, J. Controllable two-dimensional luminescence tuning in Eu2+,Mn2+ doped (Ca,Sr)9Sc(PO4)7 based on crystal field regulation and energy transfer. J. Mater. Chem. C 2018, 6, 6714–6725. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Wang, J.; Wu, M.; Wang, C. Color-tunable phosphor of Eu2+ and Mn2+ codoped Ca2Sr(PO4)2 for UV light-emitting diodes. J. Phys. Chem. C 2014, 118, 12494–12499. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wu, P.-J.; Lee, J.-F.; Chen, T.-M. (Ca,Mg,Sr)9Y(PO4)7:Eu2+,Mn2+: Phosphors for white-light near-UV LEDs through crystal field tuning and energy transfer. J. Mater. Chem. 2011, 21, 10489. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Li, P.; Cheng, J.; Li, Z.; Tian, M.; Sun, Y.; Yang, Z. Relationships between luminescence properties and polyhedron distortion in Ca9−x−y−zMgxSryBazCe(PO4)7:Eu2+, Mn2+. J. Mater. Chem. C 2017, 5, 10839–10846. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Guo, Z.; Gong, M. Luminescence and energy transfer of dual-emitting solid solution phosphors (Ca,Sr)10Li(PO4)7:Ce3+, Mn2+ for ratiometric temperature sensing. Ind. Eng. Chem. Res. 2017, 56, 890–898. [Google Scholar] [CrossRef]
- Lv, Y.; Jin, Y.; Wu, H.; Liu, D.; Xiong, G.; Ju, G.; Chen, L.; Hu, Y. An all-optical ratiometric thermometer based on reverse thermal response from interplay among diverse emission centers and traps with high-temperature sensitivity. Ind. Eng. Chem. Res. 2019, 58, 21242–21251. [Google Scholar] [CrossRef]
- Pasiński, D.; Sokolnicki, J. Broadband orange phosphor by energy transfer between Ce3+ and Mn2+ in Ca3Al2Ge3O12 garnet host. J. Alloys Compd. 2019, 786, 808–816. [Google Scholar] [CrossRef]
- Dong, R.; Liu, W.; Song, Y.; Zhang, X.; An, Z.; Zhou, X.; Zheng, K.; Sheng, Y.; Shi, Z.; Zou, H. A promising single-phase, color-tunable phosphor (Ba0.9Sr0.1)9Lu2Si6O24:Eu2+, Mn2+ for near-ultraviolet white-light-emitting diodes. J. Lumin. 2019, 214, 116585. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Z.; Wen, D.; Zhou, J.; Xu, Y.; Li, J.; Ma, C.-G.; Shi, J.; Wu, M. Crystal structure and photoluminescence tuning of novel single-phase Ca8ZnLu(PO4)7:Eu2+,Mn2+ phosphors for near-UV converted white light-emitting diodes. J. Mater. Chem. C 2019, 7, 8374–8382. [Google Scholar] [CrossRef]
- Huang, C.-H.; Kuo, T.-W.; Chen, T.-M. Novel red-emitting phosphor Ca9Y(PO4)7:Ce3+,Mn2+ with energy transfer for fluorescent lamp application. ACS Appl. Mater. Interfaces 2010, 2, 1395–1399. [Google Scholar] [CrossRef]
- Liu, S.; Liang, Y.; Li, H.; Zhang, W.; Tu, D.; Chen, Y. Color tuning of β-Ca3(PO4)2-type phosphor with enhanced quantum efficiency via self-charge compensation for healthy and warm solid state lighting application. Chem. Eng. J. 2020, 390, 124463. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Wang, Z.; Sun, Y.; Cheng, J.; Li, Z.; Tian, M.; Yang, Z. Crystal structure, luminescence properties, energy transfer and thermal properties of a novel color-tunable, white light-emitting phosphor Ca9−x−yCe(PO4)7:xEu2+,yMn2+. Phys. Chem. Chem. Phys. 2016, 18, 28661–28673. [Google Scholar] [CrossRef]
- Carrasco, I.; Piccinelli, F.; Bettinelli, M. Optical spectroscopy of Ca9Tb1−xEux(PO4)7 (x = 0, 0.1, 1): Weak donor energy migration in the whitlockite structure. J. Phys. Chem. C 2017, 121, 16943–16950. [Google Scholar] [CrossRef]
- Tong, X.; Han, J.; Zhang, X. Realization of color tunable via energy transfer in Ba1.2Ca0.8SiO4:Tb3+ phosphor. J. Lumin. 2019, 216, 116742. [Google Scholar] [CrossRef]
- Bessière, A.; Benhamou, R.A.; Wallez, G.; Lecointre, A.; Viana, B. Site occupancy and mechanisms of thermally stimulated luminescence in Ca9Ln(PO4)7 (Ln = lanthanide). Acta Mater. 2012, 60, 6641–6649. [Google Scholar] [CrossRef]
- Ju, X.; Li, X.; Li, W.; Yang, W.; Tao, C. Luminescence properties of ZnMoO4:Tb3+ green phosphor prepared via co-precipitation. Mater. Lett. 2011, 65, 2642–2644. [Google Scholar] [CrossRef]
- Reddy, G.L.; Moorthy, L.R.; Chengaiah, T.; Jamalaiah, B. Multi-color emission tunability and energy transfer studies of YAl3(BO3)4:Eu3+/Tb3+ phosphors. Ceram. Int. 2014, 40, 3399–3410. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Lu, C.-H.; Hsu, C.-H. Preparation and photoluminescence properties of novel color-tunable MgY4Si3O13:Ce3+, Tb3+ phosphors for ultraviolet light-emitting diodes. J. Am. Ceram. Soc. 2010, 93, 1838–1841. [Google Scholar] [CrossRef]
- Chen, X.; Dai, P.; Zhang, X.; Li, C.; Lu, S.; Wang, X.; Jia, Y.; Liu, Y. A Highly efficient white light (Sr3,Ca,Ba)(PO4)3Cl:Eu2+, Tb3+, Mn2+ phosphor via dual energy transfers for white light-emitting diodes. Inorg. Chem. 2014, 53, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, L.; Kumar, K.N.; Hwang, P. Tailoring ultraviolet-green to white light via energy transfer from Tb3+-Eu3+ codoped glasses for white light-emitting diodes. Scr. Mater. 2020, 187, 97–102. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.J.; Liu, C.J.; Dong, G.Y.; Dai, D.J.; Xing, Z.H.; Li, X.T.; Yang, Z.P.; Li, P.L. Tunable emitting phosphor Ca6Ce2Na2(PO4)6F2:Mn2+/Tb3+ for white LEDs:Luminescence, energy transfer and high thermal property. J. Lumin. 2020, 217, 116817. [Google Scholar] [CrossRef]
- Dai, P.P.; Ma, R. Realizing effificient Mn2+ red emission via synergistic sensitization of Eu2+ and Tb3+ for white light-emitting diodes. J. Alloys Compd. 2020, 812, 152143. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Zhang, H.; Wu, Z.-C.; Liu, Y.; Ma, L.; Wang, X.; Liu, W.-R.; Lei, B. Enhanced absorption of Sr3Lu2(BO3)4:Ce3+,Tb3+ phosphor with energy transfer for UV-pumped white LEDs. J. Alloys Compd. 2019, 789, 215–220. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, J.; Zhang, X.; Lu, S.; Ren, X.; Wang, X.-J. Mn2+ concentration manipulated red emission in BaMg2Si2O7:Eu2+, Mn2+. J. Appl. Phys. 2007, 101, 033513. [Google Scholar] [CrossRef]
- Martín-Rodríguez, R.; Valiente, R.; Rodríguez, F.; Piccinelli, F.; Speghini, A.; Bettinelli, M. Temperature dependence and temporal dynamics of Mn2+ upconversion luminescence sensitized by Yb3+ in codoped LaMgAl11O19. Phys. Rev. B 2010, 82, 075117. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Dexter, D.L.; Schulman, J.H. Theory of concentration quenching in inorganic phosphors. J. Chem. Phys. 1954, 22, 1063. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Guo, Z.; Sun, Z.; Yang, Z.; Zhang, T.; Zhang, J.; Wu, Z.-C.; Wang, Z. Dopant preferential site occupation and high efficiency white emission in K2BaCa(PO4)2:Eu2+,Mn2+ phosphors for high quality white LED applications. Inorg. Chem. Front. 2019, 6, 1289–1298. [Google Scholar] [CrossRef]
- Wang, B.; Kong, Y.; Chen, Z.; Li, X.; Wang, S.; Zeng, Q. Thermal stability and photoluminescence of Mn2+ activated green-emitting feldspar phosphor SrAl2Si2O8:Mn2+ for wide gamut w-LED backlight. Opt. Mater. 2020, 99, 109535. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, X.; Wang, Y.; Hao, Z.; Liu, Y.; Luo, Y.; Wang, X.; Zhang, J.; Liu, Y. Luminescence investigation and thermal stability study of Eu2+ and Eu2+-Mn2+ codoped (Ba,Sr)Mg2Al6Si9O30 phosphor. J. Alloys Compd. 2012, 513, 430–435. [Google Scholar] [CrossRef]
- Chen, D.; Liu, S.; Zhou, Y.; Wan, Z.; Huang, P.; Ji, Z. Dual-activator luminescence of RE/TM:Y3Al5O12 (RE = Eu3+, Tb3+, Dy3+; TM = Mn4+, Cr3+) phosphors for self-referencing optical thermometry. J. Mater. Chem. C 2016, 4, 9044–9051. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Wu, Q.; Wang, C.; Wang, Q.; Yanyan, L.; Wang, Y. Structure, photoluminescence and abnormal thermal quenching behavior of Eu2+-doped Na3Sc2(PO4)3: A novel blue-emitting phosphor for n-UV LEDs. J. Mater. Chem. C 2016, 4, 8795–8801. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Feng, Y.; Zhao, Q.; He, M.; Lv, Y. Novel Tunable Green-Red Luminescence in Mn2+ Doped Ca9Tb(PO4)7 Phosphors Based on the Mn2+ Regulation and Energy Transfer. Coatings 2020, 10, 952. https://doi.org/10.3390/coatings10100952
Yang B, Feng Y, Zhao Q, He M, Lv Y. Novel Tunable Green-Red Luminescence in Mn2+ Doped Ca9Tb(PO4)7 Phosphors Based on the Mn2+ Regulation and Energy Transfer. Coatings. 2020; 10(10):952. https://doi.org/10.3390/coatings10100952
Chicago/Turabian StyleYang, Bingwen, Yefeng Feng, Qinghu Zhao, Miao He, and Yang Lv. 2020. "Novel Tunable Green-Red Luminescence in Mn2+ Doped Ca9Tb(PO4)7 Phosphors Based on the Mn2+ Regulation and Energy Transfer" Coatings 10, no. 10: 952. https://doi.org/10.3390/coatings10100952
APA StyleYang, B., Feng, Y., Zhao, Q., He, M., & Lv, Y. (2020). Novel Tunable Green-Red Luminescence in Mn2+ Doped Ca9Tb(PO4)7 Phosphors Based on the Mn2+ Regulation and Energy Transfer. Coatings, 10(10), 952. https://doi.org/10.3390/coatings10100952



