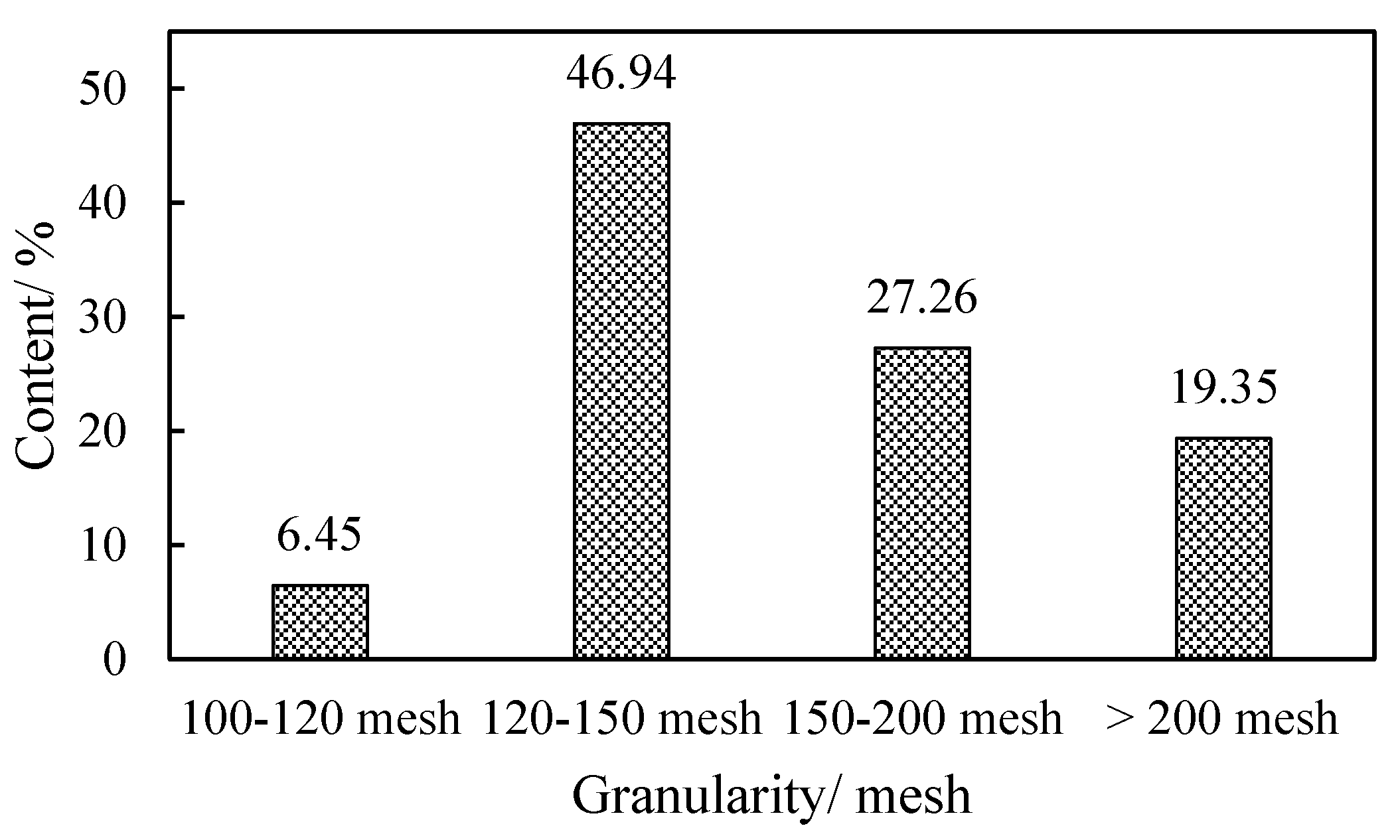
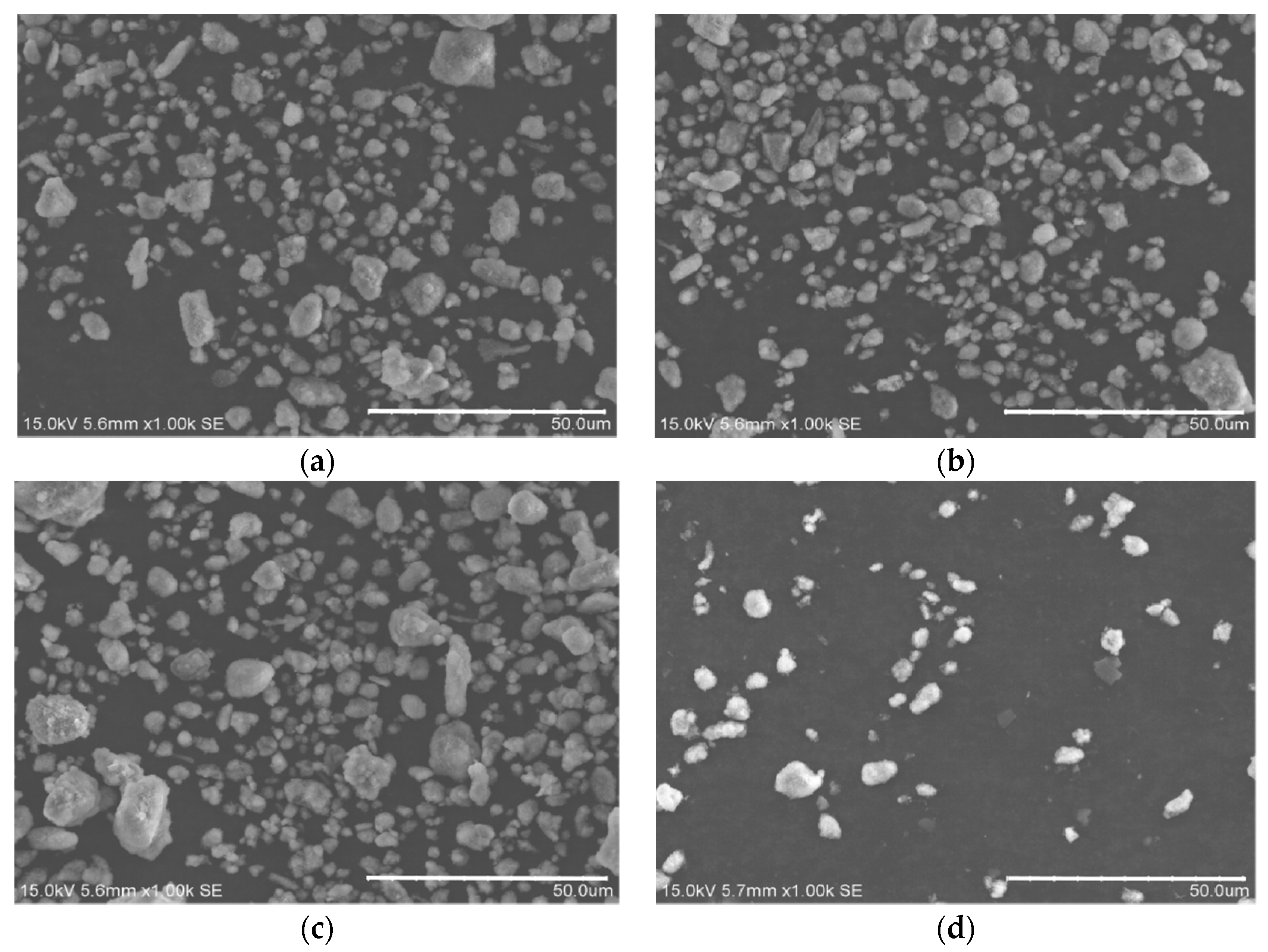
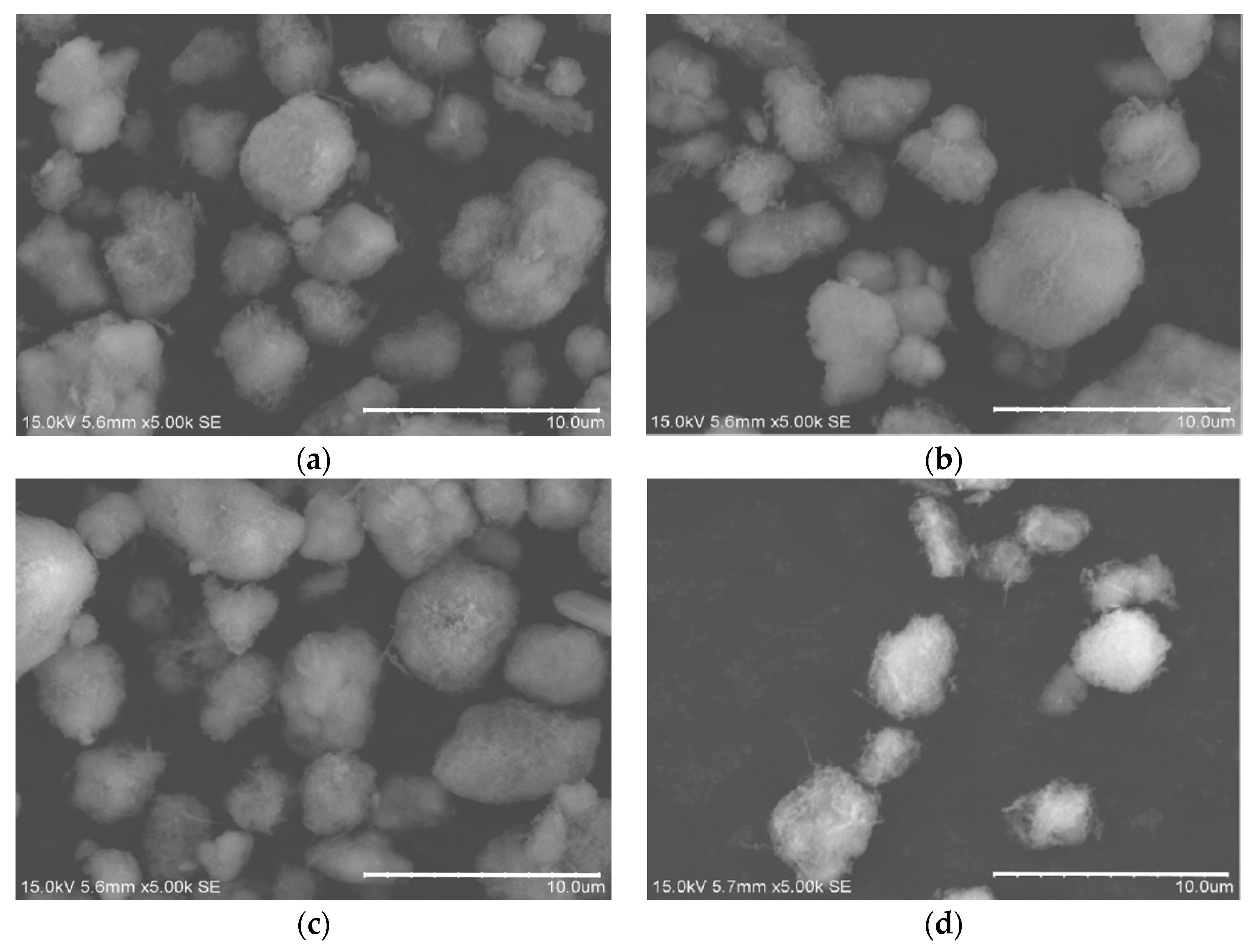
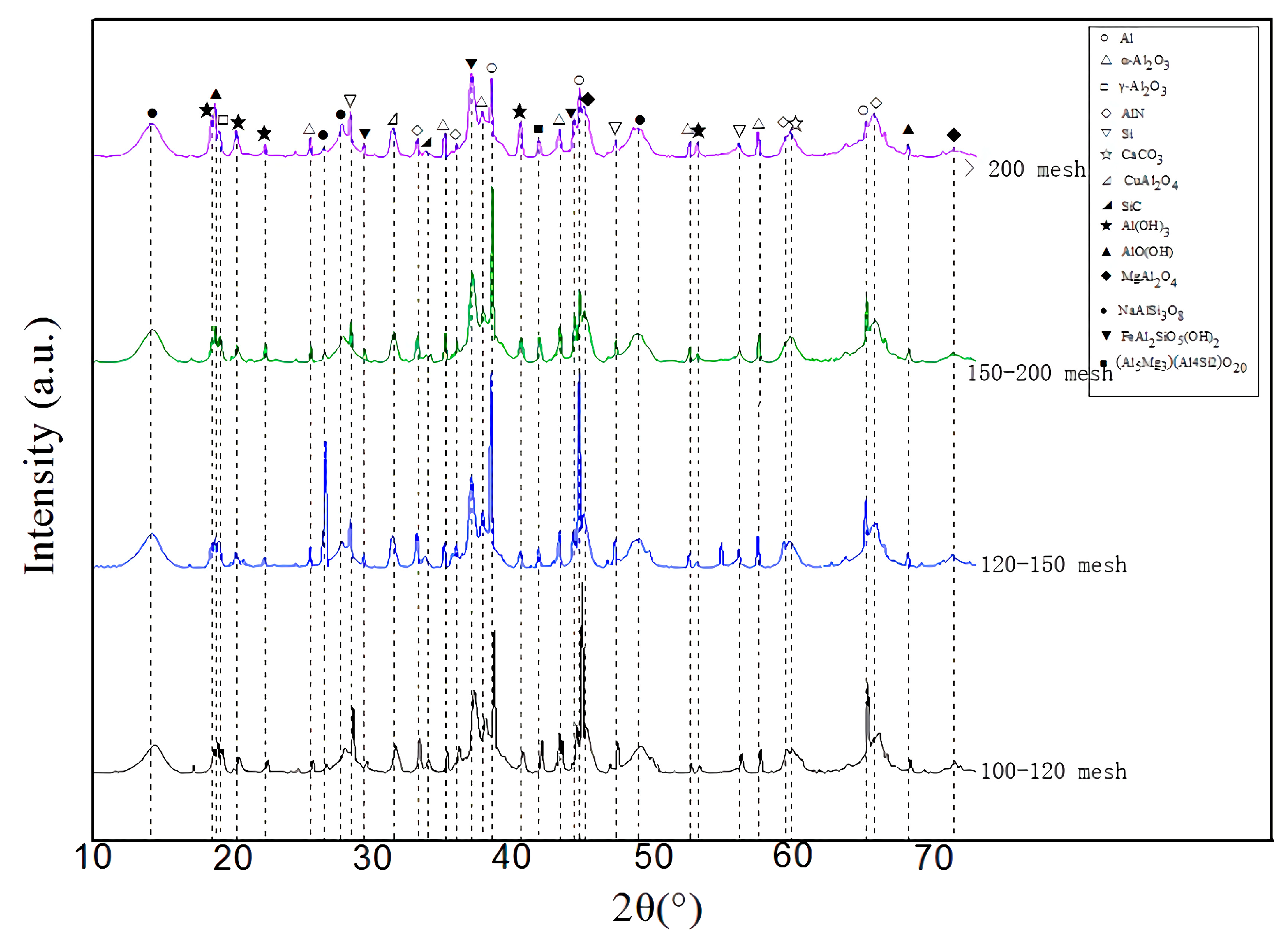
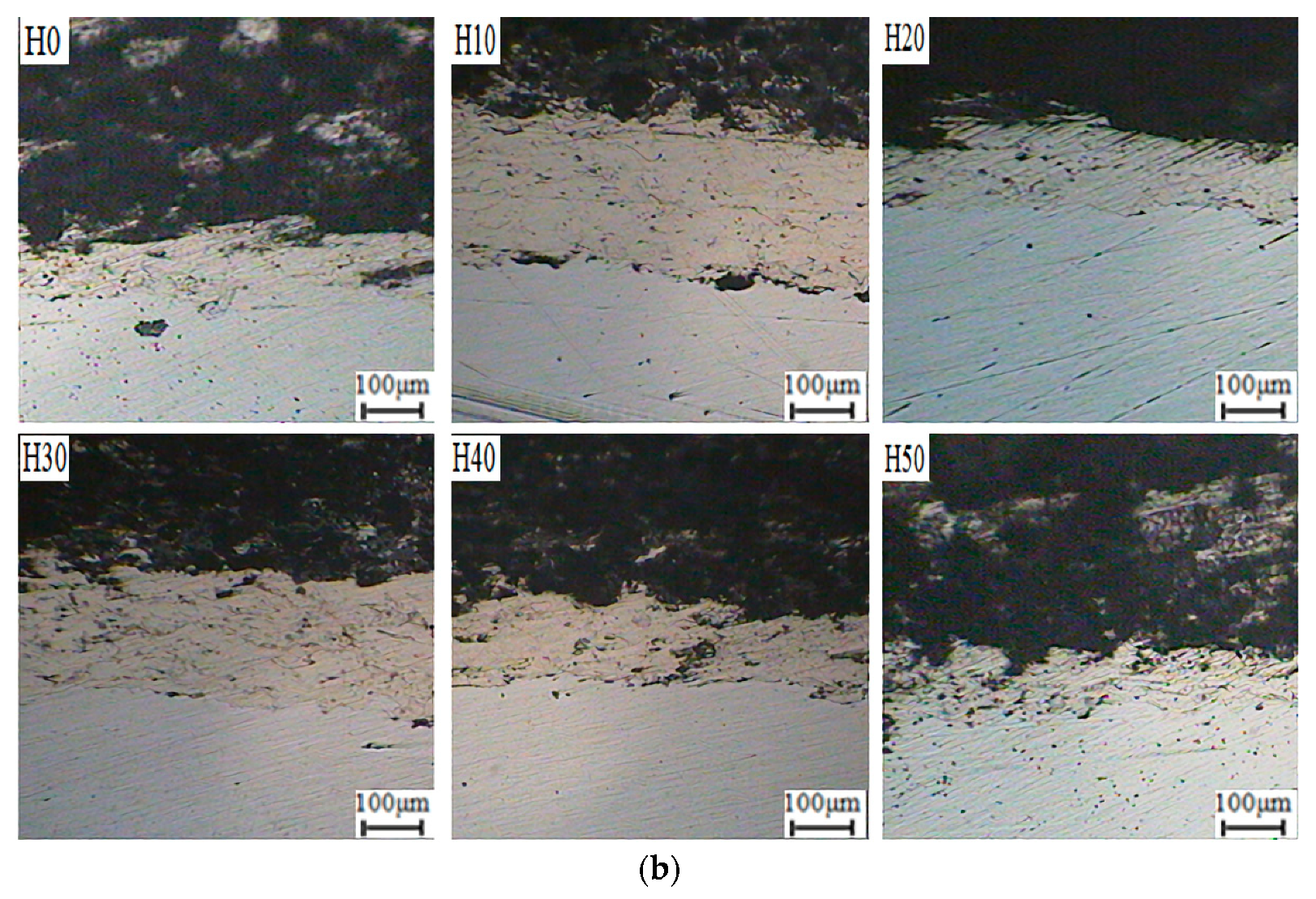
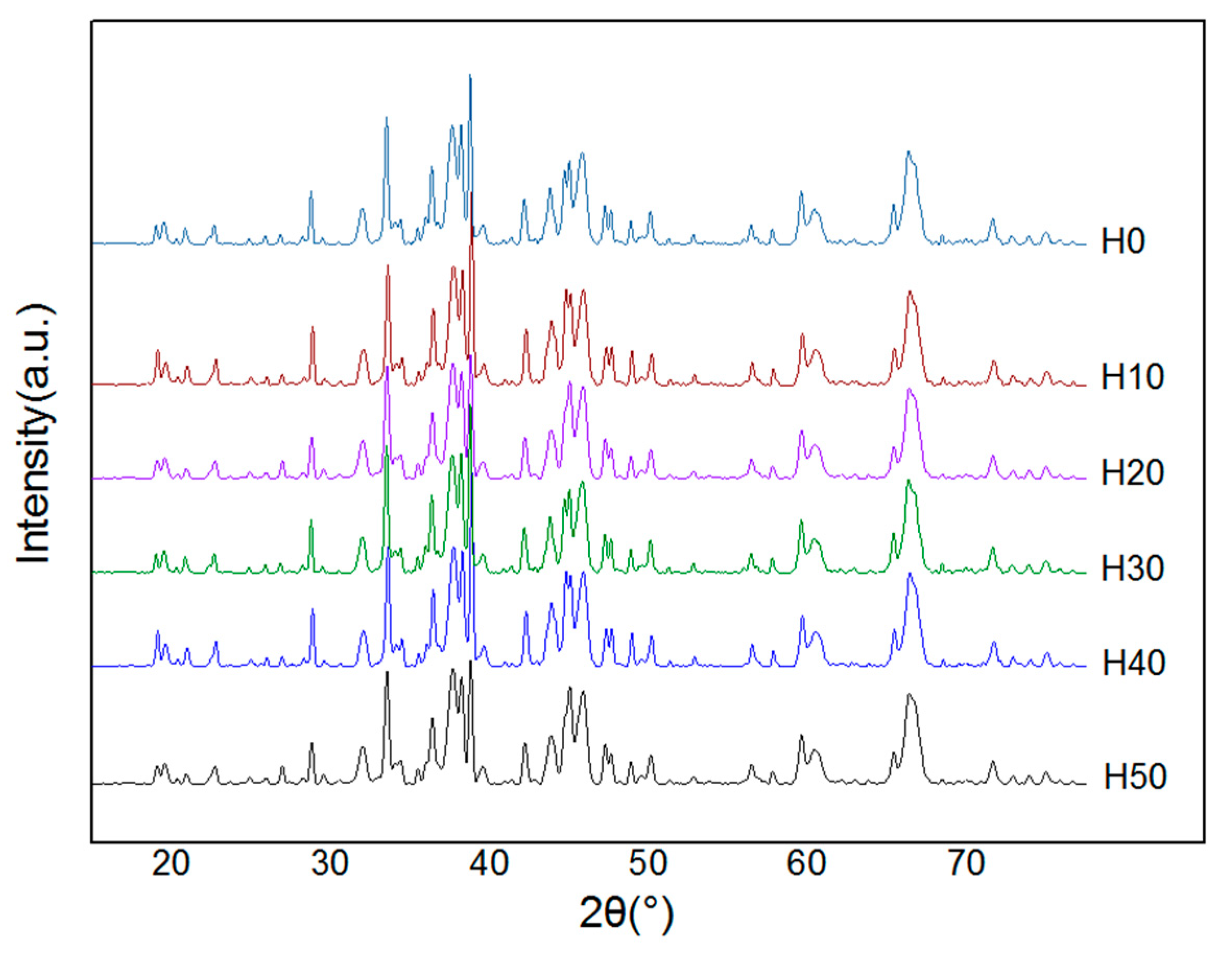
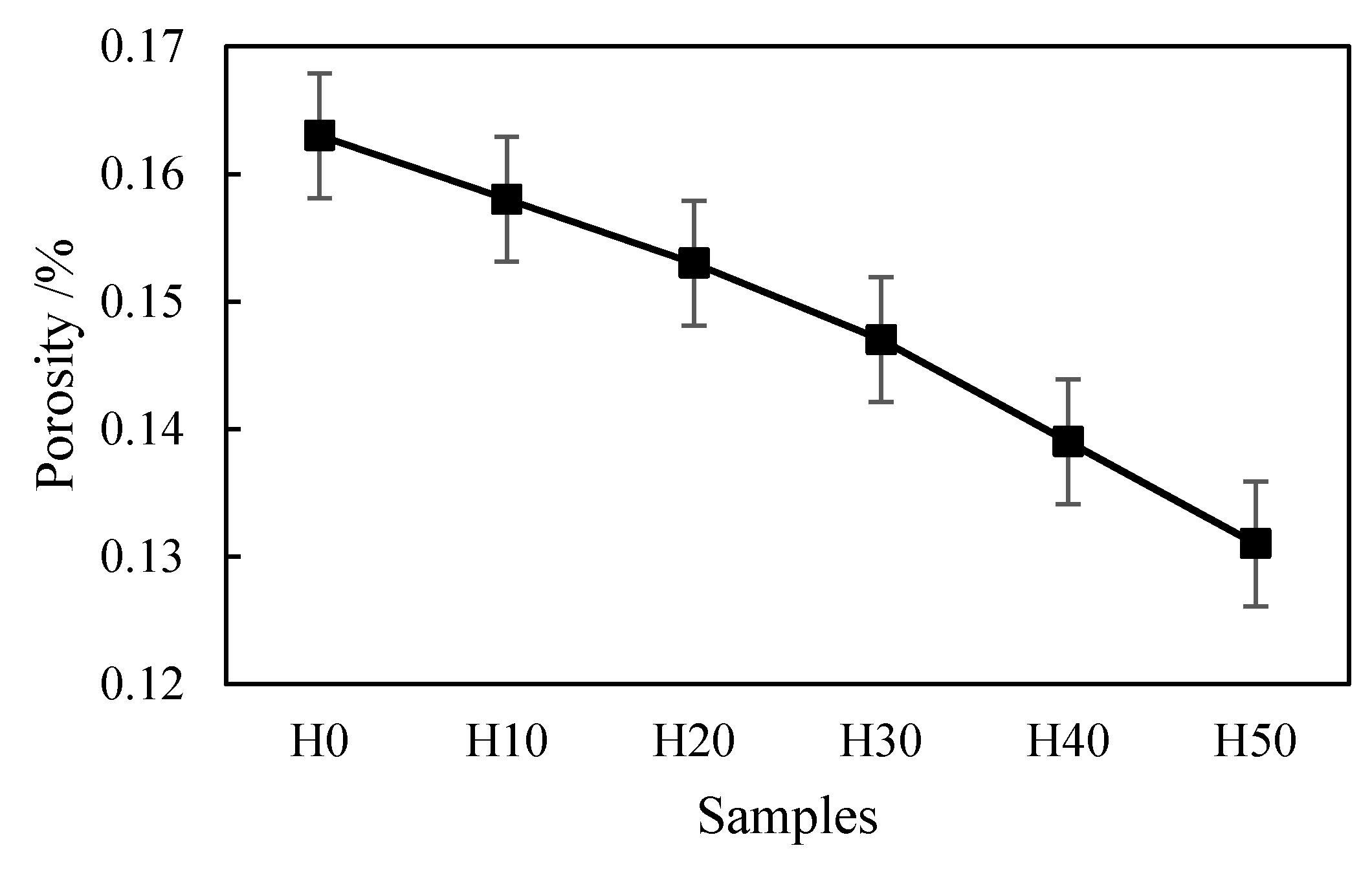
2.2.1. Experiment Equipment
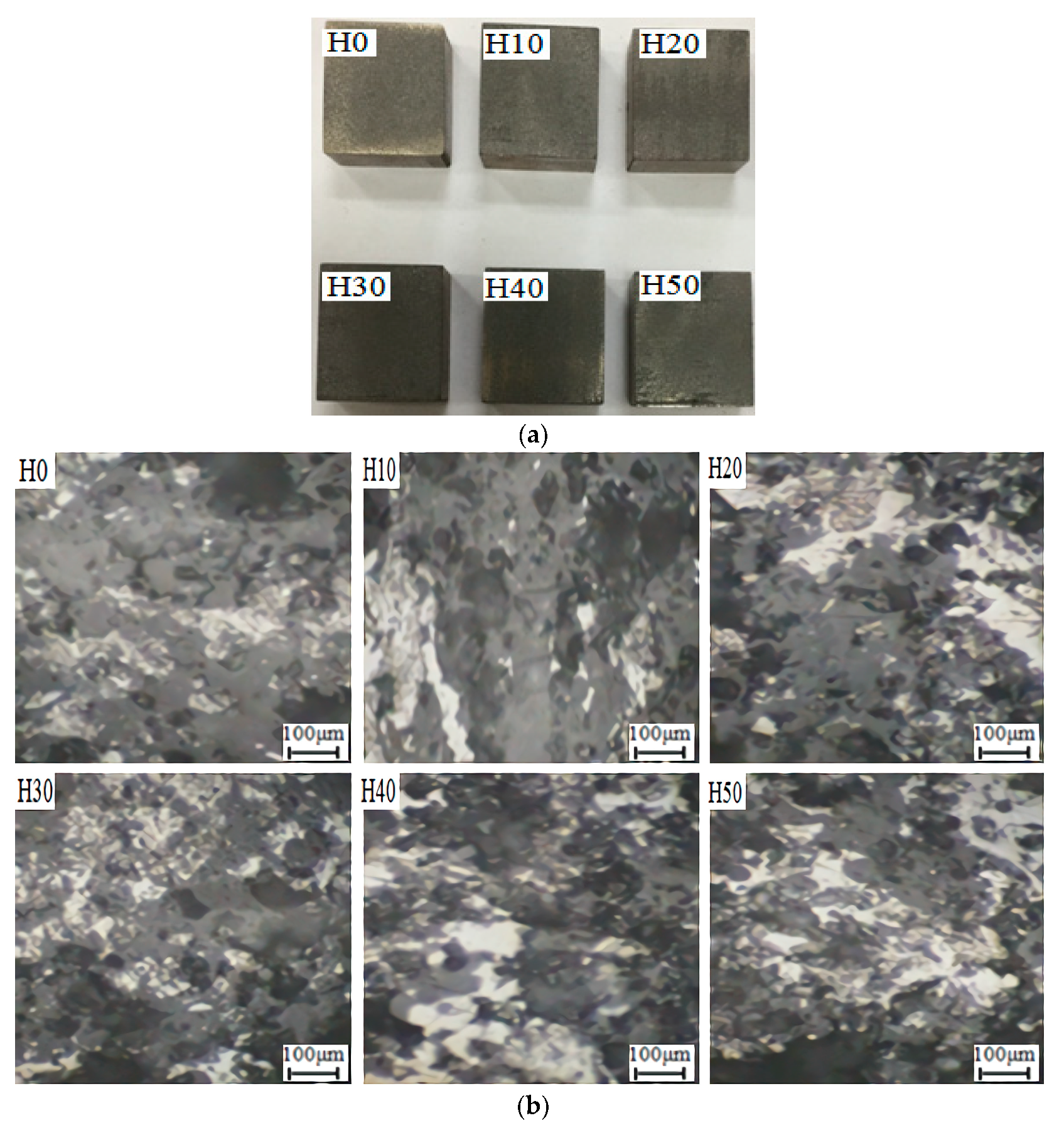
An atmospheric plasma spray equipment was used to prepare coatings on the surface of 45 steel substrates. The type of the spray equipment is FH-80, produced by Fahan Spraying Machinery Co., Ltd. (Shanghai, China). Scanning electron microscope (SEM, the S-3400 type, produced by Hitachi, Ltd., Tokyo, Japan) was used to observe the micro morphology of OAASP with different particle sizes. The surface and cross-section of the samples were polished. Then, the coating surface and cross-section morphology were observed with a metallurgical microscope (BX12C type, Shanghai Shuangxu Electronics Co., Ltd., Shanghai, China).
The X-ray diffraction (XRD, D/Max 2500PC Rigaku, Japan Science and Technology Co., Ltd., Tokyo, Japan) was used to analyze the phase and chemical composition of the coating. The Kα rays of Cu were selected, the tube voltage was 40 kV, and the tube current was 100 mA. The θ~2θ step scanning method was adopted, the step length was 0.02° (2θ), and the scanning speed was 2°~4°/min.
The Energy Dispersive Spectrometer (EDS, the Quantax75 type, produced by Japan Hitachi, Ltd., Hitachi, Japan) was used to analyze the types and contents of elements in different brightness regions of the coating. Each element has its own characteristic X-ray wavelength, and the size of the characteristic wavelength depends on the characteristic energy released during the energy level transition. The EDS used the characteristic energy of different X-ray photons of the element to perform component analysis.
2.2.2. Testing Methods
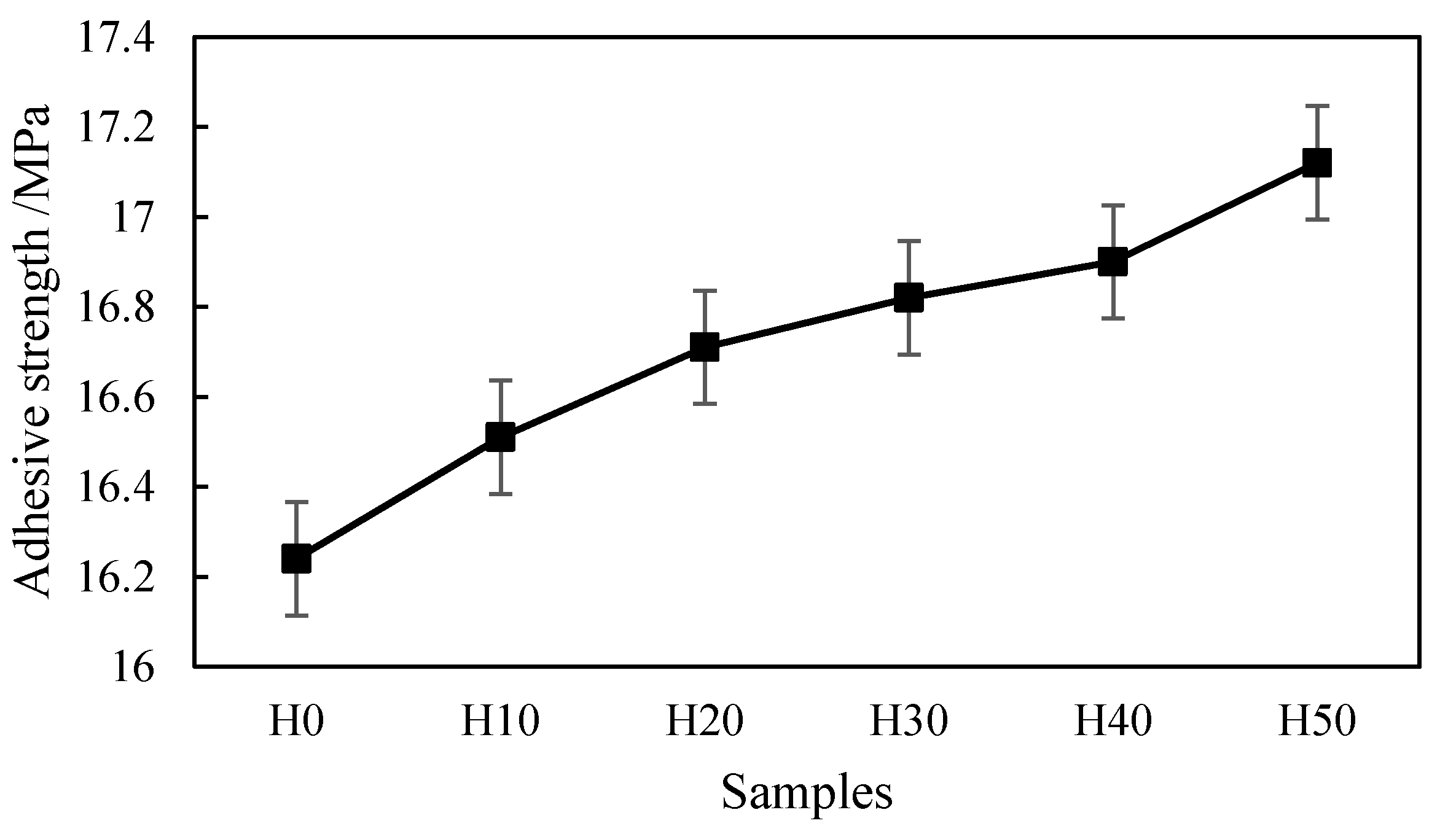
The performance of the coating was evaluated by porosity, adhesive strength, microhardness, and abrasion rate, and the angle of repose was used to evaluate the flowability of the powder.
Thermal spray coating is a layered structure formed by the stacking of molten particles, and there must be pores between different droplets. For applications with high temperature resistance and wear resistance, the fewer pores in the coating, the better the performance. The quantitative index of pores is porosity. In our experiment, the porosity was measured by Archimedes drainage method, and the calculation formula was (m2 − m0)/m1. Where m0 refers to the weight of the dried coating. Then, the dry coating was put into distilled water to obtain the mass of the discharged water m1. Furthermore, the test piece was removed from the water and weighed to obtain the mass m2. Finally, the porosity of the coating could be obtained by the above formula.
Adhesive strength is an important quantitative index for evaluating the quality of plasma sprayed coatings, and it reflects the mechanical combine ability between the bonding layer and the OAAC. With reference to the Chinese national standard of GB/T 8642-2002 “Thermal Spraying-Determination of Tensile Adhesive Strength” [
28], the tensile method was used to measure the adhesive strength. The used equipment was a universal mechanical testing machine, CMT5105 type, produced by MTS Industrial Systems Co., Ltd. (Shenzhen, China). The thickness of the coating sample used to measure the adhesive strength was about 0.5 mm, and the roughness was ≤3.2 μm after being polished. After the surface of the coating was cleaned and dried, the coating and the counterpart were glued through the epoxy resin glue. Then, the prepared sample was placed in an oven at 100~120 °C to dry. When conducting the tensile test, the tensile speed was less than 160 N/s. The formula for calculating the adhesive strength of the coating was σ
b =
F/
A. In the formula, σ
b is the adhesive strength of the coating, N/mm
2;
F is the maximum load of the sample to break, N; and
A is the area of the coating sample, mm
2.
Wear resistance is an important indicator to measure the quality of the coating. The better the wear resistance, the longer the service life of the coating. The abrasion rate was measured by a ring three-body wear tester, MMH-5 type, produced by Hansen Precision Instrument Co., Ltd. (Jinan, China). First, the sample was clamped on the mold. Then, we added a gravity block to the mold to adjust the pressure. The weight of the block could press the sample on the sandpaper to ensure that the coating was in contact with the sandpaper and the pressure was constant. After the power was turned on, the mold rotated, and the sample slid with the sandpaper, causing friction and wear. In addition, 80 mesh alumina sandpaper was selected as the sandpaper. Then, the sample was cleaned and dried. The FA-2004N electronic balance (produced by Grand Instrument and Equipment Co., Ltd., Shanghai, China) was used for weight measurement, and the average weight loss was calculated by multiple measurements. If the weight of the sample before abrasion is m1 and the weight after abrasion is m2, the calculation formula of the abrasion rate can be obtained by (m1 − m2)/t, where t is the abrasion time.
According to Chinese national standard GB 4342-84 [
29], the Vickers hardness was used to measure the microhardness of the coating. The TMV-1 digital micro Vickers hardness tester (produced by Time Group Inc., Beijing, China) was used to measure the microhardness of the coating section. The principle was to calculate the Vickers hardness according to the diagonal length of the indentation, through the formula
HV = 1.8544 ×
F/(
d2). In the formula,
HV is the Vickers microhardness of the coating, MPa;
F is the force, N; and
d is the diagonal length of the indentation, mm. Then 10 relatively smooth areas selected on the surface of the coating were tested, and the results were averaged.
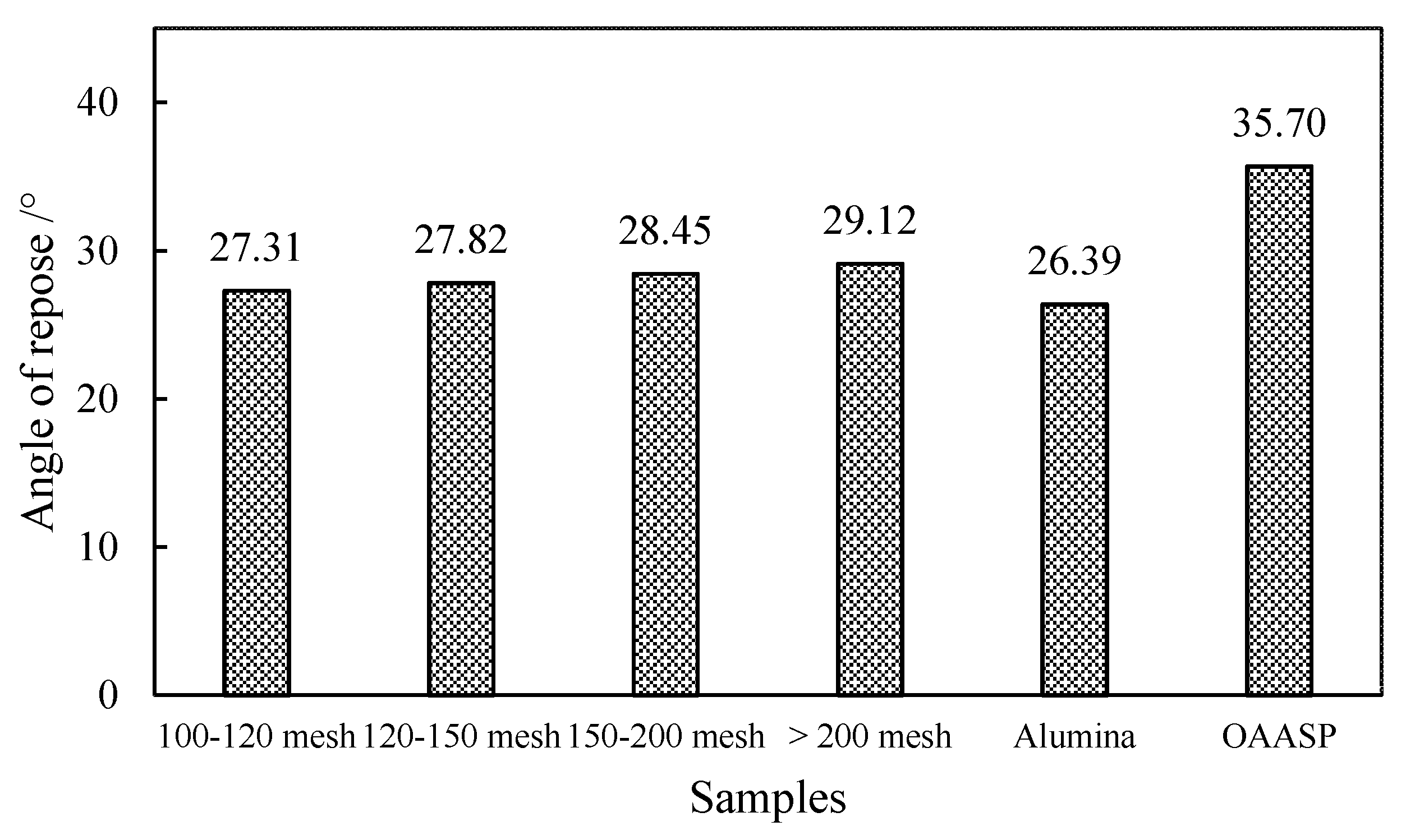
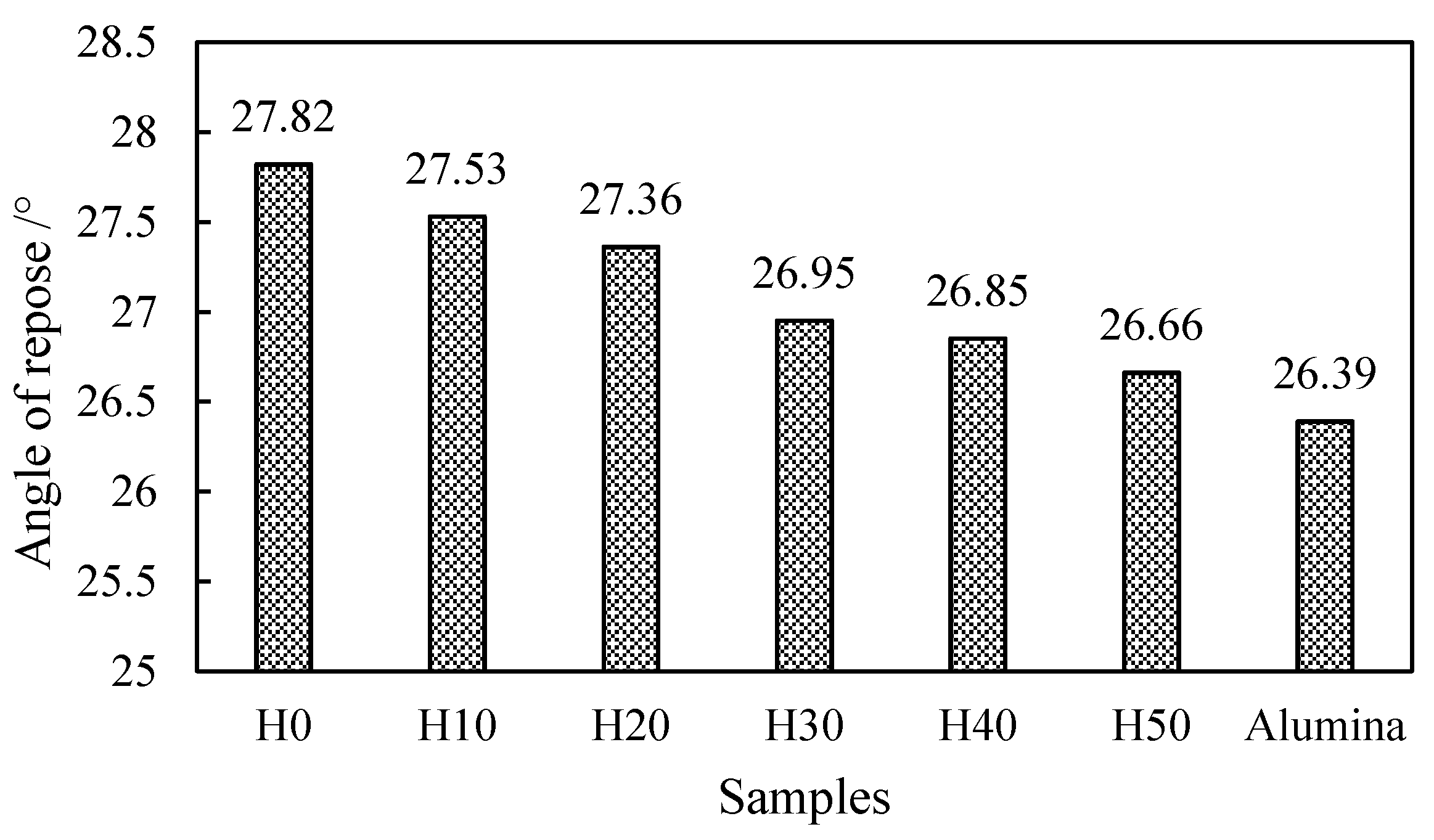
The angle of repose is the maximum angle formed by the free slope of the powder accumulation layer and the horizontal plane. The angle of repose can not only be measured directly but also can be obtained by calculation. The calculation formula was tan θ = h/r. In the formula, θ is the angle of repose, °; h is the height of the powder layer, mm; and r is the radius of the circle, mm. The smaller the angle of repose, the smaller the friction, and the better the flowability of the powder. It is generally believed that θ ≤ 40° can meet the needs of production flowability.