Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment
Abstract
1. Introduction
2. Experimental Methodology
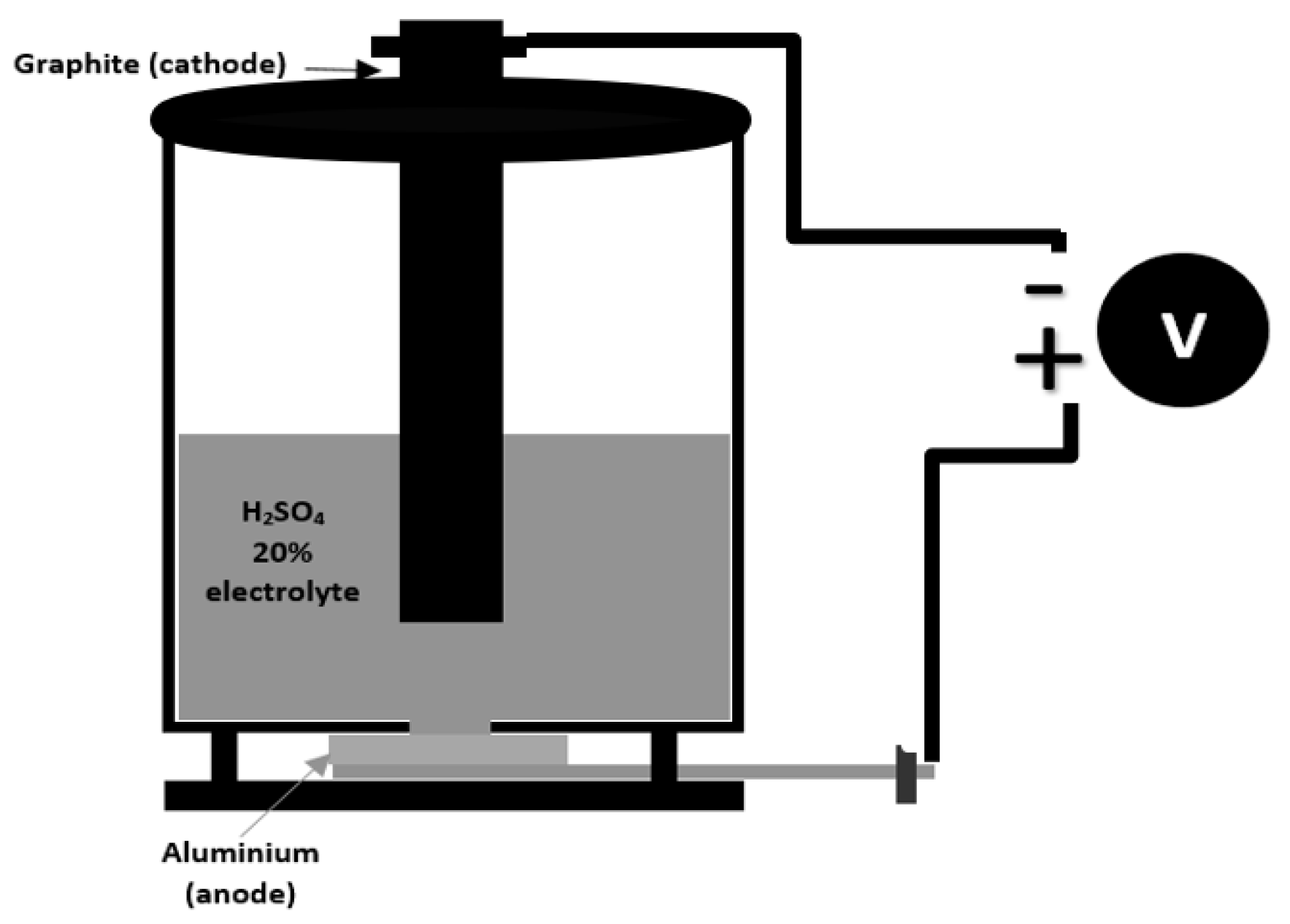
2.1. Preparation of the Anodizing Electrolyte Solution and Description of the Anodizing Procedure
2.2. Preparation of the Polymeric Polyelectrolyte Solution
- Q-2: Which involves homogenizing of solution Q-1 for 30 s, which was carried out in a Scilogex homogenizer.
- Q-3: Which involves filtration of solution Q-1 to eliminate the gel particles formed during agitation.
2.3. Polymeric Coating Electrodeposition Procedure
2.4. Scanning Electron Microscopy (SEM) Coating Characterization
2.5. Potentiodynamic Polarization Resistance Tests
3. Results
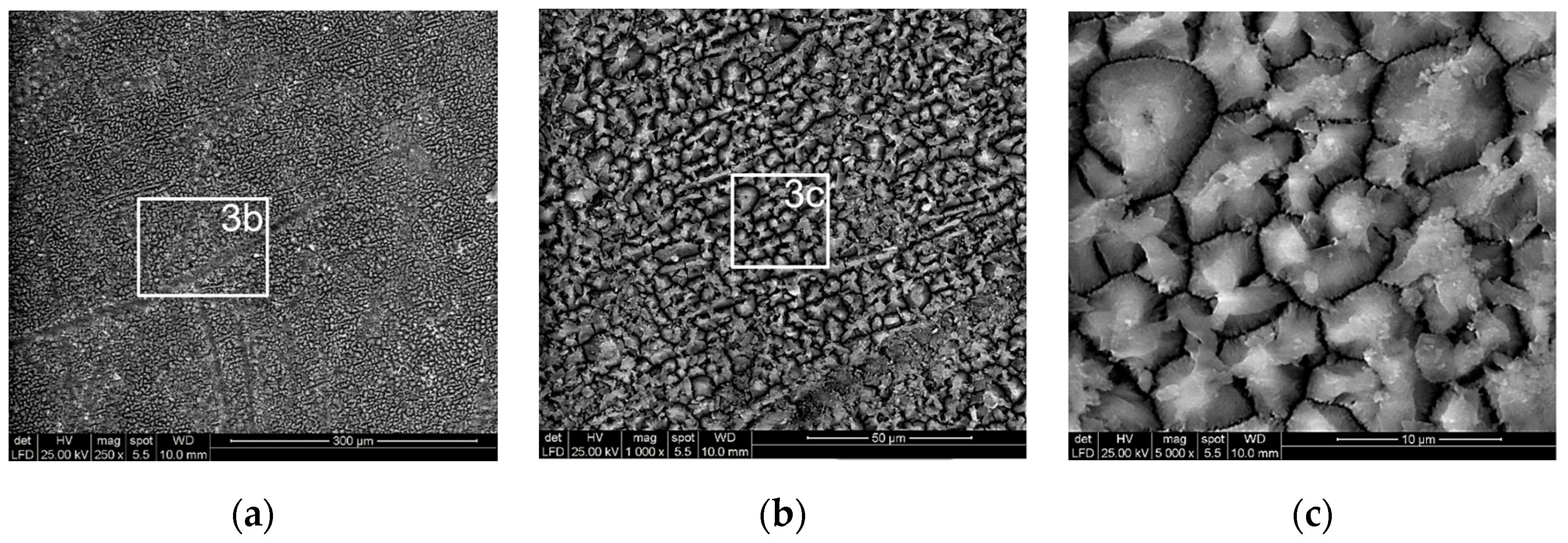
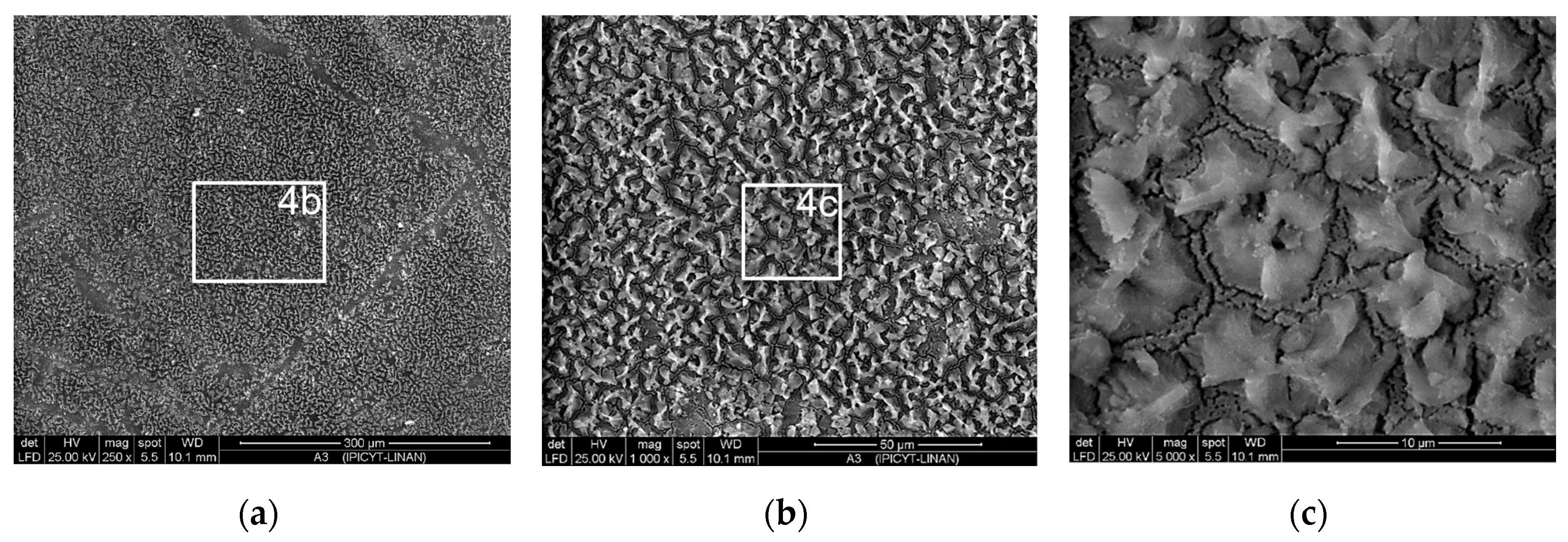
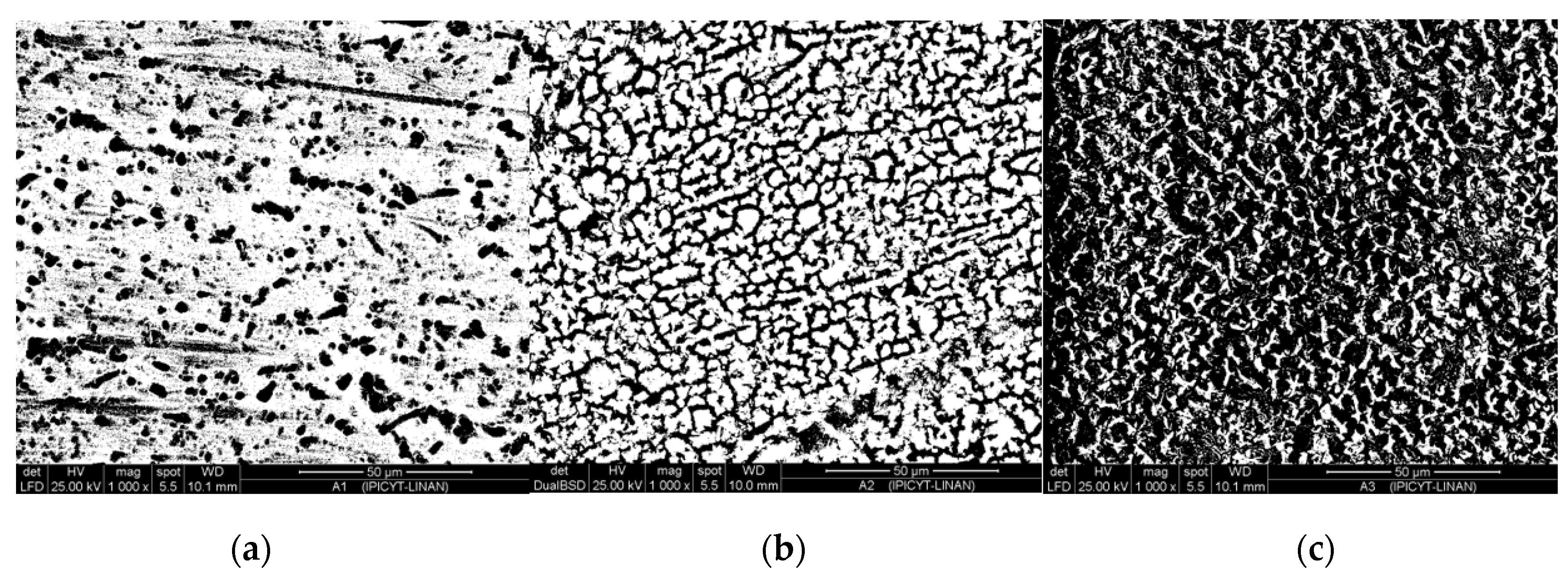
3.1. Microstructural Characterization of Inorganic-Organic Composite Coatings
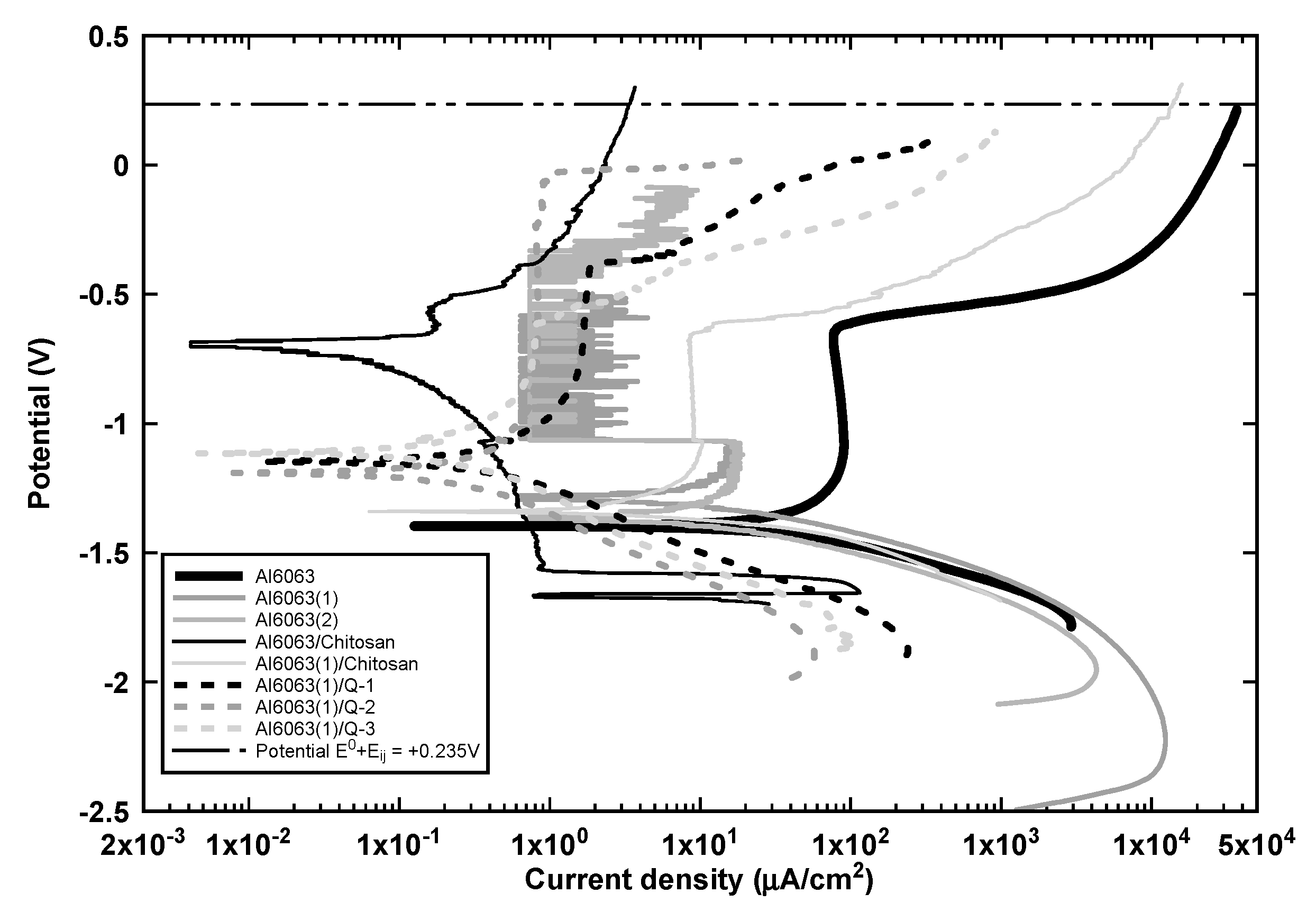
3.2. Corrosion Performance of Anodized and Coated Samples
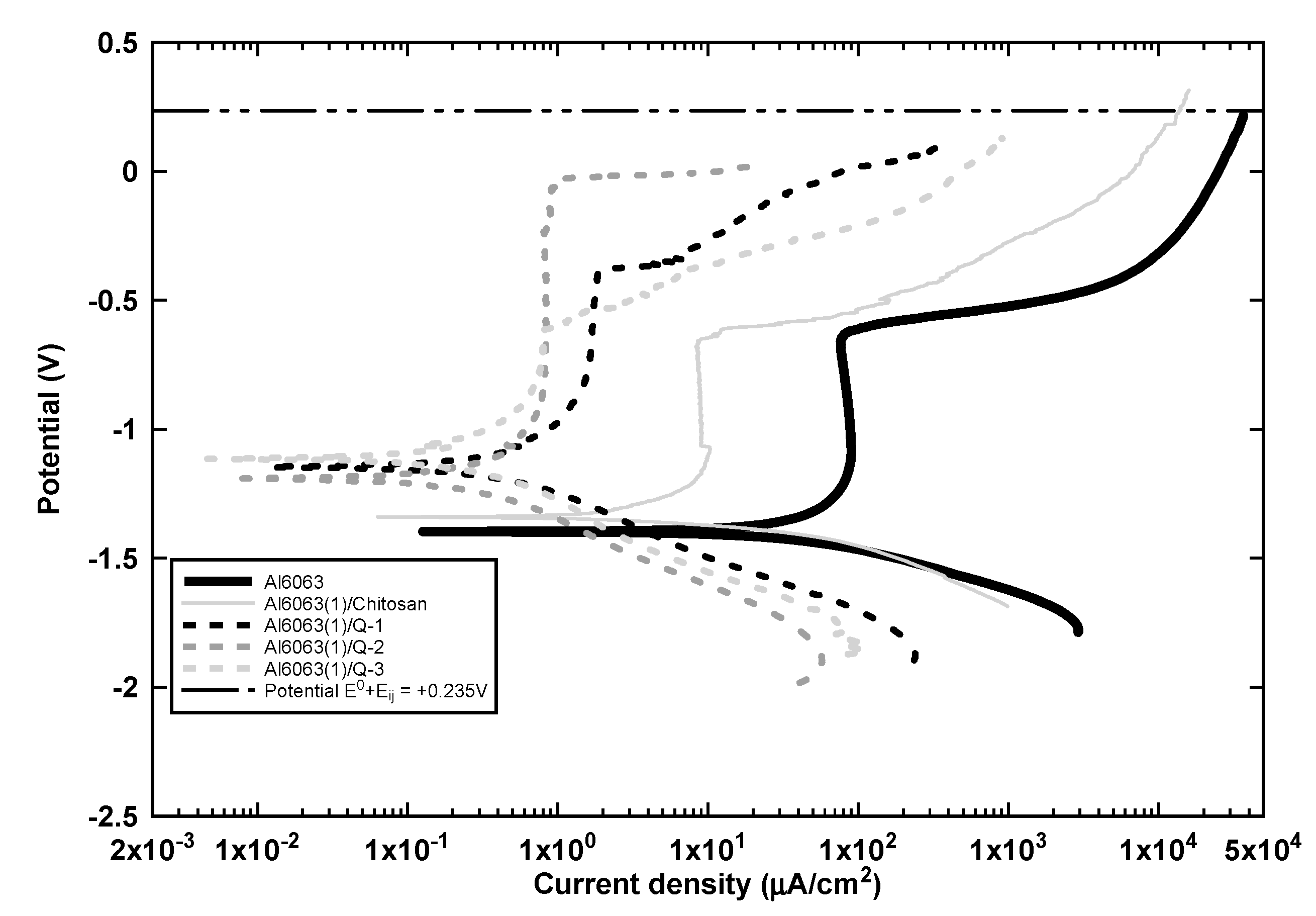
3.3. Corrosion Protection Efficiency of the Inorganic-Organic Composite Coating
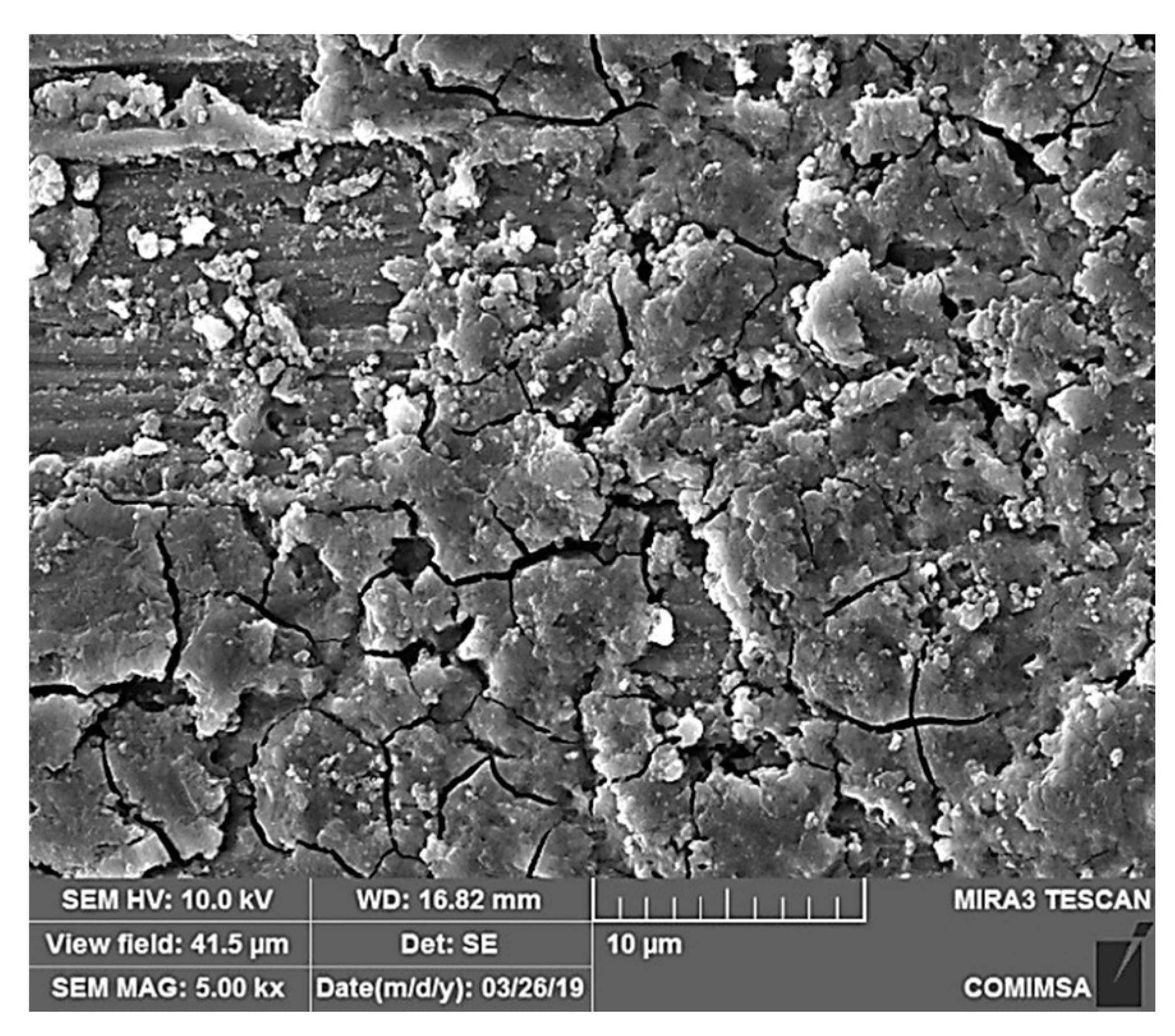
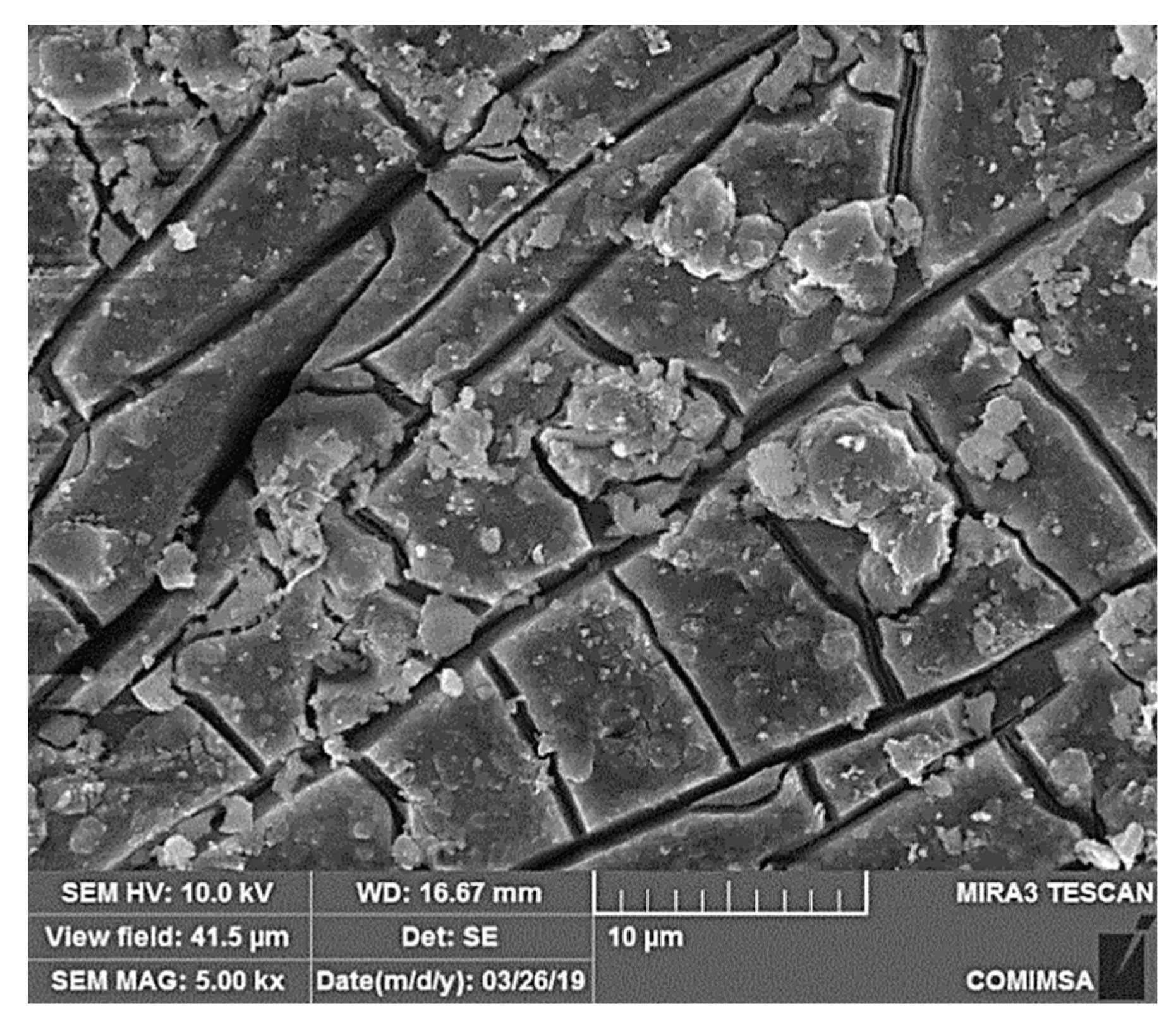
3.4. Corroded Surface Characterization after 30 Days Immersion in 3% NaCl
3.5. Corrosion Mechanism Insights
4. Conclusions
- The chitosan-coated sample exhibited the maximum polarization resistance; however, its corrosion mechanism was a continuous dissolution without a noticeable passivation behavior as shown in Figure 8 and described in Section 3.2.
- The anodized samples exhibited an almost negligible corrosion resistance improvement with an unstable passivation behavior superior to the uncoated sample as can be seen in Figure 8 at a current density range between 0.01 and 0.01 μA/cm2. Furthermore, the addition of the chitosan film stabilized the passivation behavior of the anodized samples because of the sealing of the alumina pores eliminating the chaotic behavior of anodized samples
- Finally, the anodized and chitosan-alginate coated samples exhibited a corrosion protection efficiency above 99% and increased the pitting passivation potential up to 0.0 V as depicted in Figure 9. The chitosan-alginate coated samples exhibited a passivation behavior in contrast to the chitosan-coated sample, which may be due to the blending of the two polymers that results in the spontaneous formation of polyion complex (PIC) because of the occurrence of ionic crosslinking as explained is Section 3.5.
- The main corrosion mechanism proposed is that the protonated carboxylates and amino groups are deposited on the cathodic or anodic sites, respectively during the deposition and potentiodynamic polarization tests because of the Al3+ ions that enhances the cathodic activity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Starke, E.A.; Staley, J.T. Application of modern aluminum alloys to aircraft. Prog. Aerosp. Sci. 1996, 32, 131–172. [Google Scholar] [CrossRef]
- Polmear, I.J. Light Alloy; Butterworth-Heinemann: Oxford, UK, 2017; pp. 299–365. [Google Scholar] [CrossRef]
- Davis, J.R. Aluminum and Aluminum Alloys. Light Met. Alloy. 2001, 66. [Google Scholar] [CrossRef]
- Suresh, S.; Vasudévan, A.K.; Bretz, P.E. Mechanisms of Slow Fatigue Crack Growth in High Strength Aluminum Alloys: Role of Microstructure and Environment. Metall. Trans. A 1984, 15, 369–379. [Google Scholar] [CrossRef]
- JScully, R.; Gebert, A.; Payer, J.H. Corrosion and related mechanical properties of bulk metallic glasses. J. Mater. Res. 2007, 22, 302–313. [Google Scholar] [CrossRef]
- Li, J.F.; Birbilis, N.; Li, C.X.; Jia, Z.Q.; Cai, B.; Zheng, Z.Q. Influence of retrogression temperature and time on the mechanical properties and exfoliation corrosion behavior of aluminium alloy AA7150. Mater. Charact. 2009, 60, 1334–1341. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Assem, L.; Zhu, H. Chromium Toxicological Overview; Institute of Environment and Health, Cranfield University, Health Protection Agency: Bedfordshire, UK, 2013. [Google Scholar]
- McKeen, L.W. Substrates and Substrate Preparation. In Fluorinated Coatings Finish; Elseviar: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Cha, S.C.; Erdemir, A. (Eds.) Coating Technology for Vehicle Applications; Springer: Basel, Switzerland, 2015. [Google Scholar]
- Djokić, S.S. Electrodeposition and Surface Finishing; Springer: New York, NY, USA, 2014. [Google Scholar]
- Kendig, M.W.; Buchheit, R.G. Corrosion inhibition of aluminum and aluminum alloys by soluble chromates, chromate coatings, and chromate-free coatings. Corrosion 2003, 59, 379–400. [Google Scholar] [CrossRef]
- DeVito, S.C. Present and future regulatory trends of the United States environmental protection agency. Prog. Org. Coat. 1999, 35, 55–61. [Google Scholar] [CrossRef]
- Jianqi, H.; Hong, H.; Lieping, S.; Genghua, G. Journal of Oral and Maxillofacial, Undefined 2002. Comparison of Calcium Alginate Film with Collagen Membrane for Guided Bone Regeneration in Mandibular Defects in Rabbits, Elsevier. Available online: https://www.sciencedirect.com/science/article/pii/S0278239102006432 (accessed on 8 August 2018).
- Suzuki, Y.; Nishimura, Y.; Tanihara, M.; Suzuki, K.; Nakamura, T.; Shimizu, Y.; Yamawaki, Y.; Kakimaru, Y. Evaluation of a novel alginate gel dressing: Cytotoxicity to fibroblastsin vitro and foreign-body reaction in pig skinin vivo. J. Biomed. Mater. Res. 1998, 39, 317–322. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, Y.; Tanihara, M.; Ohnishi, K.; Hashimoto, T.; Endo, K.; Nishimura, Y. Reconstruction of rat peripheral nerve gap without sutures using freeze-dried alginate gel. J. Biomed. Mater. Res. 2000, 49, 528–533. [Google Scholar] [CrossRef]
- Shilpa, A.; Agrawal, S.S.; Ray, A.R. Controlled Delivery of Drugs from Alginate Matrix. J. Macromol. Sci. Part C Polym. Rev. 2003, 43, 187–221. [Google Scholar] [CrossRef]
- Jmiai, A.; El Ibrahimi, B.; Tara, A.; El Issami, S.; Jbara, O.; Bazzi, L. Alginate biopolymer as green corrosion inhibitor for copper in 1M hydrochloric acid: Experimental and theoretical approaches. J. Mol. Struct. 2018, 1157, 408–417. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Kumar, A.M. Sodium alginate: A promising biopolymer for corrosion protection of API X60 high strength carbon steel in saline medium. Carbohydr. Polym. 2017, 178, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.D.; Gite, V.V.; Mahulikar, P.P.; Thamke, V.R.; Kodam, K.M.; Kuwar, A.S. Renewable source based non-biodegradable polyurethane coatings from polyesteramide prepared in one-pot using oleic acid. J. Am. Oil Chem. Soc. 2014, 91, 1055–1063. [Google Scholar] [CrossRef]
- Qian, B.; Song, Z.; Hao, L.; Wang, W.; Kong, D. Self-Healing Epoxy Coatings Based on Nanocontainers for Corrosion Protection of Mild Steel. J. Electrochem. Soc. 2017, 164, C54–C60. [Google Scholar] [CrossRef]
- Heise, S.; Virtanen, S.; Boccaccini, A.R. Tackling Mg alloy corrosion by natural polymer coatings—A review. J. Biomed. Mater. Res. Part A 2016, 104, 2628–2641. [Google Scholar] [CrossRef]
- Koch, G.H.; Brongers, M.P.; Thompson, N.; Virmani, Y.P.; Payer, J. Corrosion Cost and Preventative Strategies in the United States; Federal Highway Administration Washington: Washington, DC, USA, 2002.
- Jones, A.S.; Rule, J.D.; Moore, J.S.; Sottos, N.R.; White, S.R. Life extension of self-healing polymers with rapidly growing fatigue cracks. J. R. Soc. Interface 2007, 4, 395–403. [Google Scholar] [CrossRef]
- Keller, M.W.; White, S.R.; Sottos, N.R. Torsion fatigue response of self-healing poly (dimethylsiloxane) elastomers. Polymer 2008, 49, 3136–3145. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite-chitosan coatings. Mater. Charact. 2007, 58, 339–348. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Gerard, P.D.; Bergin, P.; Chestnutt, B.; Marini, M.; Ramsey, V.; Elder, S.H.; Gilbert, J.A. Chitosan: Potential use as a bioactive coating for orthopaedic and craniofacial/dental implants. J. Biomater. Sci. Polym. Ed. 2003, 14, 423–438. [Google Scholar] [CrossRef]
- Rusu, V.M.; Ng, C.H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size-controlled hydroxyapatite nanoparticles as self-organized organic-inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Z.C.; Lin, C.J. Preparation and characterization of nano-sized hydroxyapatite particles and hydroxyapatite/chitosan nano-composite for use in biomedical materials. Mater. Lett. 2002, 57, 858–861. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Iizuka, S.; Osaka, A.; Monma, H.; Tanaka, J. The effect of citric acid addition on chitosan/hydroxyapatite composites. Coll. Surf. A Physicochem. Eng. Asp. 2003, 214, 111–118. [Google Scholar] [CrossRef]
- Cheong, M.; Zhitomirsky, I. Electrodeposition of alginic acid and composite films. Aspects 2008, 328, 73–78. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Ferreira, M.G.S. Chitosan as a smart coating for corrosion protection of aluminum alloy 2024: A review. Prog. Org. Coat. 2015, 89, 348–356. [Google Scholar] [CrossRef]
- HWG, H, The Anodic Oxidation of Aluminium and its Alloys. Nature 1941, 147, 659. [CrossRef]
- Wang, Z.; Zhang, X.; Gu, J.; Yang, H.; Nie, J.; Ma, G. Electrodeposition of alginate/chitosan layer-by-layer composite coatings on titanium substrates. Carbohydr. Polym. 2014, 103, 38–45. [Google Scholar] [CrossRef]
- DropSens, Electorchemical Software. Available online: https://www.metrohm.com/es/productos/potenciostatos/dropview-software-de-electroquimica/DropView8400 (accessed on 27 December 2019).
- Sun, Y.P.; Yang, C.T.; Yang, C.G.; Xu, D.K.; Li, Q.; Yin, L.; Qiu, C.S.; Liu, D.; Yang, K. Stern—Geary Constant for X80 Pipeline Steel in the Presence of Different Corrosive Microorganisms. Acta Metall. Sin. Lett. 2019, 32, 1483–1489. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization. J. Electrochem. Soc. 1957, 104, 559. [Google Scholar] [CrossRef]
- Redepenning, J.; Venkataraman, G.; Chen, J.; Stafford, N. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates. J. Biomed. Mater. Res. A 2003, 66, 411–416. [Google Scholar] [CrossRef]
- Suay, J.J.; Giménez, E.; Rodríguez, T.; Habbib, K.; Saura, J.J. Characterization of anodized and sealed aluminium by EIS. Corros. Sci. 2003, 45, 611–624. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan–sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F. The Mechanism of Hydrogen Evolution During Anodic Polarization of Aluminium. Electrochim. Acta 2015, 180, 712–721. [Google Scholar] [CrossRef]
- Yıldız, R.; Doğan, T.; Science, I.D.-C. Evaluation of Corrosion Inhibition of Mild Steel in 0.1 M HCl by 4-amino-3-hydroxynaphthalene-1-sulphonic acid, Elsevier. Available online: https://www.sciencedirect.com/science/article/pii/S0010938X14001887 (accessed on 9 October 2018).
- El-Haddad, M.N.; Fouda, A.S. Inhibition Effect and Adsorption Behavior of New Azodye Derivatives on Corrosion of Carbon Steel in Acid Medium. J. Dispers. Sci. Technol. 2013, 34, 1471–1480. [Google Scholar] [CrossRef]
- Umoren, S.; Obot, I.; Polymers, A.M.-C. Performance Evaluation of Pectin as Ecofriendly Corrosion Inhibitor for X60 Pipeline Steel in Acid Medium: Experimental and Theoretical Approaches, Elsevier. Available online: https://www.sciencedirect.com/science/article/pii/S0144861715001502 (accessed on 9 October 2018).
- Fayomi, O.S.I.; Abdulwahab, M.; Popoola, A.P.I.; Asuke, F. Corrosion resistance of AA6063-Type Al-Mg-Si alloy by silicon carbide in sodium chloride solution for marine application. J. Mar. Sci. Appl. 2015, 14, 459–462. [Google Scholar] [CrossRef]
- Halambek, J.; Bubalo, M.C.; Redovnikovic, I.R.; Berkovic, K. Corrosion behaviour of aluminium and AA5754 alloy in 1% acetic acid solution in presence of laurel oil. Int. J. Electrochem. Sci. 2014, 9, 5496–5506. [Google Scholar]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar] [CrossRef][Green Version]
- Macías, A. Comparison of different electrochemical techniques for corrosion-rate determination of zinc-coated reinforcements in simulated concrete pore solutions. Mater. Struct. 1991, 24, 456–465. [Google Scholar] [CrossRef]
- Skold, R.V.; Larson, T.E. Measurement of the Instantaneous Corrosion Rate By Means of Polarization Data. Corrosion 1957, 13, 69–72. [Google Scholar] [CrossRef]
- Roberge, P.R. Principles and Practice. In Corrosion Engineering; Mcgraw-hill: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Magalhães, A.A.; Margarit, I.C.; Mattos, O. Electrochemical characterization of chromate coatings on galvanized steel. Electrochim. Acta 1999, 44, 4281–4287. [Google Scholar] [CrossRef]
- Zakaulla, M.; Khan, A.R.A.; Mukunda, P.G. Effect of Electroless Copper Coating on the Corrosion Behavior of Aluminium Based Metal Matrix Composites Reinforced with Silicon Carbide Particles. J. Miner. Mater. Charact. Eng. 2014, 2, 21–25. [Google Scholar] [CrossRef]
- Mandel, M.; Krüger, L. Long-term Corrosion Studies of a CFRP/EN AW-6060-T6 Self-piercing rivet Joint and a Steel/EN AW-6060-T6 Blind Rivet Joint. Mater. Today Proc. 2015, 2, 2959–2967. [Google Scholar] [CrossRef]
- Uragami, T.; Okazaki, K.; Matsugi, H.; Miyata, T. Structure and permeation characteristics of an aqueous ethanol solution of organic-Inorganic hybrid membranes composed of poly(vinyl alcohol) and tetraethoxysilane. Macromolecules 2002, 35, 9156–9163. [Google Scholar] [CrossRef]
- Uragami, T.; Katayama, T.; Miyata, T.; Tamura, H.; Shiraiwa, T.; Higuchi, A. Dehydration of an ethanol/water azeotrope by novel organic-Inorganic hybrid membranes based on quaternized chitosan and tetraethoxysilane. Biomacromolecules 2004, 5, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, M.G.M.; Huang, R.Y.M. Pervaporation dehydration of isopropanol with chitosan membranes. J. Membr. Sci. 1997, 124, 53–62. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Hasani-Sadrabadi, M.M.; Moaddel, H. Structural modification of chitosan biopolymer as a novel polyelectrolyte membrane for green power generation. Polym. Adv. Technol. 2010, 21, 726–734. [Google Scholar] [CrossRef]
- Uragami, T.; Takigawa, K. Permeation and separation characteristics of ethanol-water mixtures through chitosan derivative membranes by pervaporation and evapomeation. Polymer 1990, 31, 668–672. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Chen, G. Pervaporation characteristics and solution-diffusion behaviors through sodium alginate dense membrane. J. Appl. Polym. Sci. 1996, 61, 1387–1394. [Google Scholar] [CrossRef]
- Riccardo, R. Water-absorbent polymers: A patent survey. J. Macromol. Sci. Part C 1994, 34, 607–662. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behaviour of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Cárdenas, A.; Argüelles-Monal, W.; Goycoolea, F.M.; Higuera-Ciapara, I.; Peniche, C. Diffusion through Membranes of the Polyelectrolyte Complex of Chitosan and Alginate. Macromol. Biosci. 2003, 3, 535–539. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Mikhailov, G.P.; Tuchkov, S.V.; Lazarev, V.V.; Kulish, E.I. Complexation of chitosan with acetic acid according to Fourier transform Raman spectroscopy data. Russ. J. Phys. Chem. A 2014, 88, 936–941. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L.; Lawrie, G. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]


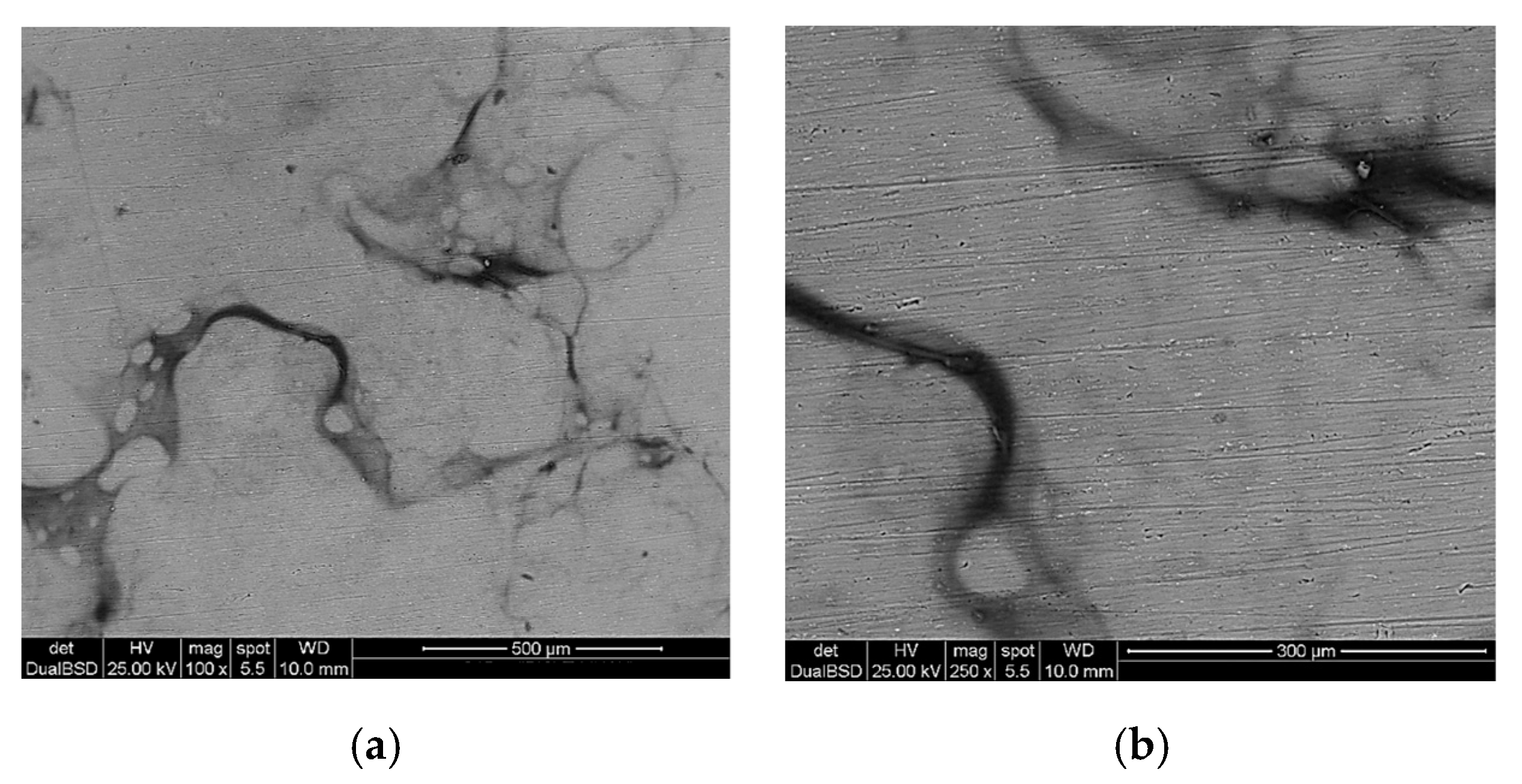




| Sample | Coating |
|---|---|
| Al-6063 | Uncoated |
| Al-6063(1) | Anodized |
| Al-6063/chitosan | Chitosan |
| Al-6063(1)/chitosan | Anodized and chitosan |
| Al-6063(1)/Q-1 | Anodized and chitosan-sodium alginate (using solution Q-1) |
| Al-6063(1)/Q-2 | Anodized and chitosan-sodium alginate (using solution Q-2) |
| Al-6063(1)/Q-3 | Anodized and chitosan-sodium alginate (using solution Q-3) |
| Sample | Current (A/cm2) |
|---|---|
| Al-AA6063 (1) | 0.1 |
| Al-AA6063 (2) | 0.2 |
| Samples | Ecorr (V) | Icorr (µA/cm2) | ba (V/dec) | bc (V/dec) | B | Rp (KOhm cm2) Eq. x | Rp (KOhm cm2) DropView | np (%) |
|---|---|---|---|---|---|---|---|---|
| Al-6063 | −1.397 | 22.36 | 0.11 | 0.45 | 38.38 | 1.717 | 1.693 | --- |
| Al-6063 (1) | −1.291 | 5.46 | 0.05 | 0.27 | 18.32 | 3.357 | 3.128 | 86.53 |
| Al-6063 (2) | −1.365 | 7.96 | 0.071 | 0.44 | 26.55 | 3.334 | 3.359 | 32.11 |
| Al-6063/chitosan | −0.698 | 0.08 | 0.30 | 0.42 | 75.99 | 971.339 | 978.454 | |
| Al-6063 (1)/chitosan | −1.34 | 2.09 | 0.08 | 0.37 | 28.56 | 13.697 | 13.132 | 81.83 |
| Al-6063 (1)/Q-1 | −1.145 | 0.23 | 0.22 | 0.33 | 57.32 | 253.546 | 254.557 | 99.01 |
| Al-6063 (1)/Q-2 | −1.192 | 0.18 | 0.22 | 0.39 | 61.08 | 334.804 | 336.806 | 98.95 |
| Al-6063 (1)/Q-3 | 1.114 | 0.30 | 0.30 | 0.90 | 97.70 | 332.308 | 335.675 | 98.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos-Melgar, A.; Serna, S.A.; Lázaro, I.; Gutiérrez-Castañeda, E.-J.; Mercado-Lemus, V.H.; Arcos-Gutierrez, H.; Hernández-Hernández, M.; Porcayo-Calderón, J.; Mayen, J.; Monroy, M.D.A. Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment. Coatings 2020, 10, 45. https://doi.org/10.3390/coatings10010045
Gallegos-Melgar A, Serna SA, Lázaro I, Gutiérrez-Castañeda E-J, Mercado-Lemus VH, Arcos-Gutierrez H, Hernández-Hernández M, Porcayo-Calderón J, Mayen J, Monroy MDA. Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment. Coatings. 2020; 10(1):45. https://doi.org/10.3390/coatings10010045
Chicago/Turabian StyleGallegos-Melgar, A., Sergio A. Serna, I. Lázaro, E.-J. Gutiérrez-Castañeda, V. H. Mercado-Lemus, H. Arcos-Gutierrez, M. Hernández-Hernández, J. Porcayo-Calderón, Jan Mayen, and M. Del Angel Monroy. 2020. "Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment" Coatings 10, no. 1: 45. https://doi.org/10.3390/coatings10010045
APA StyleGallegos-Melgar, A., Serna, S. A., Lázaro, I., Gutiérrez-Castañeda, E.-J., Mercado-Lemus, V. H., Arcos-Gutierrez, H., Hernández-Hernández, M., Porcayo-Calderón, J., Mayen, J., & Monroy, M. D. A. (2020). Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment. Coatings, 10(1), 45. https://doi.org/10.3390/coatings10010045








