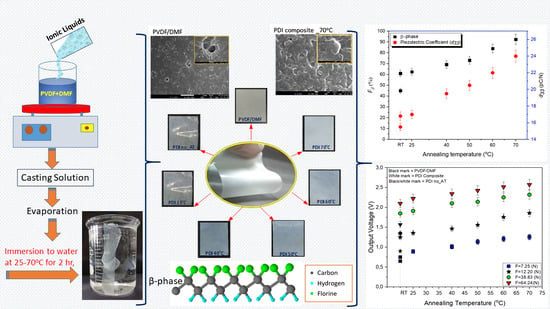
Using Annealing Treatment on Fabrication Ionic Liquid-Based PVDF Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
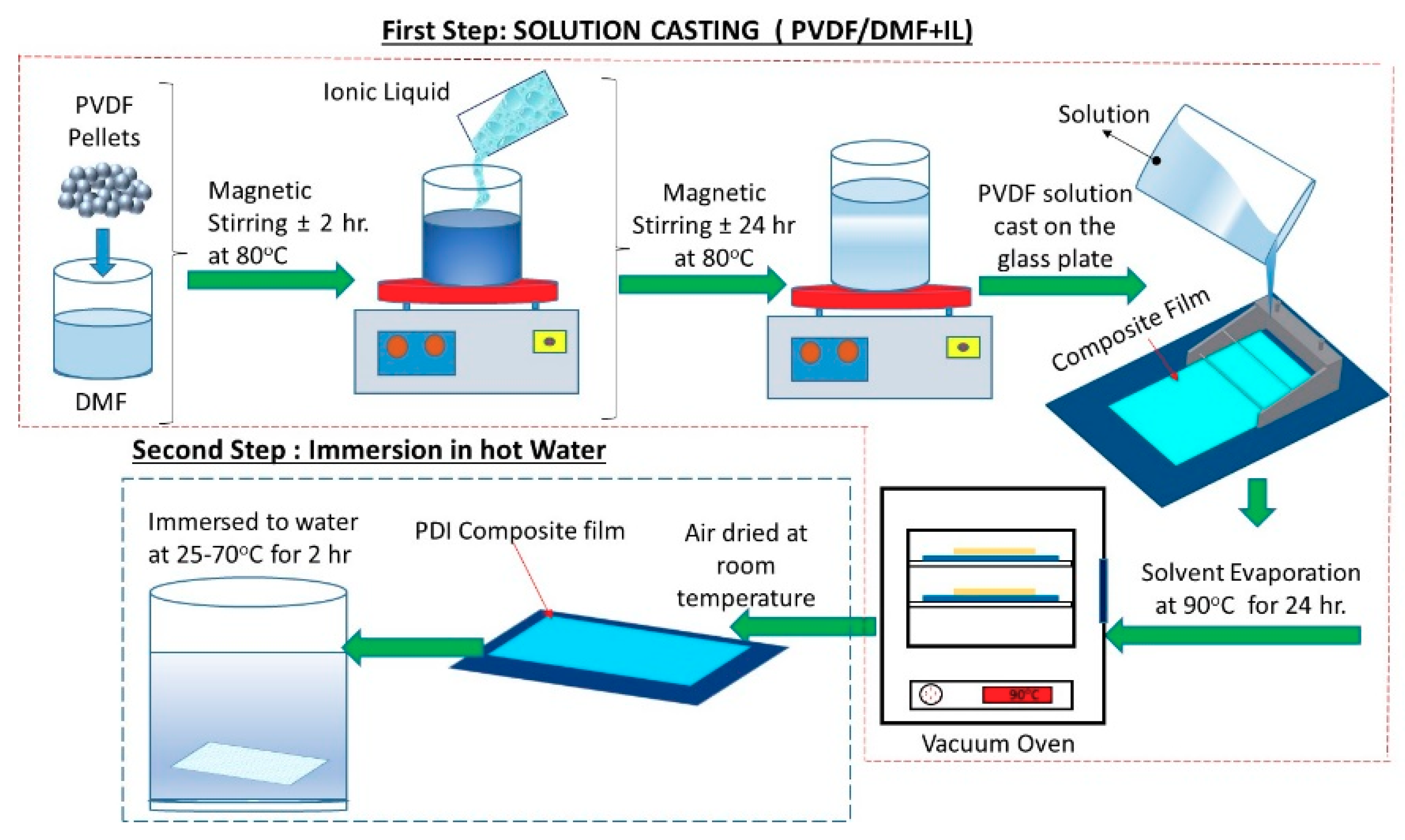
2.2. Fabrication of the PVDF Films Composite
2.3. Measurement and Characterization
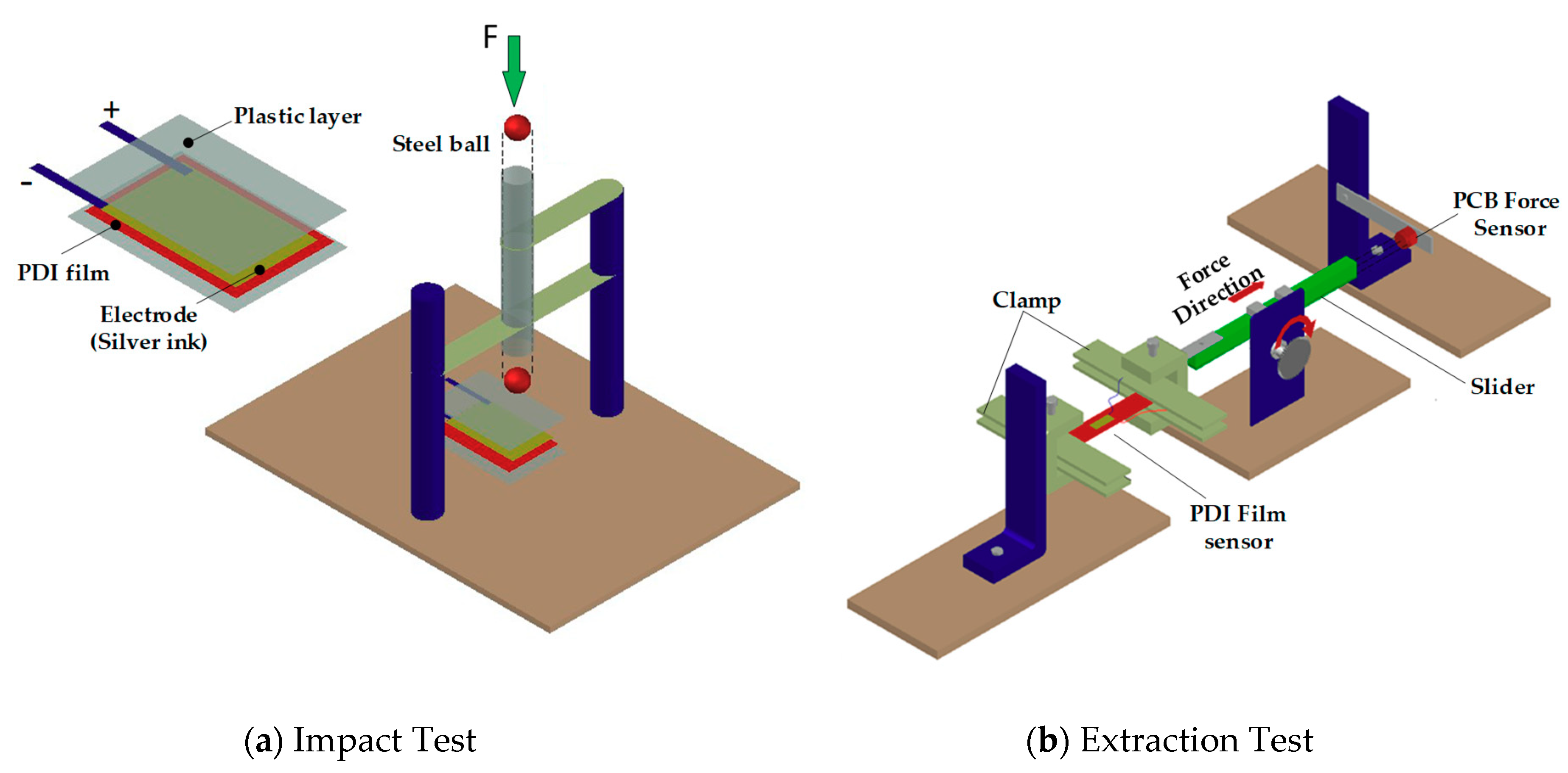
2.4. Measurement of the Piezolectricity
3. Results and Discussion
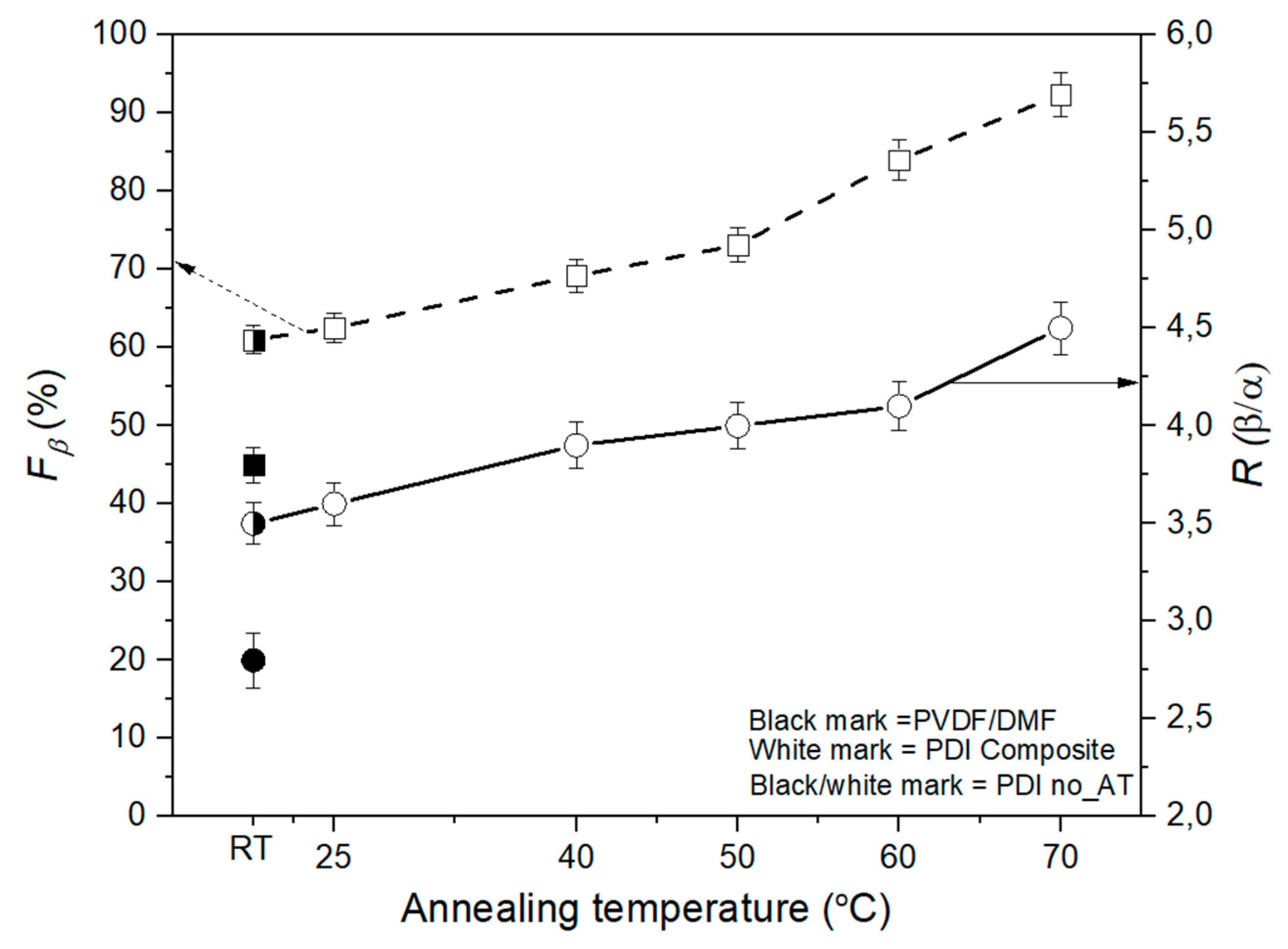
3.1. FTIR Analysis to β-Phase Fraction
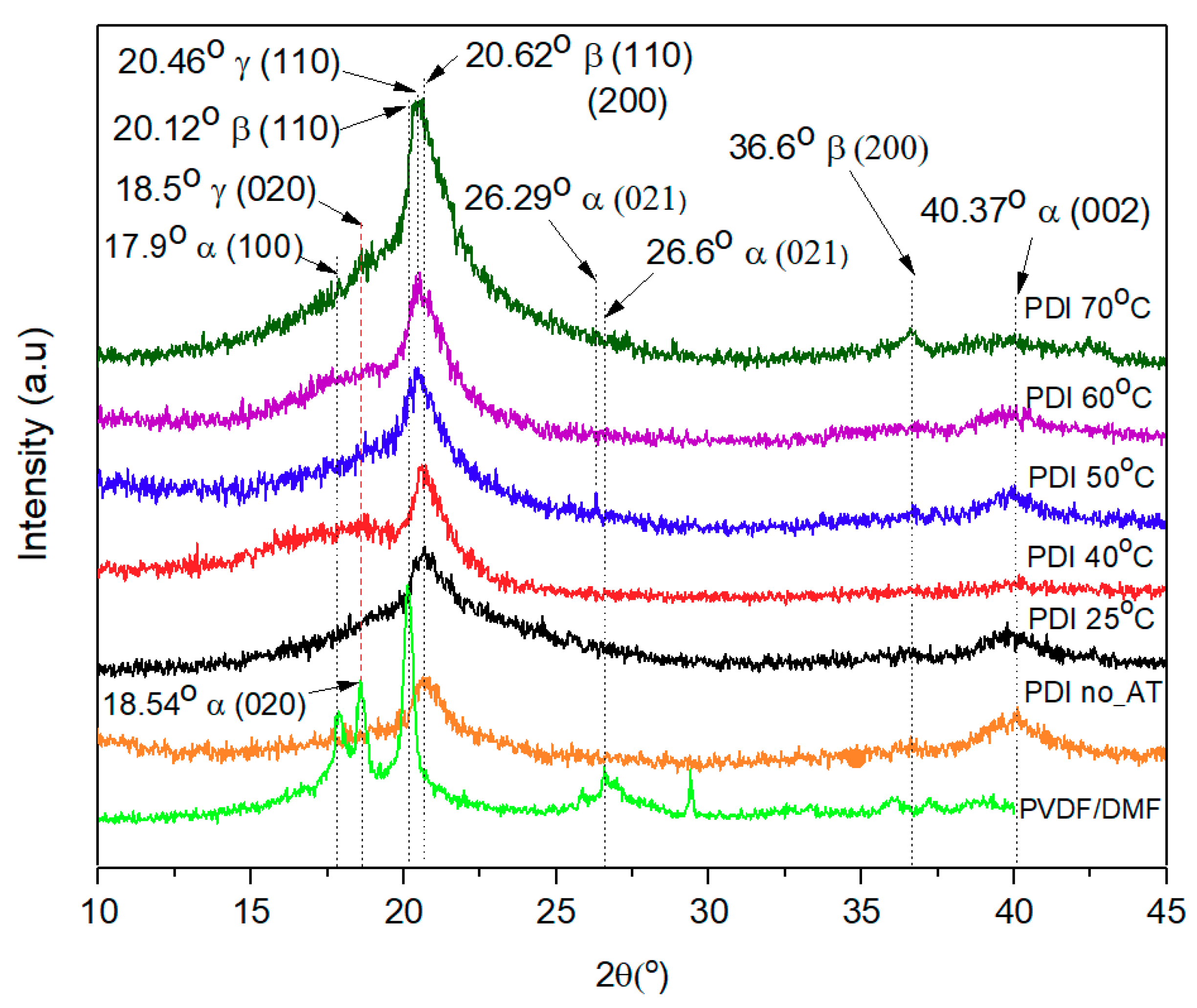
3.2. XRD Diffraction Pattern and Degree of Crystallinity
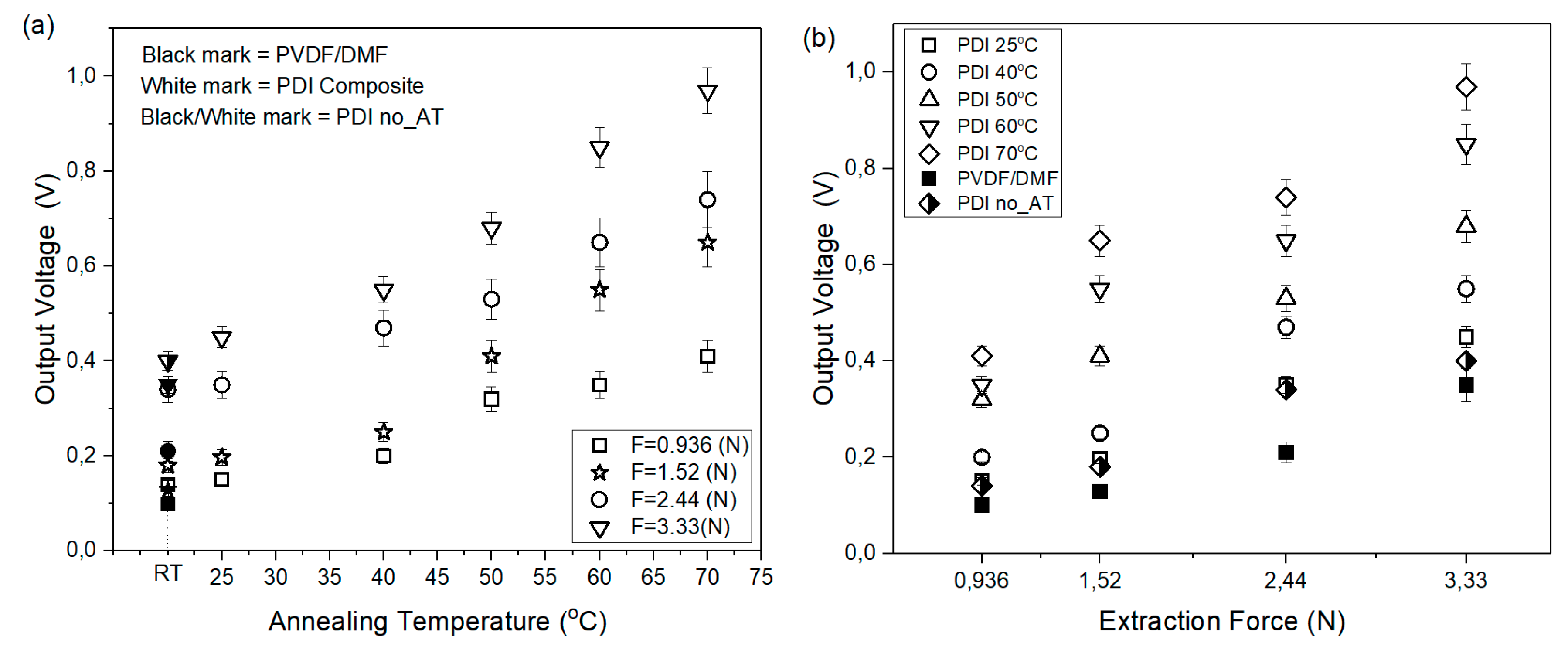
3.3. Output Voltage Response
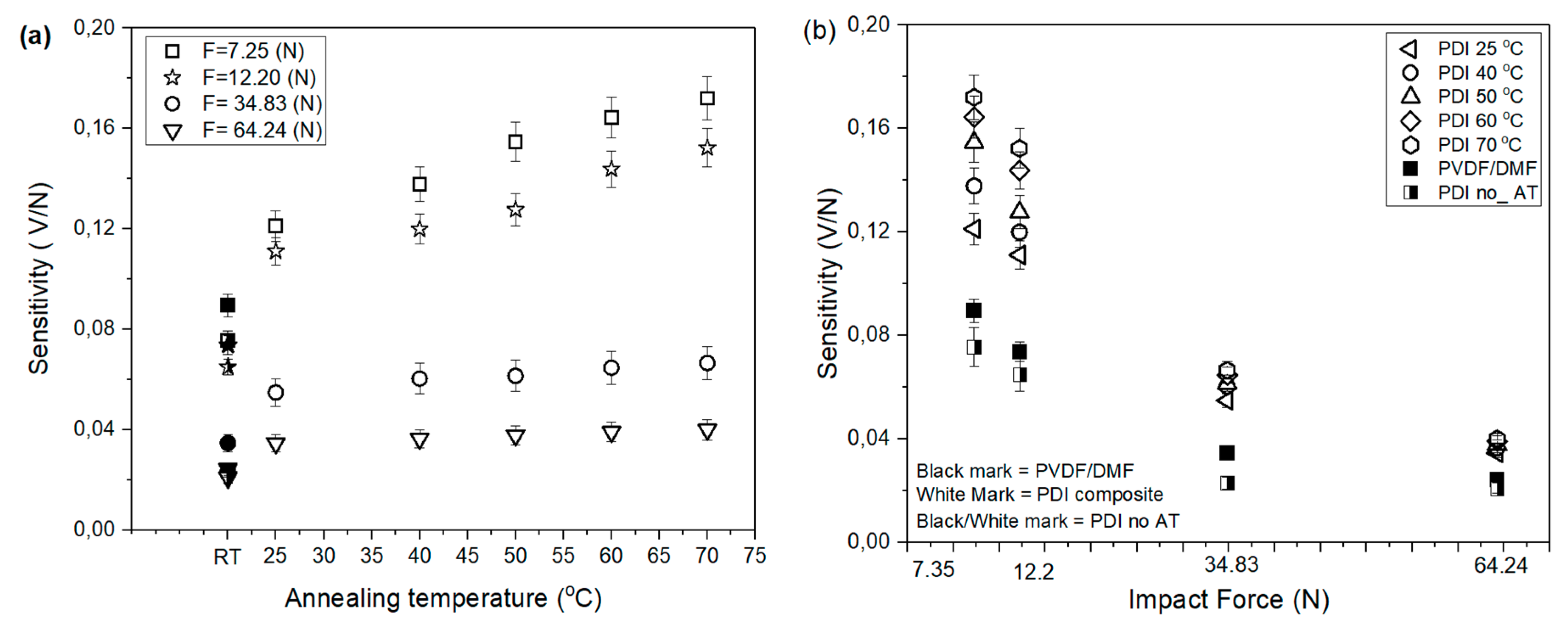
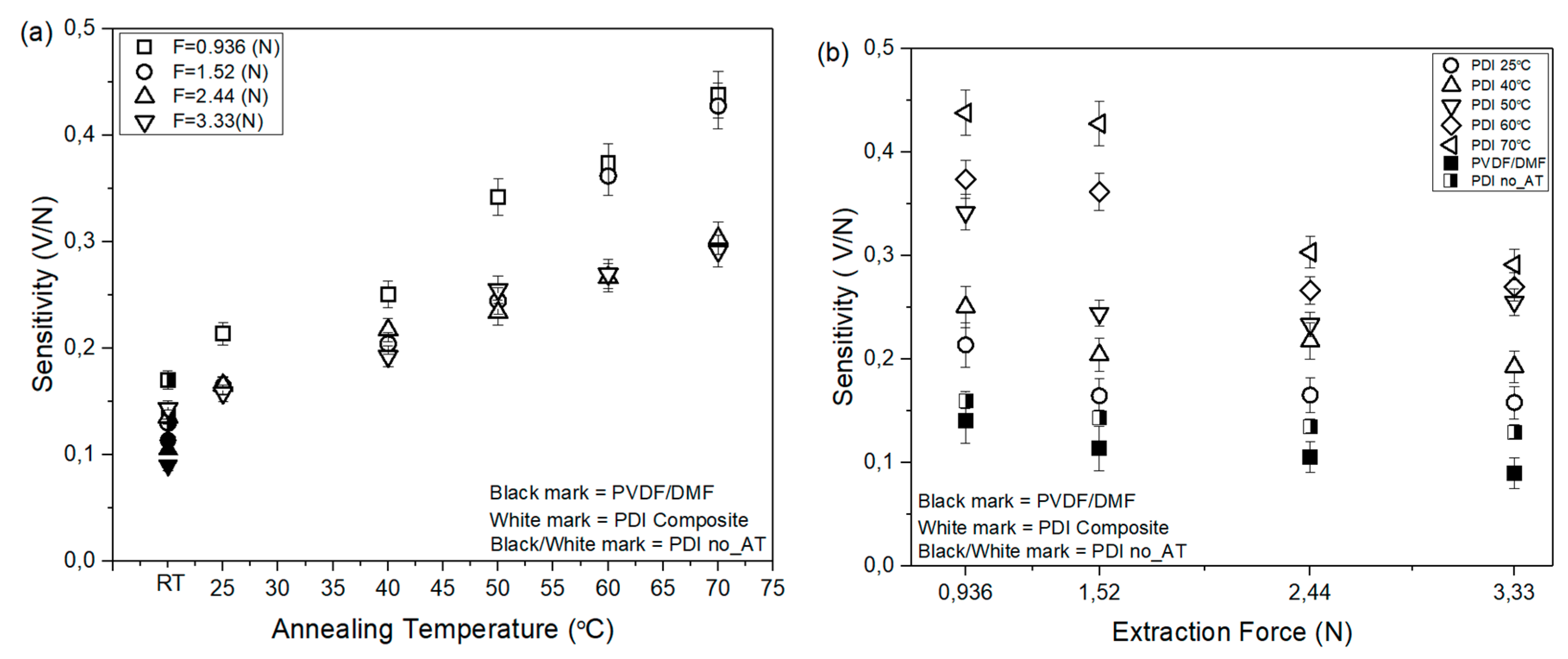
3.4. Sensitivity of PVDF Sensor
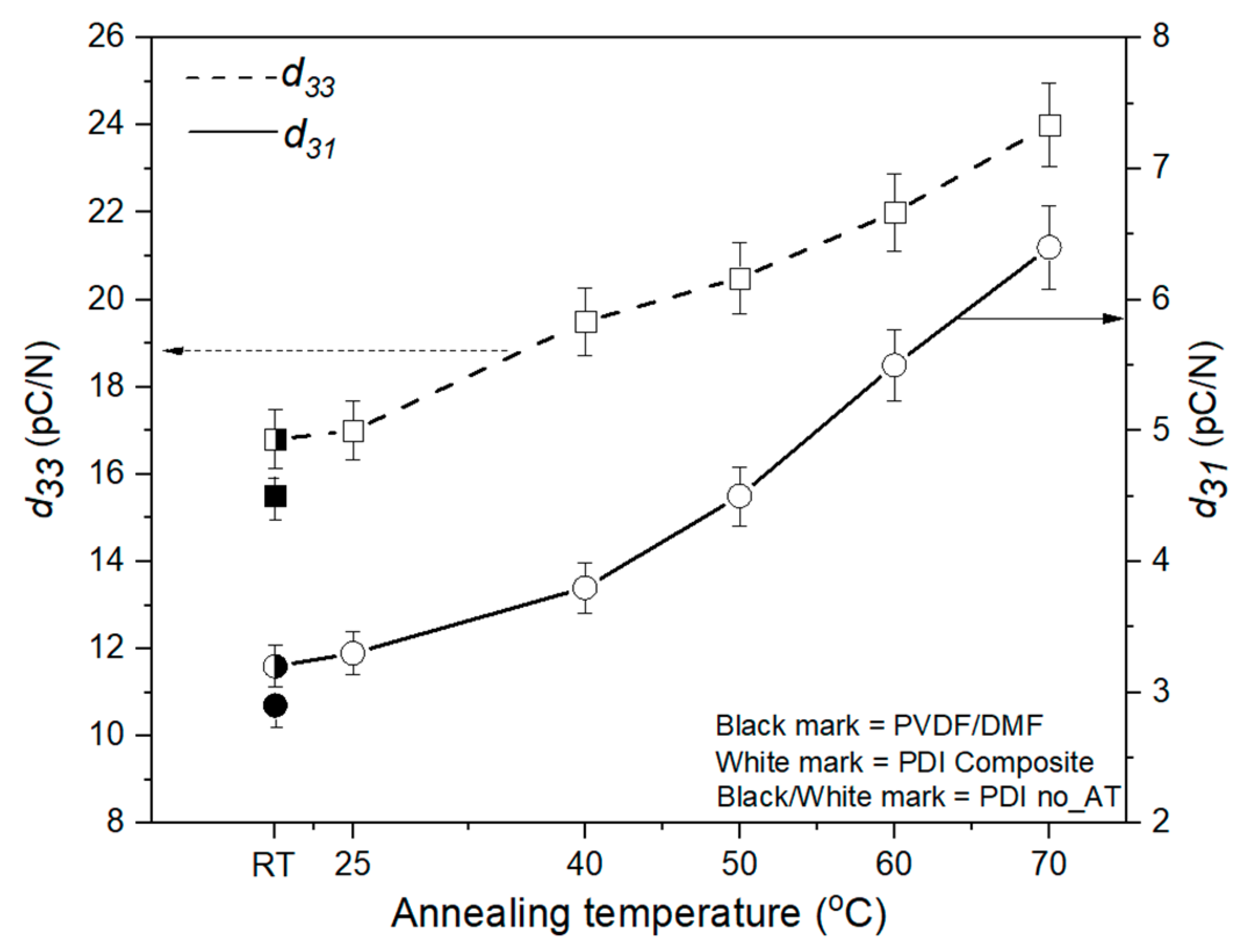
3.5. Measurement of Piezoelectric Coefficient (d33 and d31)
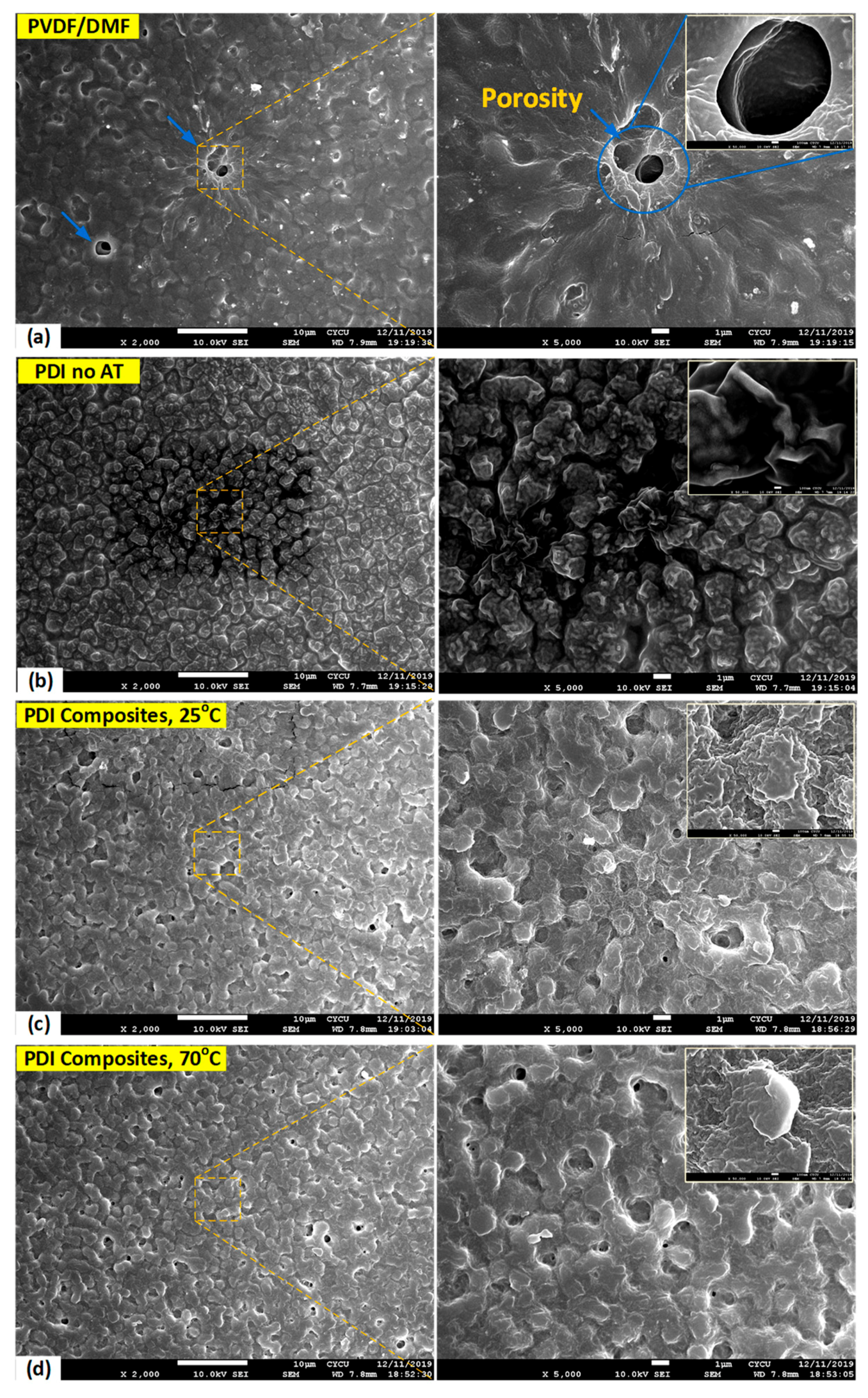
3.6. Surface Morphology
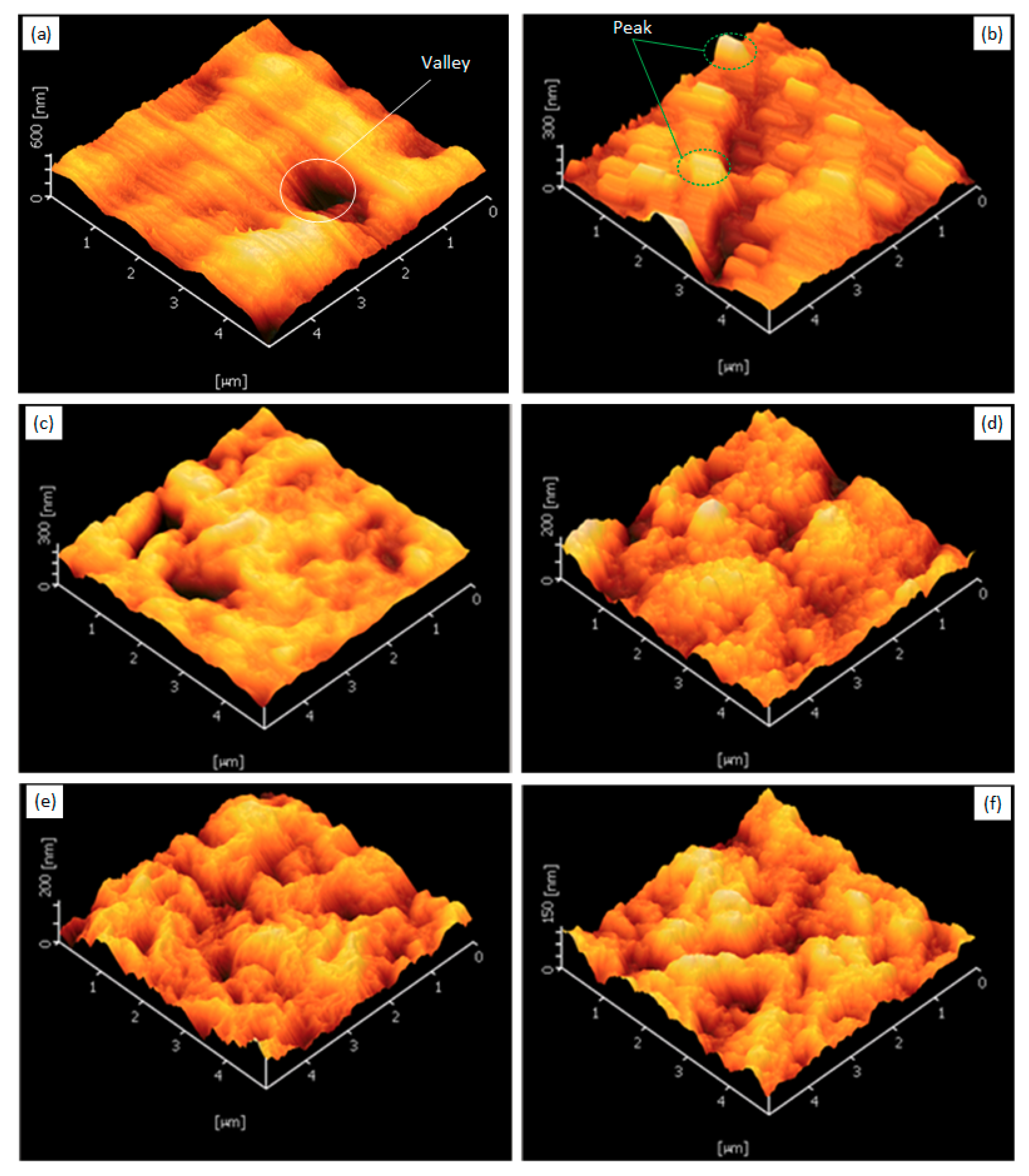
3.7. Atomic Force Microscopy (AFM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burnham-Fay, E.D.; Le, T.; Tarbutton, J.A.; Ellis, J.D. Strain characteristics of additive manufactured polyvinylidene fluoride (PVDF) actuators. Sens. Actuators A Phys. 2017, 266, 85–92. [Google Scholar] [CrossRef]
- Liu, S.Y.; Li, Y.Y.; Guo, W.; Huang, X.; Xu, L.; Lai, Y.-C.; Zhang, C.; Wu, H. Triboelectric nanogenerators enabled sensing and actuation for robotics. Nano Energy 2019, 65, 104005. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Costa, C.M.; Minas, G.; Lanceros-Mendez, S. Improving the optical and electroactive response of poly(vinylidene fluoride–trifluoroethylene) spin-coated films for sensor and actuator applications. Smart Mater. Struct. 2012, 21, 085020. [Google Scholar] [CrossRef]
- Sharma, T.; Je, S.-S.; Gill, B.; Zhang, J.X.J. Patterning piezoelectric thin film PVDF–TrFE based pressure sensor for catheter application. Sens. Actuators A Phys. 2012, 177, 87–92. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Oddo, C.M.; Zollo, L.; Silvestri, S.; Guglielmelli, E. Microfabricated tactile sensors for biomedical applications: A review. Biosensors 2014, 4, 422–448. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.-H.; Kim, J. A review of piezoelectric energy harvesting based on vibration. Int. J. Precis. Eng. Manuf. 2011, 12, 1129–1141. [Google Scholar] [CrossRef]
- Hernández-Rivera, D.; Rodríguez-Roldán, G.; Mora-Martínez, R.; Suaste-Gómez, E. A capacitive humidity sensor based on an electrospun PVDF/graphene membrane. Sensors 2017, 17, 1009. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and modification of poly (vinylidene fluoride)(PVDF) membranes–a review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Ueberschlag, P. PVDF piezoelectric polymer. Sens. Rev. 2001, 21, 118–126. [Google Scholar] [CrossRef]
- Gradys, A.; Sajkiewicz, P. Determination of the melting enthalpy of β phase of poly (vinylidene fluoride). e-Polymers 2013, 13. [Google Scholar] [CrossRef]
- Prest, W.M., Jr.; Luca, D.J. The formation of the γ phase from the α and β polymorphs of polyvinylidene fluoride. J. Appl. Phys. 1978, 49, 5042–5047. [Google Scholar] [CrossRef]
- Lovinger, A.J. Poly (vinylidene fluoride). In Developments in Crystalline Polymers—1; Springer: Berlin, Germany, 1982; pp. 195–273. [Google Scholar]
- Pan, H.; Na, B.; Lv, R.; Li, C.; Zhu, J.; Yu, Z. Polar phase formation in poly (vinylidene fluoride) induced by melt annealing. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1433–1437. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. FTIR studies of β-phase crystal formation in stretched PVDF films. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Sencadas, V.; Gregorio, R.; Lanceros-Mendez, S. α to β Phase Transformation and Microestructural Changes of PVDF Films Induced by Uniaxial Stretch. J. Macromol. Sci. Part B 2009, 48, 514–525. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Berring, J.; Walus, K.; Stoeber, B. Effect of poling time and grid voltage on phase transition and piezoelectricity of poly (vinyledene fluoride) thin films using corona poling. J. Phys. D Appl. Phys. 2013, 46, 285305. [Google Scholar] [CrossRef]
- Ting, Y.; Gunawan, H.; Zhong, J.-Z.; Chiu, C.-W. A new poling method for piezoelectric ceramics with thick film. J. Eur. Ceram. Soc. 2014, 34, 2849–2855. [Google Scholar] [CrossRef]
- Ejeian, F.; Azadi, S.; Razmjou, A.; Orooji, Y.; Kottapalli, A.; Warkiani, M.E.; Asadnia, M. Design and applications of MEMS flow sensors: A review. Sens. Actuators A Phys. 2019, 295, 483–502. [Google Scholar] [CrossRef]
- Bae, S.-H.; Kahya, O.; Sharma, B.K.; Kwon, J.; Cho, H.J.; Ozyilmaz, B.; Ahn, J.-H. Graphene-P (VDF-TrFE) multilayer film for flexible applications. ACS Nano 2013, 7, 3130–3138. [Google Scholar] [CrossRef]
- Pi, Z.; Zhang, J.; Wen, C.; Zhang, Z.-B.; Wu, D. Flexible piezoelectric nanogenerator made of poly (vinylidenefluoride-co-trifluoroethylene)(PVDF-TrFE) thin film. Nano Energy 2014, 7, 33–41. [Google Scholar] [CrossRef]
- Kim, S.; Towfeeq, I.; Dong, Y.; Gorman, S.; Rao, A.; Koley, G. P (VDF-TrFE) film on PDMS substrate for energy harvesting applications. Appl. Sci. 2018, 8, 213. [Google Scholar] [CrossRef]
- Peng, G.R.; Zhao, X.J.; Zhan, Z.J.; Ci, S.Z.; Wang, Q.; Liang, Y.J.; Zhao, M.L. New crystal structure and discharge efficiency of poly (vinylidene fluoride-hexafluoropropylene)/poly (methyl methacrylate) blend films. RSC Adv. 2014, 4, 16849–16854. [Google Scholar] [CrossRef]
- Sencadas, V.; Gregorio Filho, R.; Lanceros-Mendez, S. Processing and characterization of a novel nonporous poly (vinilidene fluoride) films in the β phase. J. Non-Cryst. Solids 2006, 352, 2226–2229. [Google Scholar] [CrossRef]
- Gregorio, R.; Borges, D.S. Effect of crystallization rate on the formation of the polymorphs of solution cast poly(vinylidene fluoride). Polymer 2008, 49, 4009–4016. [Google Scholar] [CrossRef]
- Freemantle, M. An Introduction to Ionic Liquids: Royal Society of Chemistry; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Kubisa, P. Ionic liquids as solvents for polymerization processes—progress and challenges. Prog. Polym. Sci. 2009, 34, 1333–1347. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, H.W.; Stoller, M.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S.; SUH, K.S. High-performance supercapacitors based on poly (ionic liquid)-modified graphene electrodes. ACS Nano 2010, 5, 436–442. [Google Scholar] [CrossRef]
- Fernicola, A.; Scrosati, B.; Ohno, H. Potentialities of ionic liquids as new electrolyte media in advanced electrochemical devices. Ionics 2006, 12, 95–102. [Google Scholar] [CrossRef]
- Dias, J.C.; Correia, D.M.; Costa, C.M.; Ribeiro, C.; Maceiras, A.; Vilas, J.L.; Botelho, G.; Bermudez, V.d.Z.; Lanceros-Mendez, S. Improved response of ionic liquid-based bending actuators by tailored interaction with the polar fluorinated polymer matrix. Electrochim. Acta 2019, 296, 598–607. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Prospects of ionic liquids application in electronic and bioelectronic nose instruments. TrACTrends Anal. Chem. 2017, 93, 23–36. [Google Scholar] [CrossRef]
- Muñoz-Aguirre, S.; López-Casique, A.; Alcántara-Iniesta, S.; Castillo-Mixcóatl, J.; Beltrán-Pérez, G.; Muñoz-Aguirre, N. High-resolution gas/odor sensors using high-frequency quartz crystal microbalance. Sens. Mater. 2014, 26, 131–136. [Google Scholar]
- Okada, D.; Kaneko, H.; Kato, K.; Furumi, S.; Takeguchi, M.; Yamamoto, Y. Colloidal crystallization and ionic liquid induced partial β-phase transformation of poly (vinylidene fluoride) nanoparticles. Macromolecules 2015, 48, 2570–2575. [Google Scholar] [CrossRef]
- Mejri, R.; Dias, J.; Lopes, A.; Hentati, S.B.; Silva, M.M.; Botelho, G.; Mao de Ferro, A.; Esperanca, J.M.S.S.; Maceiras, A.; Laza, J.M.; et al. Effect of ionic liquid anion and cation on the physico-chemical properties of poly (vinylidene fluoride)/ionic liquid blends. Eur. Polym. J. 2015, 71, 304–313. [Google Scholar] [CrossRef]
- Lopes, A.; Gutiérrez, J.; Barandiarán, J. Direct fabrication of a 3D-shape film of polyvinylidene fluoride (PVDF) in the piezoelectric β-phase for sensor and actuator applications. Eur. Polym. J. 2018, 99, 111–116. [Google Scholar] [CrossRef]
- Silva, M.P.; Sencadas, V.; Botelho, G.; Machado, A.V.; Rolo, A.G.; Rocha, J.G.; Lanceros-Mendez, S. α-and γ-PVDF: Crystallization kinetics, microstructural variations and thermal behaviour. Mater. Chem. Phys. 2010, 122, 87–92. [Google Scholar] [CrossRef]
- Ting, Y.; Suprapto; Nugraha, A.; Chiu, C.W.; Gunawan, H. Design and characterization of one-layer PVDF thin film for a 3D force sensor. Sens. Actuators A Phys. 2016, 250, 129–137. [Google Scholar] [CrossRef]
- Ting, Y.; Suprapto, S.; Chiu, C.W.; Gunawan, H. Characteristic analysis of biaxially stretched PVDF thin films. J. Appl. Polym. Sci. 2018, 135, 46677. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, X.; Ni, Q.; Li, L.; Ju, C. Dependence of the impact response of polyvinylidene fluoride sensors on their supporting materials’ elasticity. Sensors (Basel) 2013, 13, 8669–8678. [Google Scholar] [CrossRef]
- Basari, A.A.; Awaji, S.; Sakamoto, S.; Hashimoto, S.; Homma, B.; Suto, K.; Okada, H.; Okuno, H.; Kobayashi, K.; Kumagai, S. Study of the effect of mechanical impact parameters on an impact-mode piezoelectric ceramic power generator. Ceram. Int. 2015, 41, 12038–12044. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly (vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Mandal, D.; Henkel, K.; Schmeißer, D. The electroactive β-phase formation in poly (vinylidene fluoride) by gold nanoparticles doping. Mater. Lett. 2012, 73, 123–125. [Google Scholar] [CrossRef]
- Xu, P.; Fu, W.; Hu, Y.; Ding, Y. Effect of annealing treatment on crystalline and dielectric properties of PVDF/PEG-containing ionic liquid composites. Compos. Sci. Technol. 2018, 158, 1–8. [Google Scholar] [CrossRef]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and Applications of the β Phase Poly (vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Yousefi, A.A. Conformational changes and phase transformation mechanisms in PVDF solution-cast films. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3487–3495. [Google Scholar] [CrossRef]
- Xing, C.; Zhao, L.; You, J.; Dong, W.; Cao, X.; Li, Y. Impact of ionic liquid-modified multiwalled carbon nanotubes on the crystallization behavior of poly (vinylidene fluoride). J. Phys. Chem. B 2012, 116, 8312–8320. [Google Scholar] [CrossRef]
- Bose, P.; Roy, A.; Dutta, B.; Bhattacharya, S. Decoupling of segmental relaxation from ionic conductivity in [DEMM][TFSI] room temperature ionic liquid incorporated poly (vinylidenefluoride-co-hexafluoropropylene) membranes. Solid State Ion. 2017, 311, 75–82. [Google Scholar] [CrossRef]
- Katsyuba, S.A.; Dyson, P.J.; Vandyukova, E.E.; Chernova, A.V.; Vidiš, A. Molecular Structure, Vibrational Spectra, and Hydrogen Bonding of the Ionic Liquid 1-Ethyl-3-methyl-1H-imidazolium Tetrafluoroborate. Helv. Chim. Acta 2004, 87, 2556–2565. [Google Scholar] [CrossRef]
- Mohammadi, B.; Yousefi, A.A.; Bellah, S.M. Effect of tensile strain rate and elongation on crystalline structure and piezoelectric properties of PVDF thin films. Polym. Test. 2007, 26, 42–50. [Google Scholar] [CrossRef]
- Gregorio, R. Determination of the α, β, and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Riosbaas, M.T.; Loh, K.J.; O’Bryan, G.; Loyola, B.R. In situ phase change characterization of PVDF thin films using Raman spectroscopy. In Sensors and Smart Structures Technologies for Civil, Mechanical, and Aerospace Systems 2014; International Society for Optics and Photonics: Bellingham, WA, USA, 2014. [Google Scholar]
- Zhu, Y.; Li, C.; Na, B.; Lv, R.; Chen, B.; Zhu, J. Polar phase formation and competition in the melt crystallization of poly (vinylidene fluoride) containing an ionic liquid. Mater. Chem. Phys. 2014, 144, 194–198. [Google Scholar] [CrossRef]
- Nasir, M.; Matsumoto, H.; Minagawa, M.; Tanioka, A.; Danno, T.; Horibe, H. Formation of β-phase crystalline structure of PVDF nanofiber by electrospray deposition: Additive effect of ionic fluorinated surfactant. Polym. J. 2007, 39, 670. [Google Scholar] [CrossRef]
- Nasir, M.; Matsumoto, H.; Danno, T.; Minagawa, M.; Irisawa, T.; Shioya, M.; Tanioka, A. Control of diameter, morphology, and structure of PVDF nanofiber fabricated by electrospray deposition. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 779–786. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Wu, S.; Jaffe, M.; Arinzeh, T.L. Structural changes in PVDF fibers due to electrospinning and its effect on biological function. Biomed. Mater. 2013, 8, 045007. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pruvost, S.; Livi, S.; Duchet-Rumeau, J. Understanding of versatile and tunable nanostructuration of ionic liquids on fluorinated copolymer. Macromolecules 2015, 48, 4581–4590. [Google Scholar] [CrossRef]
- Esterly, D.M.; Love, B.J. Phase transformation to β-poly (vinylidene fluoride) by milling. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 91–97. [Google Scholar] [CrossRef]
- Du, C.-H.; Zhu, B.-K.; Xu, Y.-Y. Effects of stretching on crystalline phase structure and morphology of hard elastic PVDF fibers. J. Appl. Polym. Sci. 2007, 104, 2254–2259. [Google Scholar] [CrossRef]
- Steinmann, W.; Walter, S.; Gries, T.; Seide, G.; Roth, G. Modification of the mechanical properties of polyamide 6 multifilaments in high-speed melt spinning with nano silicates. Text. Res. J. 2012, 82, 1846–1858. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Ribelles, J.L.G.; Lanceros-Méndez, S. Influence of Processing Conditions on Polymorphism and Nanofiber Morphology of Electroactive Poly(vinylidene fluoride) Electrospun Membranes. Soft Mater. 2010, 8, 274–287. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; Lei, G.; Liu, H. Ionic liquid-gelled polyvinylidene fluoride/polyvinyl acetate polymer electrolyte for solid supercapacitor. Chem. Eng. J. 2014, 258, 320–326. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Luo, J.; Nguyen, N.T.; Walton, A.; Flewitt, A.J.; Zu, X.T.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E.; et al. Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef]
- Du, G.; Li, Z.; Song, G. A pvdf-based sensor for internal stress monitoring of a concrete-filled steel tubular (CFST) column subject to impact loads. Sensors 2018, 18, 1682. [Google Scholar] [CrossRef] [PubMed]
- Kärki, S.; Lekkala, J.; Kuokkanen, H.; Halttunen, J. Development of a piezoelectric polymer film sensor for plantar normal and shear stress measurements. Sens. Actuators A Phys. 2009, 154, 57–64. [Google Scholar] [CrossRef]
- Gusarov, B.; Gusarova, E.; Viala, B.; Gimeno, L.; Cugat, O. PVDF piezoelectric voltage coefficientin situmeasurements as a function of applied stress. J. Appl. Polym. Sci. 2016, 133, 1–6. [Google Scholar] [CrossRef]
- Bernard, F.; Gimeno, L.; Viala, B.; Gusarov, B.; Cugat, O. Direct Piezoelectric Coefficient Measurements of PVDF and PLLA under Controlled Strain and Stress. Proceedings 2017, 1, 335. [Google Scholar] [CrossRef]
- Kim, G.H.; Hong, S.M.; Seo, Y. Piezoelectric properties of poly (vinylidene fluoride) and carbon nanotube blends: β-phase development. Phys. Chem. Chem. Phys. 2009, 11, 10506–10512. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Fu, W.; Luo, X.; Ding, Y. Enhanced dc conductivity and conductivity relaxation in PVDF/ionic liquid composites. Mater. Lett. 2017, 206, 60–63. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Nithya, R.; Rajendran, S. Surface analysis studies on polymer electrolyte membranes using scanning electron microscope and atomic force microscope. In Scanning Electron Microscopy; InTech: London, UK, 2012. [Google Scholar]
- Oraby, S.E.; Alaskari, A.M. Atomic force microscopy (AFM) topographical surface characterization of multilayer-coated and uncoated carbide inserts. Microscopy 2010, 33, 38. [Google Scholar]
- Bajpai, A.K.; Bhatt, R.; Katare, R. Atomic force microscopy enabled roughness analysis of nanostructured poly (diaminonaphthalene) doped poly (vinyl alcohol) conducting polymer thin films. Micron 2016, 90, 12–17. [Google Scholar] [CrossRef]
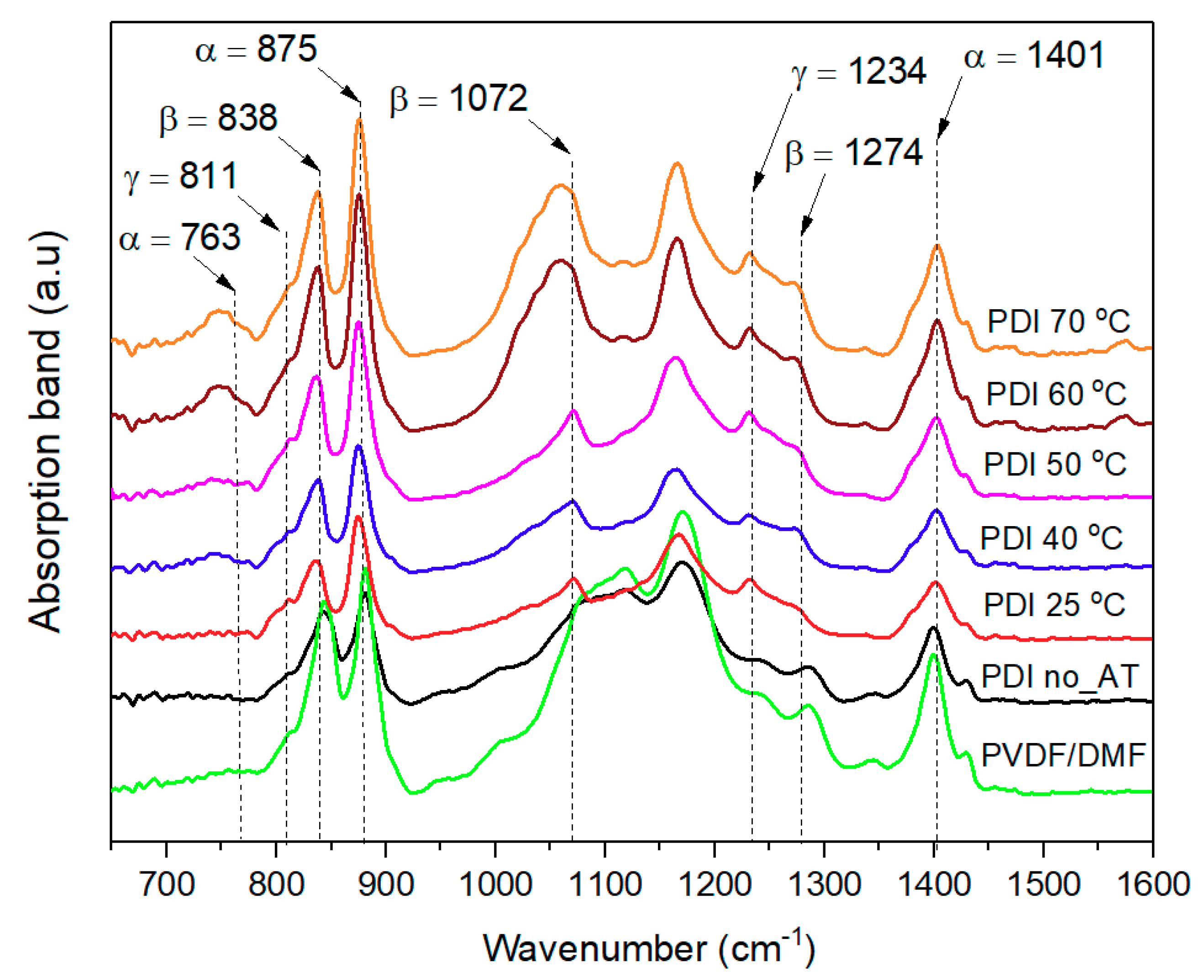


| Wave Number (cm−1) | Crystalline Phase | Group/Vibration |
|---|---|---|
| 763 | α | CF2 rocking or In-plane bending [45] |
| 811 | γ | HC out-of plane [46] |
| 838 | β + γ | CH2 rocking vibration [47] |
| 875 | α | CF2 Asymmetric stretching [48] |
| 1072 | β | CH3 rocking [34,49] |
| 1234 | β +γ | CF2 Asymmetric stretching [14,34] |
| 1274 | β | CF2 Antisymmetric stretching [14] |
| 1401 | α | CH2 wagging [34] |
| Sample | Crystalline Structure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present Study | Previous Work Ref. [14,51,53,56,57,58] | |||||||||||
| α | β | γ | α | β | γ | |||||||
| 2θ (°) | Crystal Plane | 2θ(°) | Crystal Plane | 2θ(°) | Crystal Plane | 2θ(°) | Crystal Plane | 2θ(°) | Crystal Plane | 2θ(°) | Crystal Plane | |
| PVDF/DMF | 17.9 18.54 26.6 | (100) (020) (021) | 20.12 | (110) | 18.5 | (020) | 17.66 18.3 19.9 26.56 27.8 35.7 39 41.1 | (100) (020) (110) (021) (111) (200) (002) (111) | 20.6 20.26 20.7 20.8 20.9 36.6 | (110/200) (200) (110) (200) (110) (200) | 18.30 19.20 20.0 20.04 20.03 20.3 39.0 | (020) (002) (110) (110) (110) (020) (211) |
| PDI no AT | 17.7 26.6 40.37 | (100) (021) (002) | 20.28 | (110)/(200) | 18.22 | (020) | ||||||
| PDI Composite 25 °C | 17.6 26.29 40.37 | (100) (021) (002) | 20.62 | (110)/200) | 18.17 20.3 | (020) (110) | ||||||
| 40 °C | 17.49 26.26 40.39 | (100) (021) (002) | 20.60 | (110)/(200) | 18.4 20.2 | (020) (110) | ||||||
| 50 °C | 17.5 26.24 40.39 | (100) (021) (002) | 20.62 | (110)/(200) | 18.8 20.19 | (020) (110) | ||||||
| 60 °C | 17.5 26.29 40.15 | (100) (021) (002) | 20.60 | (110)/(200) | 18.73 20.40 | (020) (110) | ||||||
| 70 °C | 17.62 26.14 40.37 | (100) (021) (002) | 20.62 36.6 | (110)/(200) (200) | 18.20 20.46 | (020) (110) | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, Y.; Suprapto; Bunekar, N.; Sivasankar, K.; Aldori, Y.R. Using Annealing Treatment on Fabrication Ionic Liquid-Based PVDF Films. Coatings 2020, 10, 44. https://doi.org/10.3390/coatings10010044
Ting Y, Suprapto, Bunekar N, Sivasankar K, Aldori YR. Using Annealing Treatment on Fabrication Ionic Liquid-Based PVDF Films. Coatings. 2020; 10(1):44. https://doi.org/10.3390/coatings10010044
Chicago/Turabian StyleTing, Yung, Suprapto, Naveen Bunekar, Kulandaivel Sivasankar, and Yopan Rahmad Aldori. 2020. "Using Annealing Treatment on Fabrication Ionic Liquid-Based PVDF Films" Coatings 10, no. 1: 44. https://doi.org/10.3390/coatings10010044
APA StyleTing, Y., Suprapto, Bunekar, N., Sivasankar, K., & Aldori, Y. R. (2020). Using Annealing Treatment on Fabrication Ionic Liquid-Based PVDF Films. Coatings, 10(1), 44. https://doi.org/10.3390/coatings10010044