Abstract
Oxidative stress may induce a series of pathophysiological modifications that are directly involved in the development of ophthalmic diseases like age-related cataract, macular degeneration or diabetic retinopathy, considered to be responsible for the majority of vision loss cases. Although various treatment options for eye diseases are available, multiple factors could limit their efficacy. Recently, the accelerated development of ophthalmic nanosystems has provided new possibilities for overcoming the limitations of existing ocular drug delivery methods. This review evaluates the current status of ophthalmic nanosystems loaded with antioxidants for the prevention and treatment of several eye diseases.
1. Introduction
Vision impairment is a major public health concern, affecting 2.2 billion people worldwide. According to a report from the World Health Organization (WHO), 65.2 million patients have a cataract and 3 million have diabetic retinopathy, diseases capable of producing a permanent vision loss [1]. A significant proportion of visually impaired patients are over 50 years old, where a constantly aging global population will likely further increase the incidence of severe eye diseases in the future [1]. The eye is an organ prone to oxidative stress due to high exposure to ultraviolet (UV) light. In addition, daily exposure to blue light (450–495 nm) from television, computer or mobile phone LED screens can also generate reactive oxygen species (ROS) in several eye structures [2,3,4,5]. Consequently, prolonged oxidative stress may induce a series of pathophysiological modifications such as lens protein precipitation or microvascular anomalies. These are directly involved in the development of ophthalmic diseases like age-related cataract or diabetic retinopathy [6,7]. Furthermore, photo-oxidative damage may contribute to retinal pigment epithelium degeneration, involved in the pathogenesis of age-related macular degeneration [8,9,10,11].
Although various treatment options for eye diseases are available, several factors could limit their availability and efficacy. Invasive treatment procedures of some ophthalmic pathologies, like classic surgery or laser therapy are usually too costly in developing countries and are often characterized by high recurrence rates of the disease [7]. Non-invasive treatments like topical drug administration are widely used in ophthalmology, but their effectiveness is limited by the unique anatomy and physiology of the eye. The eye has structural barriers that can significantly reduce the ophthalmic bioavailability of the active substance [7]. Therefore, there is a pressing need to develop new strategies for drug delivery in the management of ocular diseases with the ultimate goal of reducing the incidence of vision loss and improving patients’ quality of life.
In recent years, the development of nanotechnology has had a tremendous impact on multiple industrial sectors but also biomedical sciences. Nanocoating of biologically active substances gave them a superior capability of interacting with the organism at subcellular level, with improved specificity for designated molecular targets.
In ophthalmology, nanoformulations have provided new possibilities to overcome the limitations of existing ocular drug delivery methods. Various nanostructures like liposomes, nanospheres, or polymeric nanoparticles may successfully entrap the active substance, carrying it towards the internal structures of the eye that are usually difficult to reach using conventional drug formulations. Therefore, the inclusion of an ophthalmic drug in a nanostructure has the potential to improve the solubility, permeability, and stability of the active substance, with a clear augmentation of the ocular bioavailability [12].
Among various drugs used in ophthalmology, antioxidants are used for their capacity to scavenge free radicals and to prevent cellular and tissular damage caused by oxidative stress. Antioxidant substances may particularly benefit from being incorporated into nanosystems due to their poor pharmacokinetic properties and a clear propensity for rapid inactivation. Thus, a carefully chosen nanocarrier might protect the antioxidant drug from being prematurely degraded or removed by the eye’s protective mechanisms. This process enhances the ocular bioavailability and possibly allows a more targeted effect towards specific eye components [7].
Although nanotechnology is increasingly used today for the development of intelligent systems that are capable of releasing active principles, few articles have focused on its ophthalmic applications. Therefore, this review aims to briefly present the state of the art regarding the development of ophthalmic nanosystems loaded with antioxidants designed to obtain superior therapeutical effects by improving the biopharmaceutical properties of the drug.
2. Methodology
A search was performed in the Web of Science, PubMed, and Scopus scientific databases, covering the last ten years (2009–2019). The search terms “nanoparticles/nanosystems,” “antioxidant,” and “ophthalmic/ocular” were used for data selection. Only full-text articles in English were included in this work.
3. Results and Discussion
3.1. Pharmacokinetic Aspects of Ophthalmic Drug Administration
Despite being an easily accessible organ, the eye poses significant challenges for effective drug administration. Anatomically, the human eye is divided into two sections: the anterior segment which includes the cornea, iris, lens, and aqueous humor; and the posterior segment which includes the vitreous body, retina, choroid, and the back of the sclera [12,13,14]. The systemic administration of drugs can deliver them to the anterior segment of the eye but with very low ocular bioavailability due to the presence of an anterior blood-aqueous barrier (BAB) formed by inner ciliary epithelia, endothelia around the iris, and ciliary muscle capillaries. To reach the posterior segment of the eye, a systemically administered drug would have to pass the posterior blood-retinal barrier (BRB) formed by the endothelia of the retinal capillaries and retinal pigment epithelium. The existence of two barriers, the BAB and BRB, can impair the passage of a systemically administered drug from the blood to the aqueous humor or the retina. Additionally, a systemically administered drug may suffer from first-pass metabolism, usually in the liver, or it can generate unwanted adverse effects at multiple levels. Therefore, local (ophthalmic) administration of drugs is usually preferred, being more convenient and simpler [15].
To reach the anterior segment of the eye and to treat various ocular diseases like cataracts, dry eye syndrome, inflammatory conditions or glaucoma, a topically applied drug would have to pass the corneal barrier formed by five distinct layers: the epithelium, Bowman’s membrane, stroma, Descemet’s membrane, and endothelium. The increased hydrophobicity of the epithelium coupled with the presence of tight junctional proteins between epithelial cells significantly limits the capacity of hydrophilic drugs to penetrate the cornea. Meanwhile, a more hydrophilic environment present in the stroma restricts the passage of lipophilic drugs. Given this extremely selective corneal barrier, topically applied ophthalmic drugs enter in the anterior segment of the eye after they are absorbed via the conjunctiva/sclera pathway. The pathway has a larger surface area and superior permeability compared to the cornea. Limiting factors that can impair the drug’s capacity to access the inner segments of the eye include a possible elimination of the drug by conjunctival blood and lymphatic flow, as well as the size of the molecule rather than its lipophilicity. Moreover, the ophthalmic bioavailability of drugs is reduced further by other mechanisms such as metabolic degradation or a premature drug removal from the eye by tear clearance or nasolacrimal drainage [15,16].
Other alternative means to deliver the drug to the internal eye structures are intracameral or subconjunctival administrations, which allow access to the anterior segment of the eye. The other is intravitreal administration, which gives the drug the capacity to target retinal structures (Figure 1). The intracameral and subconjunctival routes of drug administration both avoid the cornea and BAB and have good efficacy due to the superior bioavailability of the active substance. Intravitreal administration allows a relatively good passage of the drug to the posterior eye structures, the vitreous being a more permeable barrier than the cornea. The main disadvantage of these alternative ocular routes is the discomfort and pain caused by the procedure itself, which can limit patient compliance [15,16].
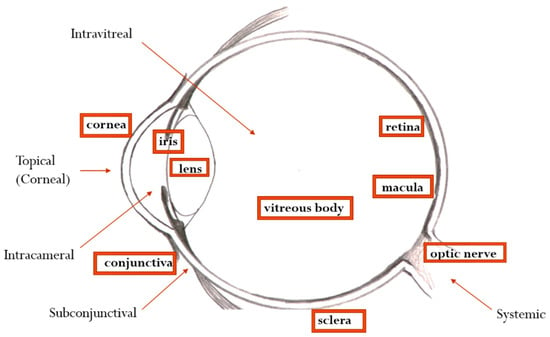
Figure 1.
Main routes of ophthalmic drug administration.
3.2. State of the Art in the Formulation of Ophthalmic Nanosystems with Antioxidants
Designing a drug delivery system to target anatomical structures of the eye is a challenging process. In the last decade, the entrapment of the active substance in a nanosystem has become a promising solution for ocular diseases by improving ophthalmic bioavailability and favoring a constant concentration of the drug in the affected eye due to a prolonged-release, thereby ensuring a superior ocular tolerance. Nanosystems represent excellent reservoirs for the encapsulation of a variety of compounds and involve colloidal drug delivery systems, such as liposomes, micelles, niosomes, dendrimers, nanoparticles (NPs), implants, in situ hydrogels, or cyclodextrines (CD) [16]. Further, other innovative nanostructured drug-delivery systems have been developed. To reduce mononuclear phagocyte system uptake and to extend biological activity of the incorporated substances, “stealth” liposomes have been synthesized with the inclusion of poly-(ethylene glycol) in liposome composition [17]. Another approach of delivering nucleic acids, small molecule therapeutic agents, quantum dots, or magnetic resonance imaging (MRI) contrast agents to their targets, involves incorporating them into cell-penetrating peptides [18]. Even a genome-editing tool like an endonuclease enzyme guided by a hybrid strand of ribonucleic acid (RNA) was incorporated successfully into nonviral vectors like cationic polymers or lipids for successful delivery to various cell types [19]. Additionally, poly-oxometalate nanoclusters have been successfully tested in vivo, protecting neurons against experimentally induced oxidative stress [20,21]. Moreover, recently published research highlighted the versatility of nanosystems (polymersomes) in incorporating complex gaseous therapeutics like nitric oxide. This ability allows for smart, light-assisted delivery of the active substance in the treatment of ocular diseases like keratitis or corneal lesions [22,23,24].
The physico-chemical properties and drug-release characteristics of the nanosystems are influenced mainly by their composition (nature of polymer: natural or synthetic, molecular weight, polymer crystallinity, lipid type, presence of stabilizers), as well as the method of manufacture [25].
An optimal particle size to avoid local irritation and ensure an easy absorption by the corneal or conjunctival routes is considered to be less than 800 nm [26,27].
Among various drugs used in ophthalmology, antioxidants are particularly susceptible to denaturation and degradation during the manufacturing process. Natural antioxidant compounds such as baicalin, β-carotene, lycopene, epigallocatechine-3-gallate (EGCG), quercetin, curcumin, as well as metal oxides, are biologically active molecules with beneficial effects in a series of eye diseases. They often present low bioavailability that can be enhanced by chemical modification and/or pharmaceutical formulation [28].
The main characteristics of the studied antioxidant-loaded ophthalmic nanosystems are presented alphabetically in Table 1.

Table 1.
Ophthalmic nanoformulations with antioxidants.
Baicalin was formulated as solid lipid nanoparticles (SLN) using the emulsification/ultrasonication method. The obtained SLN showed adequate physical characteristics and prolongation of baicalin release compared to the regular ophthalmic solutions [29]. Other solutions have been explored. In particular, vesicular nanosystems, such as liposomes, with optimal lipophilicity can improve drug permeation through the non-polar anatomical structures of the eye. They provide sustained drug release and allow good patient compliance by increasing the remanence time on the mucosa, thereby reducing the drug dose and frequency of adverse effects. Liposomes were shown to enhance the efficacy, stability, and solubility of drugs, which is a significant factor in the formulation of these systems being represented by choice of excipients, which must provide the transcorneal passage of drugs, but must also be compatible with the structures of the eye [30]. Baicalin vesicles were prepared using the thin film hydration technique. The vesicular systems showed a particle size greater than 600 nm and a negative charge. Liposomes exhibited the highest entrapment efficiency (EE) due to the lipophilicity of baicalin, which accumulated in the lipid layers of the multilamellar vesicles, as well as the presence of cholesterol which increased the hydrophobicity of the phospholipid layers [30].
Metal oxide nanoparticles have received particular attention in recent years due to their antiseptic, anti-inflammatory, and antioxidant properties. Given the increased toxicity of Ag and Au nanoparticles (NPs), the research has focused on cerium, a promising rare earth element. CeCl3 loaded mesoporous silica (CeCl3@mSiO2) NPs for use in the prevention of cataractogenesis were prepared [31]. Cerium oxide nanoparticles (nanoceria) are antioxidants and possess catalytic activities that mimic those of biological antioxidants (superoxide dismutase, catalase). They also have great potential for use as drug carriers and delivery agents due to their attractive properties, i.e., biocompatibility and small particle size that allow for easier passage through cell membranes [32].
Coenzyme Q10-loaded liposomes coated with different concentrations of N-trimethyl chitosan, an excellent absorption enhancer, were prepared. The experimental results showed a deeper penetration of coated liposomes into the corneal epithelium in comparison to the non-coated ones [33].
Various biocompatible drug carriers have been incorporated to ensure optimal drug delivery formulations at the eye level and to increase effective drug concentration in the anterior or posterior eye tissue. Albumin is an attractive macromolecular carrier because of its non-immunogenic, non-toxic, biocompatible, and biodegradable characteristics, and it is being considered as a potent drug carrier to increase the solubility of poorly soluble compounds. Albumin-based nanoformulations (bovine serum albumin) loaded with curcumin, rosmarinic acid, and ursolic acid were prepared using the desolvation method. The results confirmed that the NPs had nanosized ranges, had increased drug solubility, and better stability after 2 months of storage both in a solution state and freeze-dried powder [34].
Another antioxidant, epigallocatechin gallate (EGCG), a polyphenolic compound known for its pleiotropic effects, was encapsulated in a nanosystem for protection against undesired environmental factors (light, moisture, oxygen) and to prolong its shelf life, to avoid degradation and provide a controlled release. Huang et al. reported the preparation of epigallocatechin gallate loaded NPs using the self-assembly method [35]. The polymer used in the preparation was gelatin, a preferred raw material due to its biodegradability and biocompatibility properties [36]. The synthesized NPs were decorated on the surface with hyaluronic acid to ensure prolonged retention on the eyes by increasing the adhesion of NPs to the mucus layer. The NPs showed an increase in particle size and a decrease in zeta potential proportional to the increase in hyaluronic acid concentration. The zeta potential was positive until it reached a 62.5 μg/mL hyaluronic acid (HA) concentration, becoming negative at 125 μg/mL HA. The NPs with a particle size of 253.4 ± 7.3 nm and zeta potential of 9.2 ± 1.8 mV (corresponding to a 62.5 μg/mL HA concentration) showed a pH, osmolality, and refractive index similar to those of human tears. The NPs also demonstrated a high affinity for human corneal epithelium cells and anti-inflammatory effects after in vivo evaluation [35].
Fangueiro et al. reported the development of lipid nanoparticles (LNs), an innovative vehicle for EGCG encapsulation, for use in the future treatment of several eye diseases (dry eye syndrome, age-related macular degeneration, glaucoma, diabetic retinopathy, and macular edema). LNs represent a new generation of drug delivery systems known as safe drug carriers due to their physiological lipid contents, which are biocompatible and biodegradable. The preparation was performed via the multiple emulsion method, using cationic lipids such as cetyl-trimethyl-ammonium (CTAB) bromide and dimethyl-diocta-decyl-ammonium bromide (DDAB) and a non-ionic surfactant (Poloxamer 188) as a stabilizing agent. Moreover, the cationic properties were exploited to provide the LNs with stability and to increase their mucoadhesivity on the eye surface by electrostatic attraction. The obtained particles were in the nanometer range (<300 nm), with good EE (<90%), and they revealed higher stability at 25 °C, as well as enhanced bioavailability and physiological compatibility with ocular tissue [37].
Chang et al. reported the obtention of EGCG loaded NPs using an arginine-glycine-aspartic acid peptide, with a hyaluronic acid–conjugated complex coating the gelatine. The obtained NPs had a mean diameter smaller than 200 nm, positive charge and good EE, for use in the treatment of neovascularization of the cornea [38].
The manufacture of metal nanoparticles using plant extracts or other biomolecules has become an attractive strategy that allows avoidance of any harmful reductant, rendering it useful in biology and medicine. Dong et al. reported on the fabrication of gold nanoparticles (AuNPs) through an eco-friendly method, using a herbal polyphenol (resveratrol) as both the stabilizing and reducing agents. The obtained AuNPs were spherical with a mean particle size of 20 nm [39].
A natural carotenoid pigment, lutein, was also incorporated in several nanosystems. Liu et al. combined lutein loaded lipid nanocarriers (NLCs) with 2% β-cyclodextrins (Table 1) using the hot sonication method and they obtained an increase of the EE and lower cytotoxicity in the bovine cornea cells [40]. Bodoki et al. reported lutein loaded PLGA NPs preparation. The obtained NPs were incorporated in a bioadhesive thermosensitive gel based on Poloxamer 407 and polyethylene oxide. This process ensured a prolonged adherence to the mucosa after topical application in the eye. The combination of drug-loaded nanosystems with bioadhesive polymers can bring important benefits to the drug delivery system. Mucoadhesives attach to the mucin layer that covers the conjunctiva and the cornea, ensuring intimate contact between the drug and the biological tissues. This process results in a high local concentration of the drug, as well as its increased absorption through the biological membranes [41].
An interesting approach in the preparation of antioxidant-based pharmaceutical systems is the combination of several compounds with antioxidant action in the same formulation. Natesan et al. reported on the preparation of NPs loaded with resveratrol, alone or associated with quercetin, designed for the treatment of glaucoma. The NPs were prepared by the ionic gelation method, using chitosan and sodium tri-polyphosphate (TPP) as a polyanionic cross-linking agent. The NPs were modified with PEG (different molecular weights and in different concentrations). Spherical nanoparticles with a mean diameter greater than 100 nm were obtained. The association of quercetin in NPs with resveratrol increased the particle size and led to a decrease in the EE. It was observed that the increase of the concentration and molecular mass of the PEG caused the increase of the particle size and the decrease of the EE. This effect was probably because of reductions in the available space for the incorporation of the drugs. On the other hand, increasing the concentration of PEG leads to a decrease in the positive charge of the NPs (PEG and drugs can reduce the ionic interactions between the chitosan and cross-linking agent), thereby improving the biocompatibility and stability in the physiological fluids. Chitosan, being a cationic polyelectrolyte, can provide an improved residence of the preparation on the mucosa and, consequently, a prolonged release of the active principle. This is done by performing electrostatic interactions with the sialic acid residues in the mucus or with the negative charges on the surface of the epithelium [42].
Anbukkarasi et al. reported the successful green-synthesizing of silver NPs (AgNPs) using an ethanolic extract of the leaves of Tabernaemontana divaricata, intended for use in anticataractogenic potential [43].
Liposomes loaded with α-tocopherol were obtained to improve the stability and tolerability of vitamin E. The in vitro characterization revealed physiological values concerning pH, osmolality, and viscosity. The resuspension of liposomes in an isotonic solution of 0.2% sodium hyaluronate led to the best tolerance demonstrated in vivo after application by instillation [44].
Cationic liposomes based on chitosan were formulated to improve the ocular bioavailability of vitamin A by increasing retention time in the eye. Vitamin A-loaded obtained liposomes were then dispersed in an in-situ gel-forming matrix, consisting of Poloxamer 407 25%. Studies have shown that colloidal dispersions did not influence the gelation temperature, and by dispersing them in the in situ forming gel, a delayed release of the drug and an increased corneal retention time were obtained [45].
An interesting strategy aimed at the reduction of adverse reactions (keratitis, acute red eye) and discomfort caused by the use of contact lenses is based on coating the contact lenses with metal-oxide nanoparticles and functionalization of the surface with hydrophilic polymers. Hoyo et al. reported the simultaneous depositing of an aqueous suspension containing ZnO nanoparticles (antibacterial activity by formation of reactive oxygen species that affect bacterial membrane), chitosan (natural polymer known for biocompatibility, biodegradability, wettability, and antibacterial properties), and gallic acid (antioxidant activity) in a one-step sonochemical coating process. The ternary hybrid coating improved the surface wettability and antioxidant activity by 95% and showed high antibacterial efficiency [46].
3.3. Preclinical In Vivo and Ex-Vivo Studies with Ophthalmic Nanosystems with Antioxidants
This review of published data showed that ocular nanosystems with antioxidants presented significant protective effects against multiple ocular pathologies, demonstrated in specific in vivo or ex-vivo animal models. The studies by Li et al. [47], Bodoki et al. [41], and Anbukkarasi et al. [43] used the selenite-induced cataract model in rodents, obtaining a reduction of the disease progression under treatment with nano entrapped antioxidants like baicalin, lutein, or silver. In this model, the antioxidant molecules protected the lens against oxidative damage caused by a toxic substance-sodium selenite. Several in vivo studies evaluated the protective effect of nanoceria against light-induced retinal damage or in genetically modified animals like tubby mice or Vldr knockout mice. It demonstrated good effects in preventing retinal degeneration by a scavenging effect of ROS and a reduction of MAP kinase activity in the retina [48,49,50]. Kyosseva et al. [51] also found that the protective effect of nanoceria against retinal lesions is due to the inhibition of some pro-angiogenic factors. Huang et al. [35] proved that an ophthalmic nanosystem with an epigallocatechin gallate might alleviate the dry eye syndrome in rabbits through antioxidant and anti-inflammatory mechanisms. Furthermore, Natesan et al. [42] found that nano entrapped resveratrol can reduce intraocular pressure in rabbits.
Although preclinical data concerning the ophthalmic effects of antioxidants encapsulated in nanosystems are very promising, further research is necessary to ascertain if these results can be translated into human medicine. The antioxidant-loaded ophthalmic nanosystems are presented alphabetically, the main findings of the studies were also presented, alongside the route of administration and the applied doses (Table 2).

Table 2.
In vivo and ex-vivo studies using ophthalmic nanosystems with antioxidants.
3.4. Mechanisms of Action of Antioxidants Entrapped in Nanosystems with Ophthalmic Application
An antioxidant is a molecule that can significantly decrease the oxidation of specific substrates (proteins, lipids, DNA), thereby reducing the destructive cellular consequences of oxidative stress [7]. Extensive research has demonstrated that highly reactive oxygen species like the superoxide anion radical, hydrogen peroxide, or hydroxyl radical, are generated in the intracellular environment under certain conditions and may cause significant cellular damage. They are involved in the pathogenesis of a variety of diseases, including cardiovascular disorders, chronic inflammation, diabetes, or neurodegenerative disorders. In ophthalmology, they are linked to a series of conditions like cataract, macular degeneration, or retinopathy, making them attractive pharmacological targets. Therefore, antioxidant molecules capable of correcting the oxidative stress imbalance are becoming increasingly crucial to the prevention and treatment of various eye diseases [7]. Although important information regarding the molecular mechanisms of action of antioxidants has emerged from experimental studies, further research is necessary to clarify their complex biological effects (Figure 2).
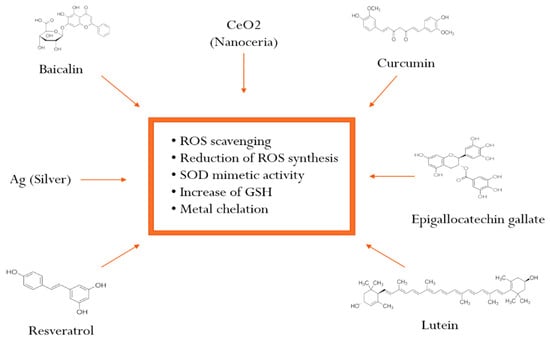
Figure 2.
Mechanisms of action of antioxidants entrapped in ophthalmic nanosystems (Abbreviations: ROS: reactive oxygen species; SOD: superoxide dismutase; GSH: glutathione).
3.4.1. Baicalin
Baicalin is a flavonoid from the plant species Scutellaria baicalensis, a herbal remedy used for millennia in traditional Chinese medicine. A recent study showed significant antioxidant and anti-inflammatory properties of baicalin but also consistent antiviral effects [52]. In a recent study, solid lipid nanoparticles with baicalin proved to be an effective ROS scavenger by augmenting superoxide dismutase (SOD) activity, as well as the level of glutathione [47].
3.4.2. Cerium
Cerium is a rare earth element that can exist both in +3 and +4 oxidation states, with important antioxidant enzyme-mimetic activity, as well as ROS and RNS scavenging capability, especially in its nanoparticulated form, nanoceria. The scavenging properties of nanoceria are augmented if the size of the nanoparticles decreases, with the smaller particles being extremely reactive. The unique chemical, biological, and surface properties of nanoceria make them essential in several economic sectors like metallurgy or the oil industry, but they are also attractive for a variety of biomedical applications including ophthalmology [53].
Korsvik et al. [54] demonstrated the SOD-mimetic activity of nanoceria synthesized with a higher Ce3+/Ce4+ surface ratio which favored the catalytic degradation of superoxide anion. Further, Rzigalinski et al. [55] showed catalase (CAT)-mimetic activity due to the regenerative nature of the Ce3+/Ce4+ redox couple on the nanoparticle surface.
In addition, several studies demonstrated a significant reduction of nitrosative stress both in vitro and in vivo. Xue et al. [56] proved that nanoceria could effectively scavenge hydroxyl radicals in vitro more effectively at higher Ce3+/Ce4+ surface ratios. Meanwhile, Zhang et al. [57] and Dowding et al. [58] showed a protective effect against the damage of cellular structures caused by aggressive free radicals ·OH and ·NO.
3.4.3. Curcumin
Curcumin is a water-insoluble pigment extracted from Curcuma longa (turmeric), a plant species extensively used as a spice in South-East Asia. In recent years, extensive research confirmed that curcumin could downregulate genes involved in uncontrolled cellular proliferation by inhibiting nuclear factor Kappa B (NF-kB) activation. In addition, curcumin reduces the synthesis of free radicals; therefore, it has antioxidant properties. In ophthalmology, curcumin showed beneficial effects in dry eye syndrome and some forms of retinopathy due to its inhibition of interleukin (IL)-1 beta production and c-Jun N-terminal kinase (JNK) activation [59].
In another study, curcumin was able to reduce the progression of retinopathy in diabetic mice treated with streptozotocin by inhibiting the expression of vascular endothelial growth factor (VEGF). The anti-angiogenic properties of curcumin could be useful not only in retinopathies but also in age-related macular degeneration [60].
3.4.4. Epigallocatechin Gallate (EGCG)
Epigallocatechin gallate (EGCG) is the most abundant and potent catechin of green tea. Chemically, it is a polyphenolic compound that is able to successfully scavenge reactive oxygen species (ROS) due to the presence of the galloyl group on the B and D rings. It inactivates superoxide anion via a reaction leading to the oxidation of the D ring. EGCG may also inhibit lipid peroxidation and damage to ATPases, but it can also decrease the formation of hydrogen peroxide and damage to DNA caused by UVB irradiation [61].
EGCG can also indirectly act as an antioxidant by increasing the level of phase II antioxidant enzymes like superoxide dismutase or glutathione peroxidase. New research claims that it can interact with signal transduction pathways and transcription factors, which can explain its complex effects [62].
3.4.5. Lutein
Lutein is a natural carotenoid pigment, uniquely concentrated in the lens where it can reduce the photochemical and oxidative damage by filtering high-energy UV light and by scavenging reactive oxygen species. Therefore, it has a potential role in the prevention of cataract [63].
Several in vitro studies have shown that in addition to quenching reactive oxygen species directly, lutein may prevent protein, lipid, or DNA from suffering oxidative damage. Several mechanisms are responsible for the protection against oxidative damage, including reduction of the level of H2O2-induced protein carbonyl and MDA, increase in the level of GSH, and an increase of the GSH:GSSG ratio [64].
In animal models of selenite and diabetes-induced cataract, lutein managed to slow down the cataract development, due to its capacity to inhibit lipid peroxidation [41,65]. In humans, a recently published meta-analysis demonstrated that increased blood concentrations of lutein and zeaxanthin might be associated with the reduced risk of nuclear cataract, but the efficacy is reduced by their low systemic bioavailability [66].
3.4.6. Resveratrol
Resveratrol is a natural polyphenolic compound present in a variety of plant species, especially in some grape varieties. Several studies have confirmed the anti-inflammatory, antitumoral, antiviral, and neuroprotective effects of this molecule. In addition, it is a powerful antioxidant that acts as an ROS scavenger but also as a metal chelator. Resveratrol can neutralize hydroxyl and hydroperoxyl radicals and can protect the cells against oxidative stress and prolong their survival. Moreover, indirect antioxidant effects are presumed for this compound by the modulation of cellular antioxidant pathways [67].
3.4.7. Silver
Silver ions are often used for medical purposes due to their antibacterial and immunomodulatory effects. Silver disrupts multiple bacterial processes, including disulfide bond formation and iron homeostasis. It can also expand the antibacterial spectrum of existing antibiotics [68]. In recent years, silver nanoparticles synthesized using vegetal extract proved to be powerful antioxidants. Das et al. demonstrated the clear ROS scavenging potential for silver nanoparticles synthesized with an extract from Ananas comosus but also nitric oxide scavenging activity [69].
4. Conclusions
Recently, there has been growing interest in the development of nanosystems loaded with antioxidants for ophthalmic use. Several nanosystems have been studied preclinically in vivo, with good results, and the properties of the formulations have been optimized to achieve some significant characteristics required by the ophthalmic tissues. The characteristics include pH, osmolality, and viscosity. Further development of the technology is still necessary to obtain safe, economical, and reproducible antioxidant-loaded nanosystems, capable of preventing or treating ophthalmic diseases.
Author Contributions
Conceptualization, E.D. and O.V.; methodology, O.V. and O.S.; software, E.B.; validation, E.D., O.V., and E.B.; formal analysis, O.S.; investigation, O.V.; resources, E.B.; data curation, E.D.; writing—original draft preparation, E.D. and O.V.; writing—review and editing, B.S.; visualization, E.B.; supervision, E.B.; project administration, E.B.; funding acquisition, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Blindness and Vision Impairment. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 8 October 2019).
- Contin, M.A.; Arietti, M.M.; Benedetto, M.M.; Bussi, C.; Guido, M.E. Photoreceptor damage induced by low-intensity light: Model of retinal degeneration in mammals. Mol. Vis. 2013, 19, 1614–1625. [Google Scholar] [PubMed]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 2014, 4, 5223. [Google Scholar] [CrossRef] [PubMed]
- Jaadane, I.; Boulenguez, P.; Chahory, S.; Carre, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Retinal damage induced by commercial light emitting diodes (LEDs). Free Radic. Biol. Med. 2015, 84, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Jaadane, I.; Villalpando Rodriguez, G.E.; Boulenguez, P.; Chahory, S.; Carre, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Effects of white light-emitting diode (LED) exposure on retinal pigment epithelium in vivo. J. Cell. Mol. Med. 2017, 21, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.-S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, S.B.; Borges, S.; Ramos, O.; Pintado, M.; Ferreira, D.; Sarmento, B. Treating retinopathies-nanotechnology as a tool in protecting antioxidants agents. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer Verlag: Berlin/Heidelberg, Germany, 2014; pp. 3539–3555. [Google Scholar]
- Brandstetter, C.; Mohr, L.K.M.; Latz, E.; Holz, F.G. Light induces NLRP3 inflammasome activation in retinal pigment epithelial cells via lipofuscin-mediated photooxidative damage. J. Mol. Med. 2015, 93, 905–916. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Fishkin, N.; Zhou, J.; Cai, B.; Jang, Y.P.; Krane, S.; Itagaki, Y.; Nakanishi, K. A2E, a byproduct of the visual cycle. Vis. Res. 2003, 43, 2983–2990. [Google Scholar] [CrossRef]
- Wihlmark, U.; Wrigstad, A.; Roberg, K.; Nilsson, S.E.; Brunk, U.T. Lipofuscin accumulation in cultured retinal pigment epithelial cells causes enhanced sensitivity to blue light irradiation. Free Radic. Biol. Med. 1997, 22, 1229–1234. [Google Scholar] [CrossRef]
- Marie, M.; Bigot, K.; Angebault, C.; Barrau, C.; Gondouin, P.; Pagan, D.; Fouquet, S.; Villette, T.; Sahel, J.-A.; Lenaers, G.; et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018, 9, 287. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier mediated retinal drug delivery: Overcoming ocular barriers to treat posterior eye diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1473. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.G. The blood-ocular barriers: Past, present and future. Doc. Ophthalmol. 1997, 93, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Rimpela, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef]
- Sahel, D.K.; Mittal, A.; Chitkara, D. CRISPR/Cas system for genome editing progress and prospects as a therapeutic tool. J. Pharmacol. Exp. Ther. 2019, 370, 725–735. [Google Scholar] [CrossRef]
- Ni, D.; Jiang, D.; Valdovinos, H.F.; Ehlerding, E.B.; Yu, B.; Barnhart, T.E.; Huang, P.; Cai, W. Bioresponsive polyoxometalate cluster for redox-activated photoacoustic imaging-guided photothermal cancer therapy. Nano Lett. 2017, 17, 3282–3289. [Google Scholar] [CrossRef]
- Li, S.; Jiang, D.; Ehlerding, E.B.; Rosenkrans, Z.T.; Engle, J.W.; Wang, Y.; Liu, H.; Ni, D.; Cai, W. Intrathecal administration of nanoclusters for protecting neurons against oxidative stress in cerebral ischemia/reperfusion injury. ACS Nano 2019, 13, 13382–13389. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, J.; Kim, J.; Kim, Y.; Song, H.B.; Kim, J.H.; Kim, K.; Kim, W.J. Light-induced acid generation on a gatekeeper for smart nitric oxide delivery. ACS Nano 2016, 10, 4199–4208. [Google Scholar] [CrossRef]
- Jeong, H.; Park, J.-H.; Shin, J.H.; Yoo, J.-C.; Park, C.Y.; Hong, J.P. Prolonged release period of nitric oxide gas for treatment of bacterial keratitis by amine-rich polymer decoration of nanoparticles. Chem. Mater. 2018, 30, 8528–8537. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Li, X.; Zhang, G.; Zhang, G.; Hu, J. Light-triggered nitric oxide (NO) release from photoresponsive polymersomes for corneal wound healing. Chem. Sci. 2020, 11, 186–194. [Google Scholar] [CrossRef]
- Giri, T.K.; Choudhary, C.; Ajazuddin, A.; Hemant, B.; Dulal, K.T. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharm. J. 2013, 21, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Alonso, M.J.; Vila-Jato, J.L.; Robinson, J.R. Improved ocular bioavailability of indomethacin by novel ocular drug carriers. J. Pharm. Pharmacol. 1996, 48, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Qaddoumi, M.G.; Ueda, H.; Yang, J.; Davda, J.; Labhasetwar, V.; Lee, V.H. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm. Res. 2004, 21, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A. Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, X.; Wu, H.; Li, J.; Shu, L.; Liu, R.; Li, L.; Li, N. Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo. Drug Dev. Ind. Pharm. 2011, 37, 475–481. [Google Scholar] [CrossRef]
- Ashraf, O.; Nasr, M.; Nebsen, M.; Said, A.M.A.; Sammour, O. In vitro stabilization and in vivo improvement of ocular pharmacokinetics of the multi-therapeutic agent baicalin: Delineating the most suitable vesicular systems. Int. J. Pharm. 2018, 539, 83–94. [Google Scholar] [CrossRef]
- Yang, J.; Gong, X.; Fang, L.; Fan, Q.; Cai, L.; Qiu, X.; Zhang, B.; Chang, J.; Lu, Y. Potential of CeCl3@mSiO2 nanoparticles in alleviating diabetic cataract development and progression. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1147–1155. [Google Scholar] [CrossRef]
- Kyosseva, S.V.; McGinnis, J.F. Cerium oxide nanoparticles as promising ophthalmic therapeutics for the treatment of retinal diseases. World J. Ophthalmol. 2015, 5, 23–30. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Jiang, T.; Zheng, L.; Wang, Z.; Zhang, J.; Yu, P. Protective effect of Coenzyme Q10 against oxidative damage in human lens epithelial cells by novel ocular drug carriers. Int. J. Pharmaceut. 2011, 403, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Maharjan, P.; Jin, M.; Park, T.; Maharjan, A.; Amatya, R.; Yang, J.; Min, K.A.; Shin, M.C. Potential albumin-based antioxidant nanoformulations for ocular protection against oxidative stress. Pharmaceutics 2019, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Wang, M.-C.; Chen, Z.-Y.; Chiu, W.-Y.; Chen, K.-H.; Lin, I.-C.; Yang, W.-C.V.; Wu, C.-C.; Tseng, C.-L. Gelatin-epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 31, 7251–7273. [Google Scholar] [CrossRef] [PubMed]
- Djagny, V.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Andreani, T.; Fernandes, L.; Garcia, M.L.; Egea, M.A.; Silva, A.M.; Souto, E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces 2014, 123, 452–460. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of arginine-glycine-aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization. Int. J. Nanomed. 2017, 12, 279–294. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.H.; Li, F.; Peng, G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B. Biol. 2019, 195, 51–57. [Google Scholar] [CrossRef]
- Liu, C.H.; Chiu, H.C.; Wu, W.C.; Sahoo, S.L.; Hsu, C.Y. Novel Lutein Loaded Lipid Nanoparticles on Porcine Corneal Distribution. J. Ophthalmol. 2014, 2014, 304694. [Google Scholar] [CrossRef]
- Bodoki, E.; Vostinaru, O.; Samoila, O.; Dinte, E.; Bodoki, A.E.; Swetledge, S.; Astete, C.E.; Sabliov, C.M. Topical nanodelivery system of lutein for the prevention of selenite-induced cataract. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 188–197. [Google Scholar] [CrossRef]
- Natesan, S.; Pandian, S.; Ponnusamy, C.; Palanichamy, R.; Muthusamy, S.; Kandasamy, R. Co-encapsulated resveratrol and quercetin in chitosan and PEG modified chitosan nanoparticles: For efficient intraocular pressure reduction. Int. J. Biol. Macromol. 2017, 104, 1837–1845. [Google Scholar] [CrossRef]
- Anbukkarasi, M.; Thomas, P.A.; Sheu, J.-R.; Geraldine, P. In vitro antioxidant and anticataractogenic potential of silver nanoparticles biosynthesized using an ethanolic extract of Tabernaemontana divaricata leaves. Biomed. Pharmacother. 2017, 91, 467–475. [Google Scholar] [CrossRef]
- Vicario-de-la-Torre, M.; Benítez-del-Castillo, J.M.; Vico, E.; Guzmán, M.; de-Las-Heras, B.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Design and characterization of an occular topical liposomal preparation to replenish the lipids of the tear film. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7839–7847. [Google Scholar] [CrossRef]
- He, W.; Guo, X.; Feng, M.; Mao, N. In vitro and in vivo studies on ocular vitamin A palmitate cationic liposomal in situ gels. Int. J. Pharm. 2013, 458, 305–314. [Google Scholar] [CrossRef]
- Hoyo, J.; Ivanova, K.; Guaus, E.; Tzanov, T. Multifunctional ZnO NPs-chitosan-gallic acid hybrid nanocoating to overcome contact lenses associated conditions and discomfort. J. Colloid Interface Sci. 2019, 543, 114–121. [Google Scholar] [CrossRef]
- Li, N.; Han, Z.; Li, L.; Zhang, B.; Liu, Z.; Li, J. The anti-cataract molecular mechanism study in selenium cataract rats for baicalin ophthalmic nanoparticles. Drug Des. Dev. Ther. 2018, 12, 1399–1411. [Google Scholar] [CrossRef]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef]
- Cai, X.; Sezate, S.A.; Seal, S.; McGinnis, J.F. Sustained protection against photoreceptor degeneration in tubby mice by intravitreal injection of nanoceria. Biomaterials 2012, 33, 8771–8781. [Google Scholar] [CrossRef]
- Zhou, X.; Wong, L.L.; Karakoti, A.S.; Seal, S.; McGinnis, J.F. Nanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the Vldr knockout mouse. PLoS ONE 2011, 6, e16733. [Google Scholar] [CrossRef]
- Kyosseva, S.V.; Chen, L.; Seal, S.; McGinnis, J.F. Nanoceria inhibit expression of genes associated with inflammation and angiogenesis in the retina of Vldr null mice. Exp. Eye Res. 2013, 116, 63–74. [Google Scholar] [CrossRef]
- Shi, H.; Ren, K.; Lu, B.; Zhang, W.; Zhao, Y.; Tan, R.X.; Li, E. Baicalin from Scutellaria baicalensis blocks respiratory syncitial virus (RSV) infections and reduces inflammatory infiltration and lung injury in mice. Sci. Rep. 2016, 6, 35851. [Google Scholar]
- Nelson, B.C.; Johnson, M.E.; Walker, M.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy. engineered ceria nanoparticles. Chem. Commun. 2007, 10, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Rzigalinski, B.A.; Meehan, K.; Davis, R.M.; Xu, Y.; Miles, W.C.; Cohen, C.A. Radical nanomedicine. Nanomedicine 2006, 1, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Luan, Q.F.; Yang, D.; Yao, X.; Zhou, K.B. Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J. Phys. Chem. C 2011, 115, 4433–4438. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, K.B.; Zhai, Y.W.; Qin, F.; Pan, L.L.; Yao, X. Crystal plane effects of nano-CeO2 on its. antioxidant activity. RSC Adv. 2014, 4, 50325–50330. [Google Scholar] [CrossRef]
- Dowding, J.M.; Dosani, T.; Kumar, A.; Seal, S.; Self, W.T. Cerium oxide nanoparticles scavenge nitric oxide radical ((no)-n-center dot). Chem. Commun. 2012, 48, 4896–4898. [Google Scholar] [CrossRef]
- Pescosolido, N.; Giannotti, R.; Plateroti, A.M.; Pascarella, A.; Nebbioso, M. Curcumin: Therapeutical Potential in Ophthalmology. Planta Med. 2014, 80, 249–254. [Google Scholar] [CrossRef]
- Mrudula, T.; Suryanarayana, P.; Srinivas, P.N.; Reddy, G.B. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem. Biophys. Res. Commun. 2007, 361, 528–532. [Google Scholar] [CrossRef]
- Wei, H.C.; Zhang, X.S.; Zhao, J.F.; Wang, Z.Y.; Bickers, D.; Lebwohl, M. Scavenging of hydrogen peroxide and inhibition of ultraviolet light-induced oxidative DNA damage by aqueous extracts from green and black teas. Free Radic. Biol. Med. 1999, 26, 1427–1435. [Google Scholar] [CrossRef]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the gren tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Krinsky, N.I. Possible biologic mechanisms for a protective role of xantophylls. J. Nutr. 2002, 132, 540S–542S. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.; Chen, C.Y.O.; Yeum, K.J.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar] [PubMed]
- Arnal, E.; Miranda, M.; Almansa, I.; Muriach, M.; Barcia, J.M.; Romero, F.J.; Diaz-Llopis, M.; Bosch-Morell, F. Lutein prevents cataract development and progression in diabetic rats. Graefes Arch. Clin. Exp. Ophtalmol. 2009, 247, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Yu, R.-B.; Liu, R.; Hao, Z.-X.; Han, C.-C.; Zhu, Z.-H.; Ma, L. Association between lutein and zeaxanthin status and the risk of cataract: A meta-analysis. Nutrients 2014, 6, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Tsouh Foukou, P.V.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against Gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.-S. Investigation of antioxidant, antibacterial, antidiabetic and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus. PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).