Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria
Abstract
:1. Introduction
2. Results
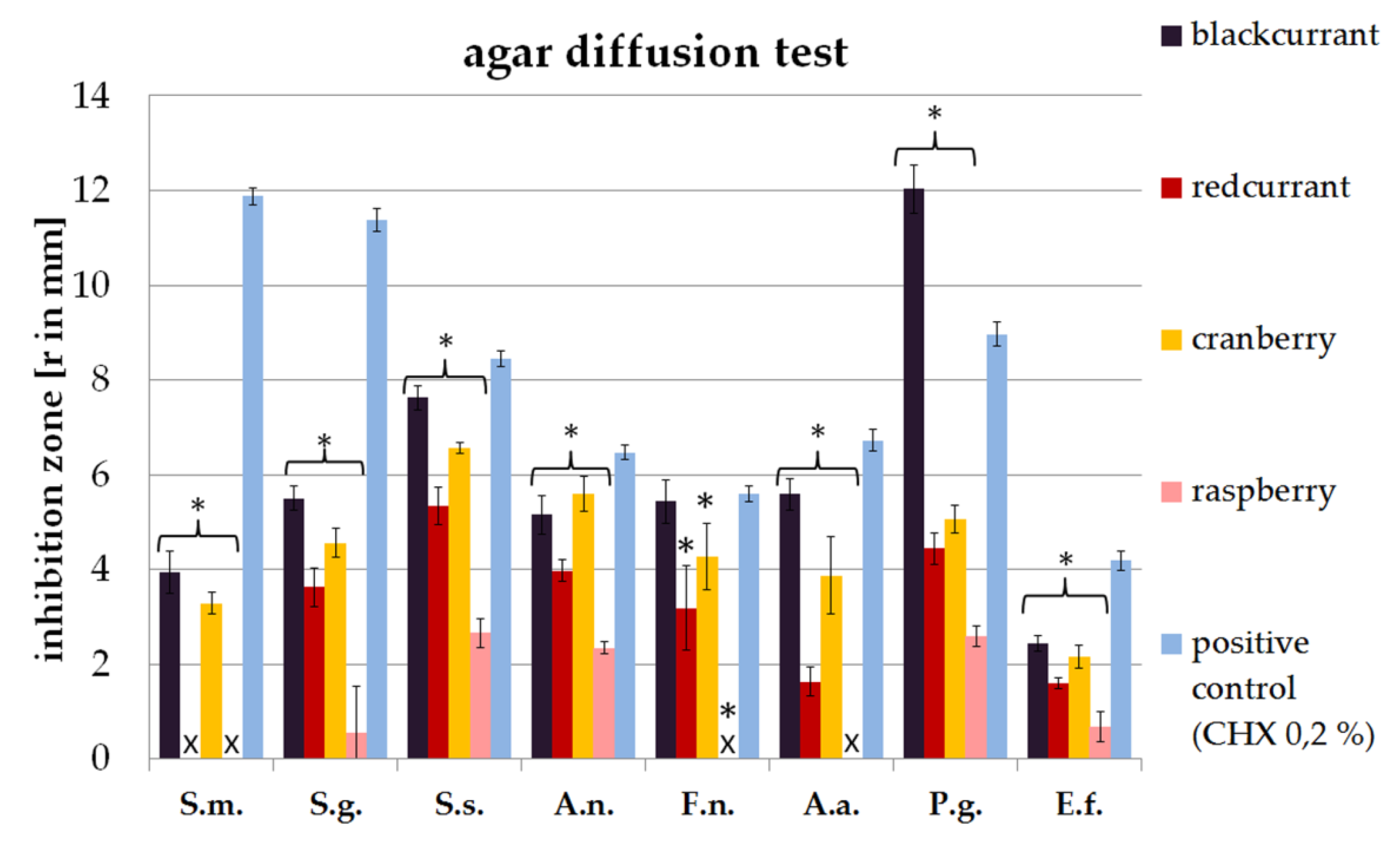
2.1. Agar Diffusion Assay
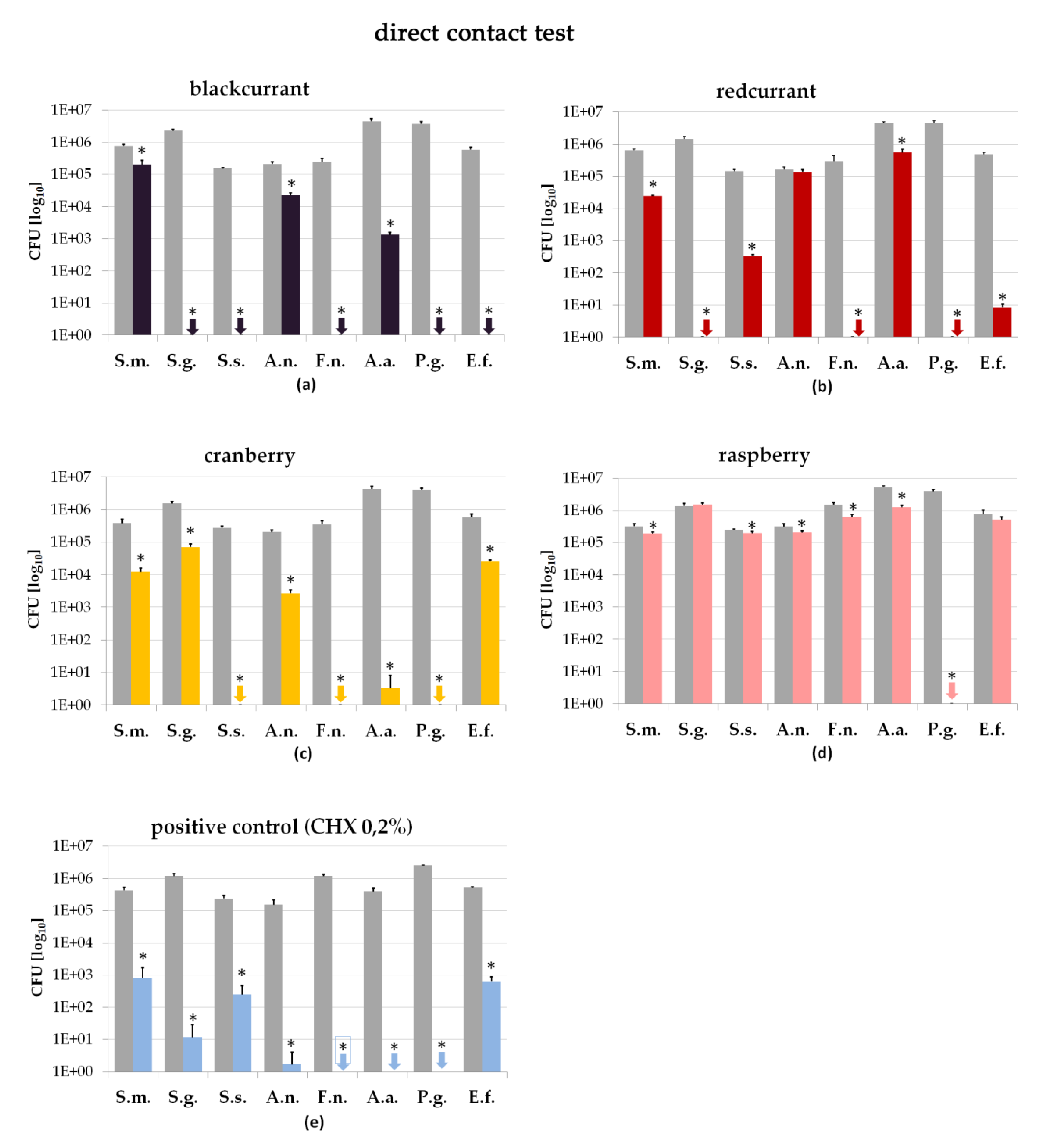
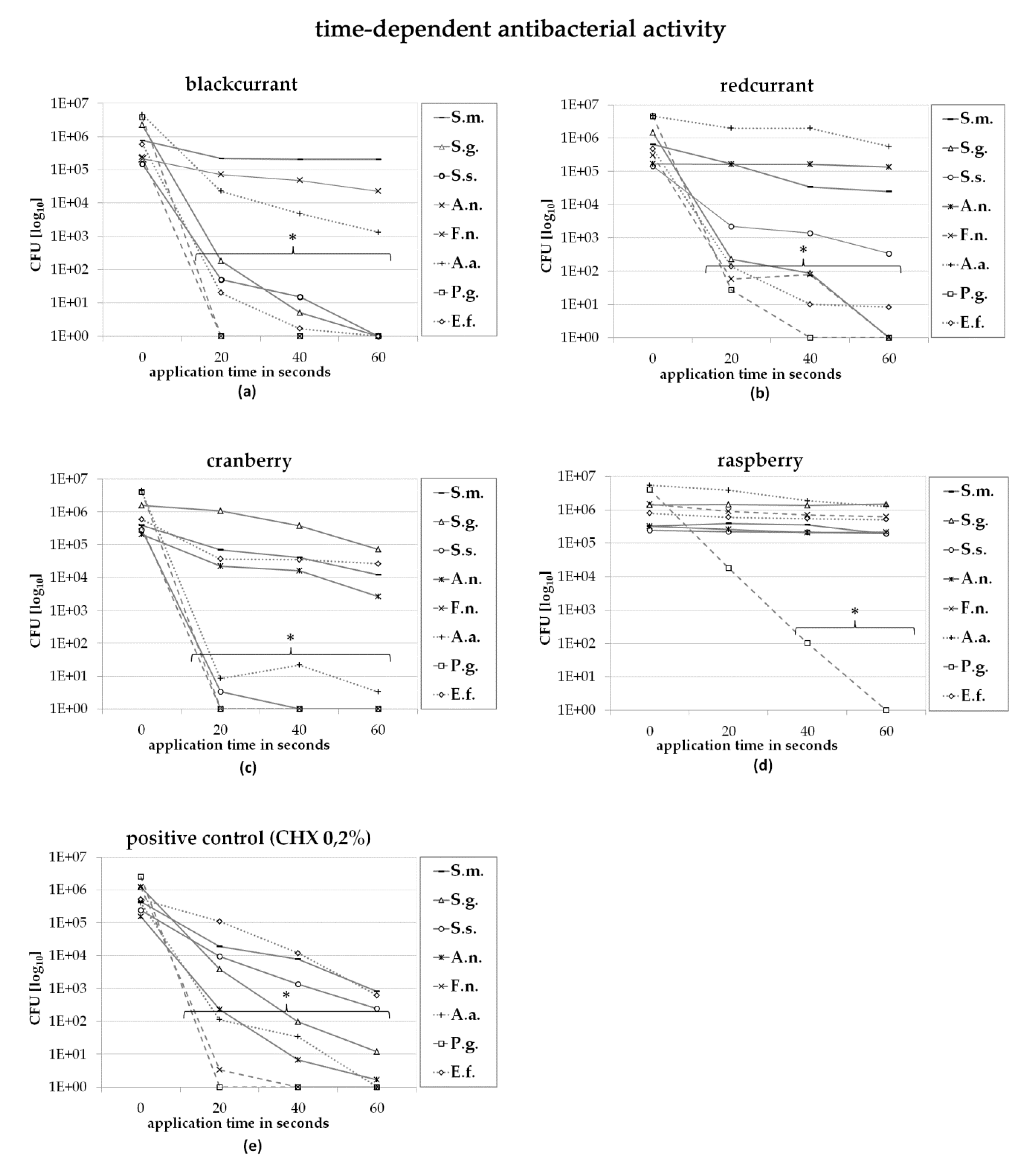
2.2. Direct Contact Test
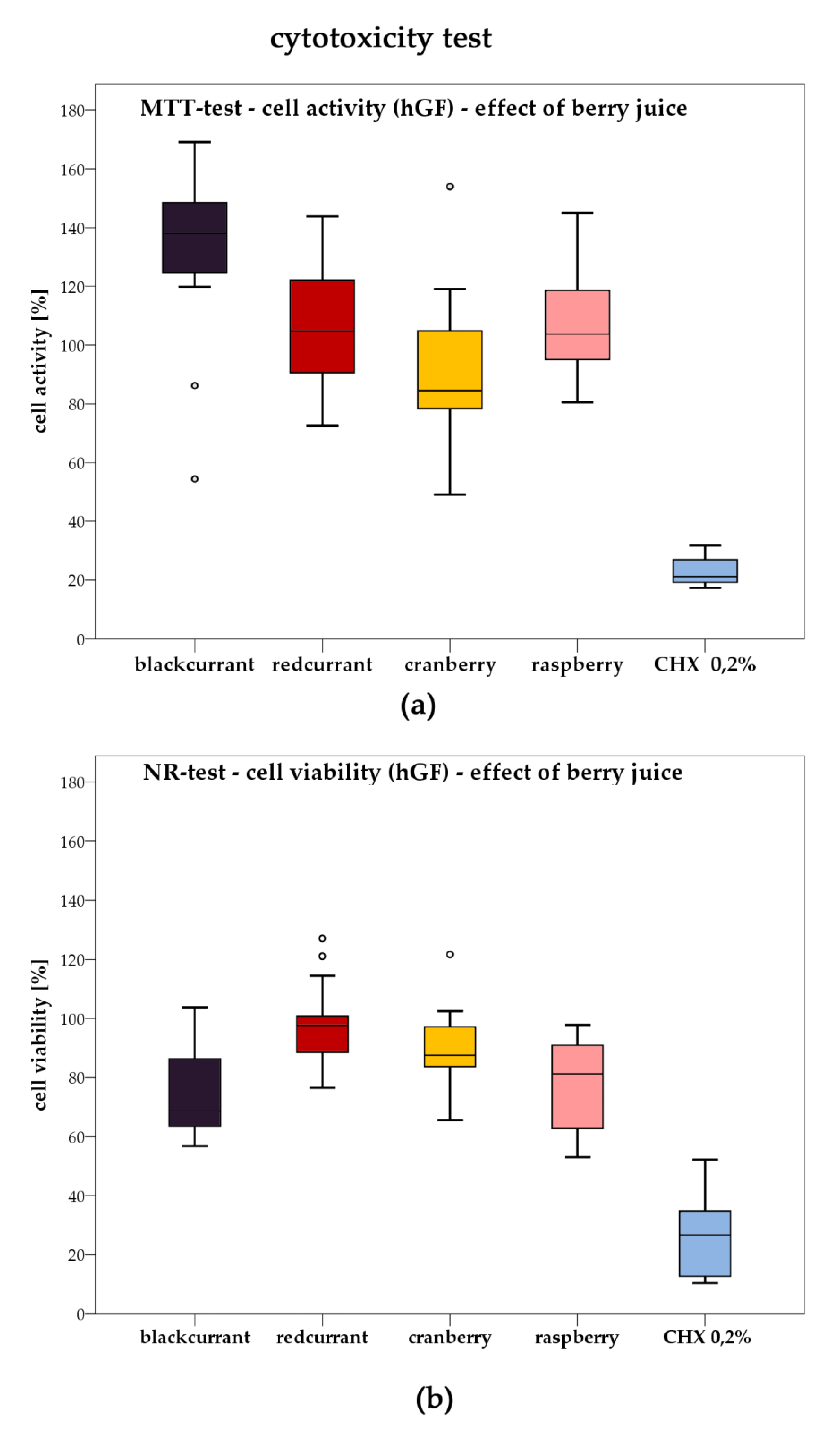
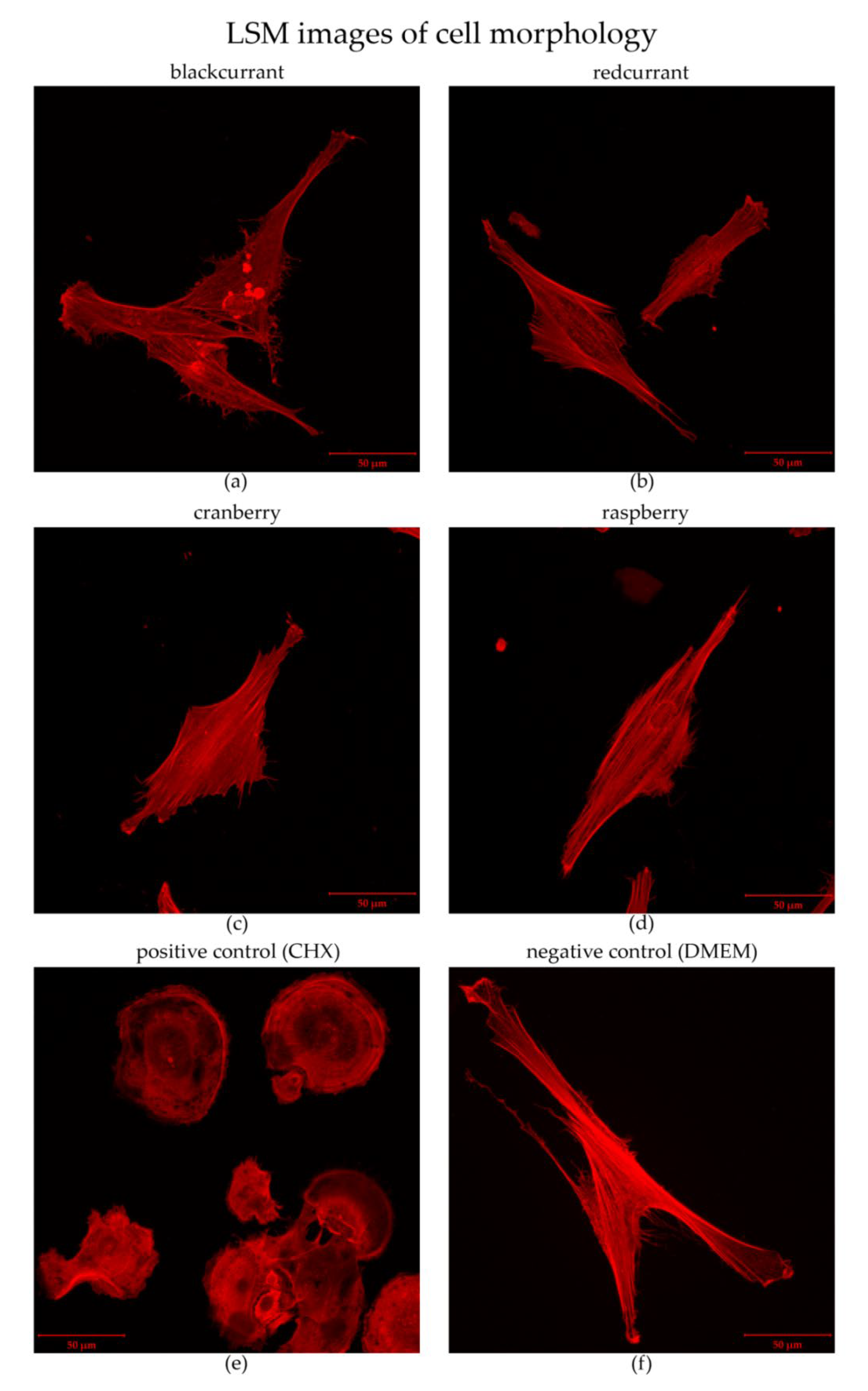
2.3. Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Preparation of Berry Juices
4.2. Bacterial Species
4.3. Agar Diffusion Assay
4.4. Direct Contact Test
4.5. Cytotoxicity Test
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cortez, R.E.; de Mejia, E.G. Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. J. Food Sci. 2019, 84, 2387–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of Flavonoid and Phenolic Antioxidants in Black Currants, Blueberries, Raspberries, Red Currants, and Cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Četojević-Simin, D.D.; Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Ćetković, G.S.; Šaponjac, V.T.T.; Vulić, J.; Čanadanović-Brunet, J.M.; Djilas, S.M. Bioactivity of Meeker and Willamette raspberry (Rubus idaeus L.) pomace extracts. Food Chem. 2015, 166, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Miladinović, B.; Kostic, M.; Šavikin, K.; Đorđević, B.; Mihajilov-Krstev, T.; Živanović, S.; Kitić, D. Chemical Profile and Antioxidative and Antimicrobial Activity of Juices and Extracts of 4 Black Currants Varieties (Ribes nigrum L.). J. Food Sci. 2014, 79, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, J.J. The world health report 2002—Reducing risks, promoting healthy life. Educ. Health (Abingdon) 2003, 16, 230. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant Properties and Phenolic Compounds of Vitamin C-Rich Juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef]
- Sigusch, B.W. The role of vitamin C (ascorbic acid) in the prevention and therapy of oral diseases. Arch. Oral Biol. 2013, 58, 905–906. [Google Scholar] [CrossRef]
- Staudte, H.; Güntsch, A.; Völpel, A.; Sigusch, B.W. Vitamin C attenuates the cytotoxic effects of Porphyromonas gingivalis on human gingival fibroblasts. Arch. Oral Biol. 2010, 55, 40–45. [Google Scholar] [CrossRef]
- Staudte, H.; Kranz, S.; Völpel, A.; Schütze, J.; Sigusch, B.W. Comparison of nutrient intake between patients with periodontitis and healthy subjects. Quintessence Int. 2012, 43, 907–916. [Google Scholar]
- Staudte, H.; Sigusch, B.W.; Glockmann, E. Grapefruit consumption improves vitamin C status in periodontitis patients. Br. Dent. J. 2005, 199, 213–217. [Google Scholar] [CrossRef]
- Tada, A.; Miura, H. The Relationship between Vitamin C and Periodontal Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef] [Green Version]
- Bishayee, A.; Haznagy-Radnai, E.; Mbimba, T.; Sipos, P.; Morazzoni, P.; Darvesh, A.S.; Bhatia, D.; Hohmann, J. Anthocyanin-rich blackcurrant extract suppresses the growth of human hepatocellular carcinoma cells. Nat. Prod. Commun. 2010, 5, 1613–1618. [Google Scholar] [PubMed] [Green Version]
- He, X.; Liu, R.H. Cranberry Phytochemicals: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidant Activities. J. Agric. Food Chem. 2006, 54, 7069–7074. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.; Bandara, H.; Leishman, S.J.; Walsh, L.J. Inhibitory effects of fruit berry extracts on Streptococcus mutans biofilms. Eur. J. Oral Sci. 2019, 127, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Franco, C.M.; Vazquez, B.I. Natural Compounds as Antimicrobial Agents. Antibiotics 2020, 9, 217. [Google Scholar] [CrossRef]
- Puupponen-Pimiaä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Maatta-Riihinen, K.; Oksman-Caldentey, K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef]
- Weiss, E.I.; Kozlovsky, A.; Steinberg, D.; Lev-Dor, R.; Greenstein, R.B.N.; Feldman, M.; Sharon, N.; Ofek, I. A high molecular mass cranberry constituent reduces mutans streptococci level in saliva and inhibits in vitro adhesion to hydroxyapatite. FEMS Microbiol. Lett. 2004, 232, 89–92. [Google Scholar] [CrossRef]
- Weiss, E.I.; Lev-Dor, R.; Sharon, N.; Ofek, I. Inhibitory Effect of a High-Molecular-Weight Constituent of Cranberry on Adhesion of Oral Bacteria. Crit. Rev. Food Sci. Nutr. 2002, 42, 285–292. [Google Scholar] [CrossRef]
- Bansal, K.; Marwaha, M.; Gupta, A. Effect of high-molecular-weight component of Cranberry on plaque and salivary Streptococcus mutans counts in children: An in vivo study. J. Indian Soc. Pedod. Prev. Dent. 2015, 33, 128. [Google Scholar] [CrossRef]
- Ambrosio, R.L.; Gratino, L.; Mirino, S.; Cocca, E.; Pollio, A.; Anastasio, A.; Palmieri, G.; Balestrieri, M.; Genovese, A.; Gogliettino, M. The Bactericidal Activity of Protein Extracts from Loranthus europaeus Berries: A Natural Resource of Bioactive Compounds. Antibiotics 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Tarce, M.; Lodi, G.; Carrassi, A. Chlorhexidine (CHX) in dentistry: State of the art. Minerva Stomatol. 2012, 61, 399–419. [Google Scholar] [PubMed]
- Van Maanen-Schakel, N.W.; Slot, D.E.; Bakker, E.W.; van der Weijden, G.A. The effect of an oxygenating agent on chlorhexidine-induced extrinsic tooth staining: A systematic review. Int. J. Dent. Hyg. 2012, 10, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannelli, M.; Chellini, F.; Margheri, M.; Tonelli, P.; Tani, A. Effect of chlorhexidine digluconate on different cell types: A molecular and ultrastructural investigation. Toxicol. Vitro 2008, 22, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Biva, I.J.; Ndi, C.P.; Semple, S.J.; Griesser, H.J. Antibacterial Performance of Terpenoids from the Australian Plant Eremophila lucida. Antibiotics 2019, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Milia, E.; Usai, M.; Szotáková, B.; Elstnerová, M.; Králova, V.; D’Hallewin, G.; Spissu, Y.; Barberis, A.; Marchetti, M.; Bortone, A.; et al. The Pharmaceutical Ability of Pistacia lentiscus L. Leaves Essential Oil Against Periodontal Bacteria and Candida sp. and Its Anti-Inflammatory Potential. Antibiotics 2020, 9, 281. [Google Scholar] [CrossRef]
- Yano, A.; Kikuchi, S.; Takahashi, T.; Kohama, K.; Yoshida, Y. Inhibitory effects of the phenolic fraction from the pomace of Vitis coignetiae on biofilm formation by Streptococcus mutans. Arch. Oral Biol. 2012, 57, 711–719. [Google Scholar] [CrossRef]
- Ikuta, K.; Hashimoto, K.; Kaneko, H.; Mori, S.; Ohashi, K.; Suzutani, T. Anti-viral and anti-bacterial activities of an extract of blackcurrants (Ribes nigrum L.). Microbiol. Immunol. 2012, 56, 805–809. [Google Scholar] [CrossRef]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef] [Green Version]
- Laplante, K.L.; Sarkisian, S.A.; Woodmansee, S.; Rowley, D.C.; Seeram, N.P. Effects of Cranberry Extracts on Growth and Biofilm Production of Escherichia coli and Staphylococcus species. Phytother. Res. 2012, 26, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Cesoniene, L.; Jasutiene, I.; Sarkinas, A. Phenolics and anthocyanins in berries of European cranberry and their antimicrobial activity. Medicina 2009, 45, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitão, D.P.; Polizello, A.C.; Ito, I.Y.; Spadaro, A.C. Antibacterial Screening of Anthocyanic and Proanthocyanic Fractions from Cranberry Juice. J. Med. Food 2005, 8, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, J.; Bodet, C.; Chandad, F.; Grenier, D. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J. Antimicrob. Chemother. 2006, 58, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Weiss, E.I.; Lev-Dor, R.O.; Kashamn, Y.; Goldhar, J.; Sharon, N.; Ofek, I. Inhibiting Interspecies Coaggregation of Plaque Bacteria with A Cranberry Juice Constituent. J. Am. Dent. Assoc. 1998, 129, 1719–1723. [Google Scholar] [CrossRef]
- Cavanagh, H.M.; Hipwell, M.; Wilkinson, J.M. Antibacterial Activity of Berry Fruits Used for Culinary Purposes. J. Med. Food 2003, 6, 57–61. [Google Scholar] [CrossRef]
- Paredes-López, O.; Cervantes-Ceja, M.L.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving Human Health and Healthy Aging, and Promoting Quality Life—A Review. Plant Foods Hum. Nutr. 2010, 65, 299–308. [Google Scholar] [CrossRef]
- Puupponen-Pimiaä, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Dastidar, S.G.; Kristiansen, J.E.; Molnar, J.; Amaral, L. Role of Phenothiazines and Structurally Similar Compounds of Plant Origin in the Fight against Infections by Drug Resistant Bacteria. Antibiotics 2013, 2, 58–72. [Google Scholar] [CrossRef] [Green Version]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; de Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno-Silva, B.; de Alencar, S.M.; Koo, H.; Ikegaki, M.; da Silva, G.V.; Napimoga, M.H.; Rosalen, P.L. Anti-Inflammatory and Antimicrobial Evaluation of Neovestitol and Vestitol Isolated from Brazilian Red Propolis. J. Agric. Food Chem. 2013, 61, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Papetti, A.; Grisoli, P.; Aceti, C.; Dacarro, C.; Gazzani, G. Antibacterial Activity of Red and White Wine against Oral Streptococci. J. Agric. Food Chem. 2007, 55, 5038–5042. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.; Urnezis, P.; Tian, M. Compressed Mints and Chewing Gum Containing Magnolia Bark Extract Are Effective against Bacteria Responsible for Oral Malodor. J. Agric. Food Chem. 2007, 55, 9465–9469. [Google Scholar] [CrossRef] [PubMed]
- Papetti, A.; Pruzzo, C.; Daglia, M.; Grisoli, P.; Bacciaglia, A.; Repetto, B.; Dacarro, C.; Gazzani, G. Effect of Barley Coffee on the Adhesive Properties of Oral Streptococci. J. Agric. Food Chem. 2007, 55, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Stauder, M.; Papetti, A.; Mascherpa, D.; Schito, A.M.; Gazzani, G.; Pruzzo, C.; Daglia, M. Antiadhesion and Antibiofilm Activities of High Molecular Weight Coffee Components against Streptococcus mutans. J. Agric. Food Chem. 2010, 58, 11662–11666. [Google Scholar] [CrossRef]
- Choi, H.-A.; Cheong, D.-E.; Lim, H.-D.; Kim, W.-H.; Ham, M.-H.; Oh, M.-H.; Wu, Y.; Shin, H.-J.; Kim, G.-J. Antimicrobial and Anti-Biofilm Activities of the Methanol Extracts of Medicinal Plants against Dental Pathogens Streptococcus mutans and Candida albicans. J. Microbiol. Biotechnol. 2017, 27, 1242–1248. [Google Scholar] [CrossRef]
- Saquib, S.A.; Alqahtani, N.A.; Ahmad, I.; Kader, M.A.; Al Shahrani, S.S.; Asiri, E.A. Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal pathobionts: An in vitro Microbiological Study. Antibiotics 2019, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Bajrami, D.; Hoxha, V.; Gorduysus, O.; Muftuoglu, S.; Zeybek, N.D.; Küçükkaya, S. Cytotoxic effect of endodontic irrigants in vitro. Med. Sci. Monit. Basic Res. 2014, 20, 22. [Google Scholar] [CrossRef] [Green Version]
- Faria, G.; Cardoso, C.R.; Larson, R.E.; Silva, J.S.; Rossi, M.A. Chlorhexidine-induced apoptosis or necrosis in L929 fibroblasts: A role for endoplasmic reticulum stress. Toxicol. Appl. Pharmacol. 2009, 234, 256–265. [Google Scholar] [CrossRef]
| Concentration-Dependent Antibacterial Activity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Test Substance | S.m. | S.g. | S.s. | A.n. | F.n. | A.a. | P.g. | E.f. |
| 90% concentration | ||||||||
| blackcurrant | - | ++ | ++ | - | ++ | + | ++ | ++ |
| redcurrant | - | ++ | - | - | ++ | - | ++ | + |
| cranberry | - | - | ++ | - | ++ | + | ++ | - |
| raspsberry | - | - | - | - | - | - | ++ | - |
| positive control (CHX 0.2%) | - | + | - | + | ++ | ++ | ++ | - |
| 50% concentration | ||||||||
| blackcurrant | - | - | - | - | + | - | ++ | + |
| redcurrant | - | - | - | - | - | - | - | + |
| cranberry | - | - | - | - | + | - | ++ | - |
| raspsberry | - | - | - | - | - | - | - | - |
| positive control (CHX 0.2%) | - | + | - | + | ++ | + | ++ | - |
| 20% concentration | ||||||||
| blackcurrant | - | - | - | - | - | - | - | - |
| redcurrant | - | - | - | - | - | - | - | - |
| cranberry | - | - | - | - | - | - | - | - |
| raspsberry | - | - | - | - | - | - | - | - |
| positive control (CHX 0.2%) | - | + | - | - | + | + | ++ | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranz, S.; Guellmar, A.; Olschowsky, P.; Tonndorf-Martini, S.; Heyder, M.; Pfister, W.; Reise, M.; Sigusch, B. Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics 2020, 9, 533. https://doi.org/10.3390/antibiotics9090533
Kranz S, Guellmar A, Olschowsky P, Tonndorf-Martini S, Heyder M, Pfister W, Reise M, Sigusch B. Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics. 2020; 9(9):533. https://doi.org/10.3390/antibiotics9090533
Chicago/Turabian StyleKranz, Stefan, André Guellmar, Philipp Olschowsky, Silke Tonndorf-Martini, Markus Heyder, Wolfgang Pfister, Markus Reise, and Bernd Sigusch. 2020. "Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria" Antibiotics 9, no. 9: 533. https://doi.org/10.3390/antibiotics9090533
APA StyleKranz, S., Guellmar, A., Olschowsky, P., Tonndorf-Martini, S., Heyder, M., Pfister, W., Reise, M., & Sigusch, B. (2020). Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics, 9(9), 533. https://doi.org/10.3390/antibiotics9090533






