Antimicrobial Property and Mode of Action of the Skin Peptides of the Sado Wrinkled Frog, Glandirana susurra, against Animal and Plant Pathogens
Abstract
1. Introduction
2. Results
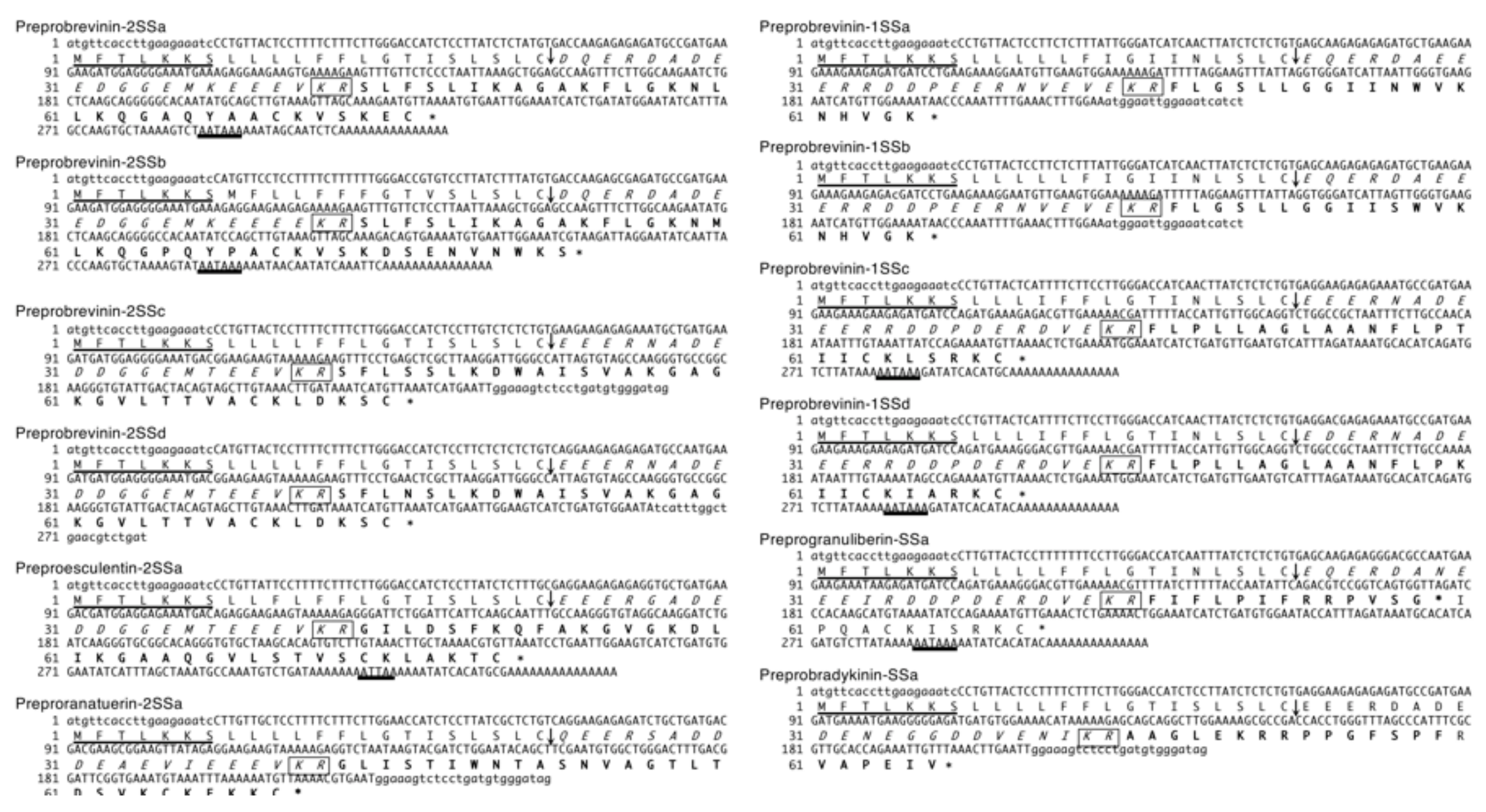
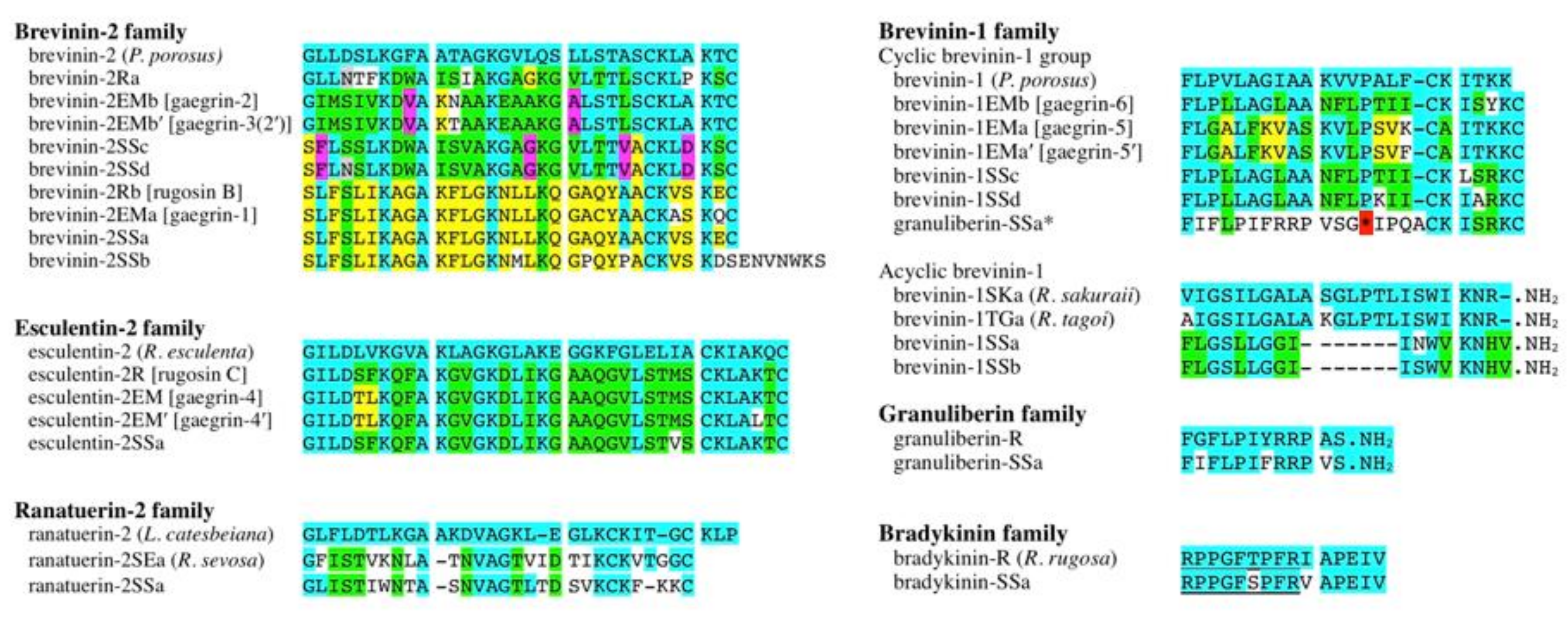
2.1. Nucleotide and Amino Acid Sequence Analyses
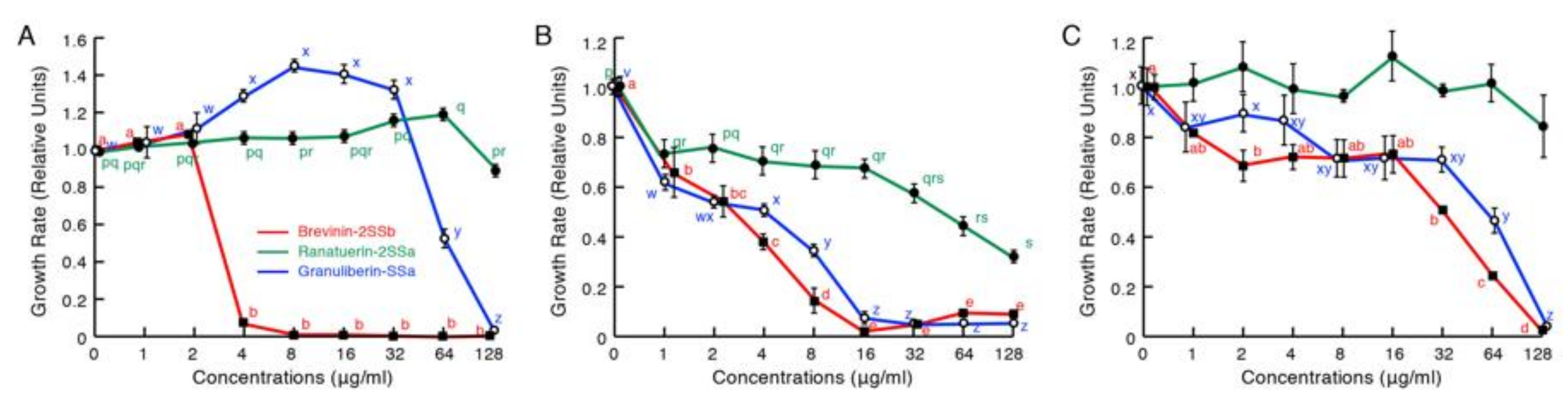
2.2. Antimicrobial Activity Assays
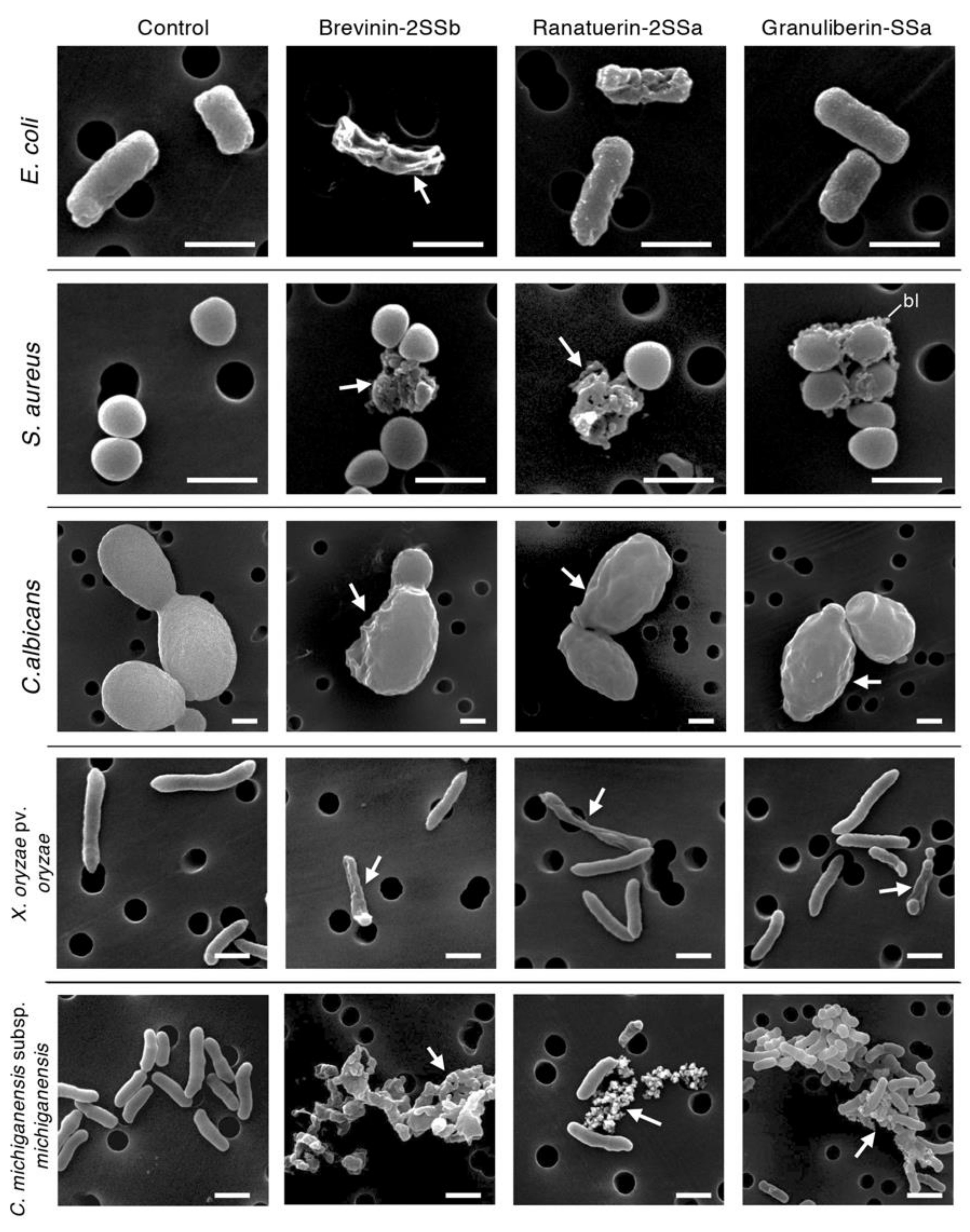
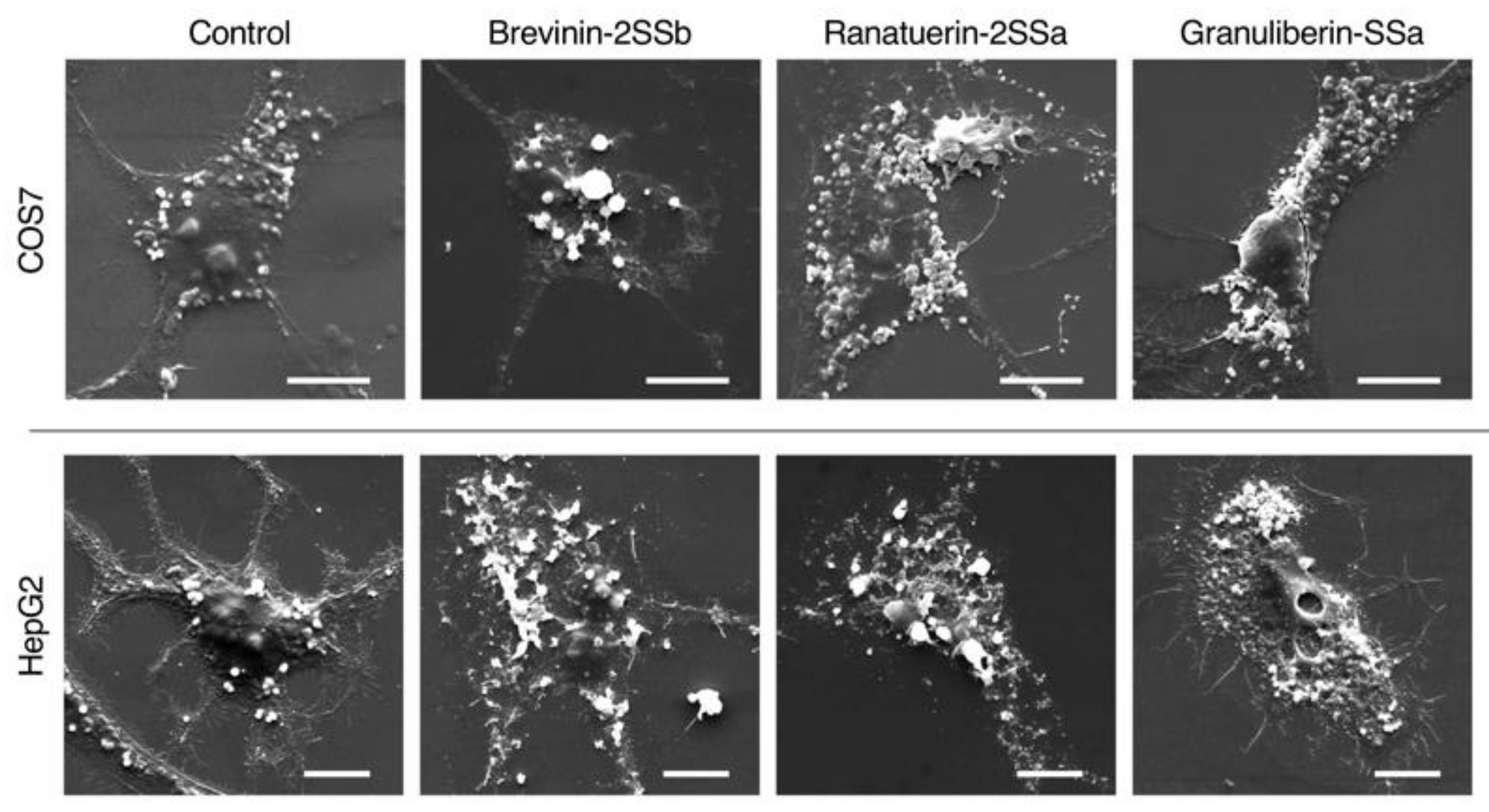
2.3. Morphological Observations
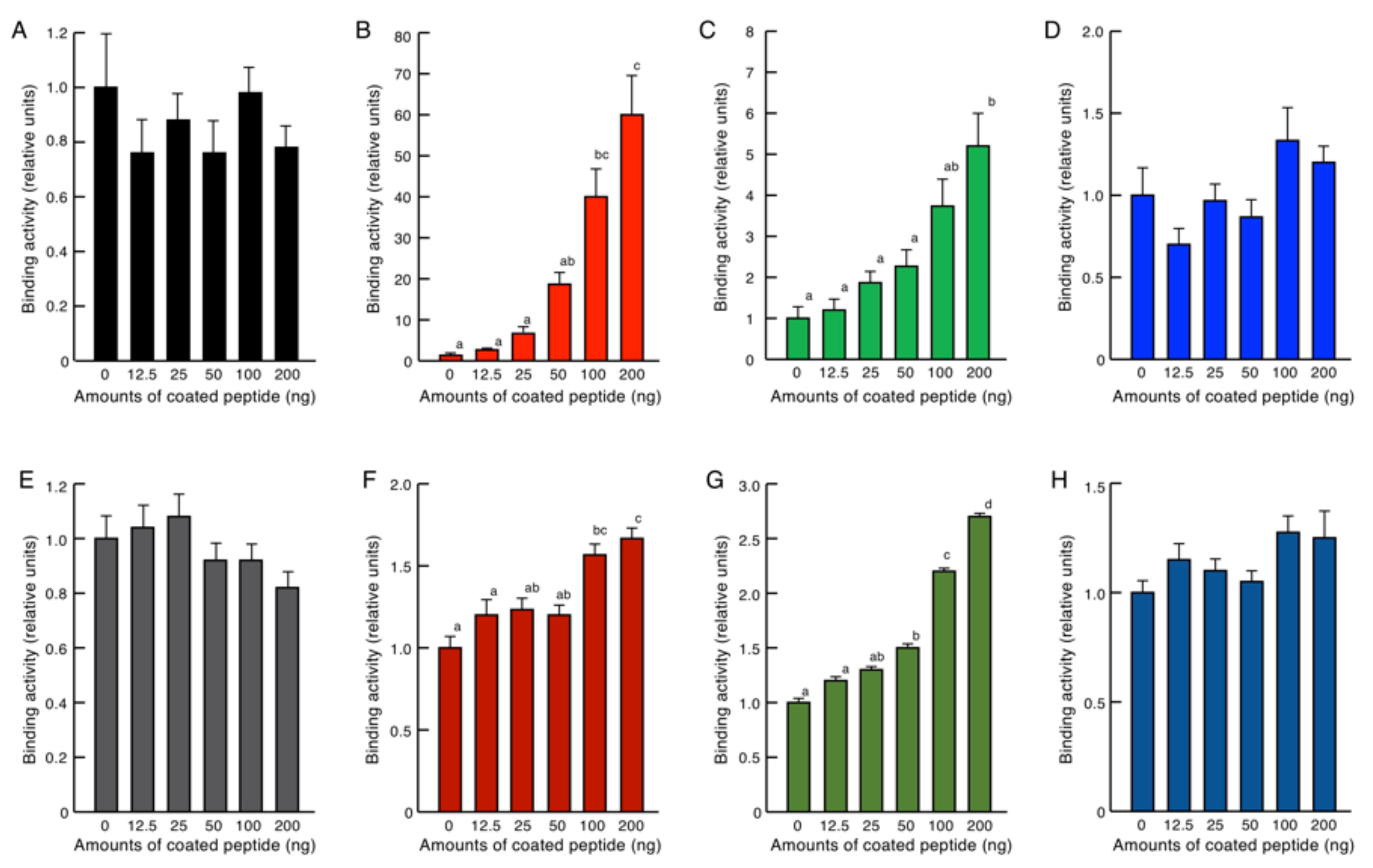
2.4. Endotoxin Binding Assay
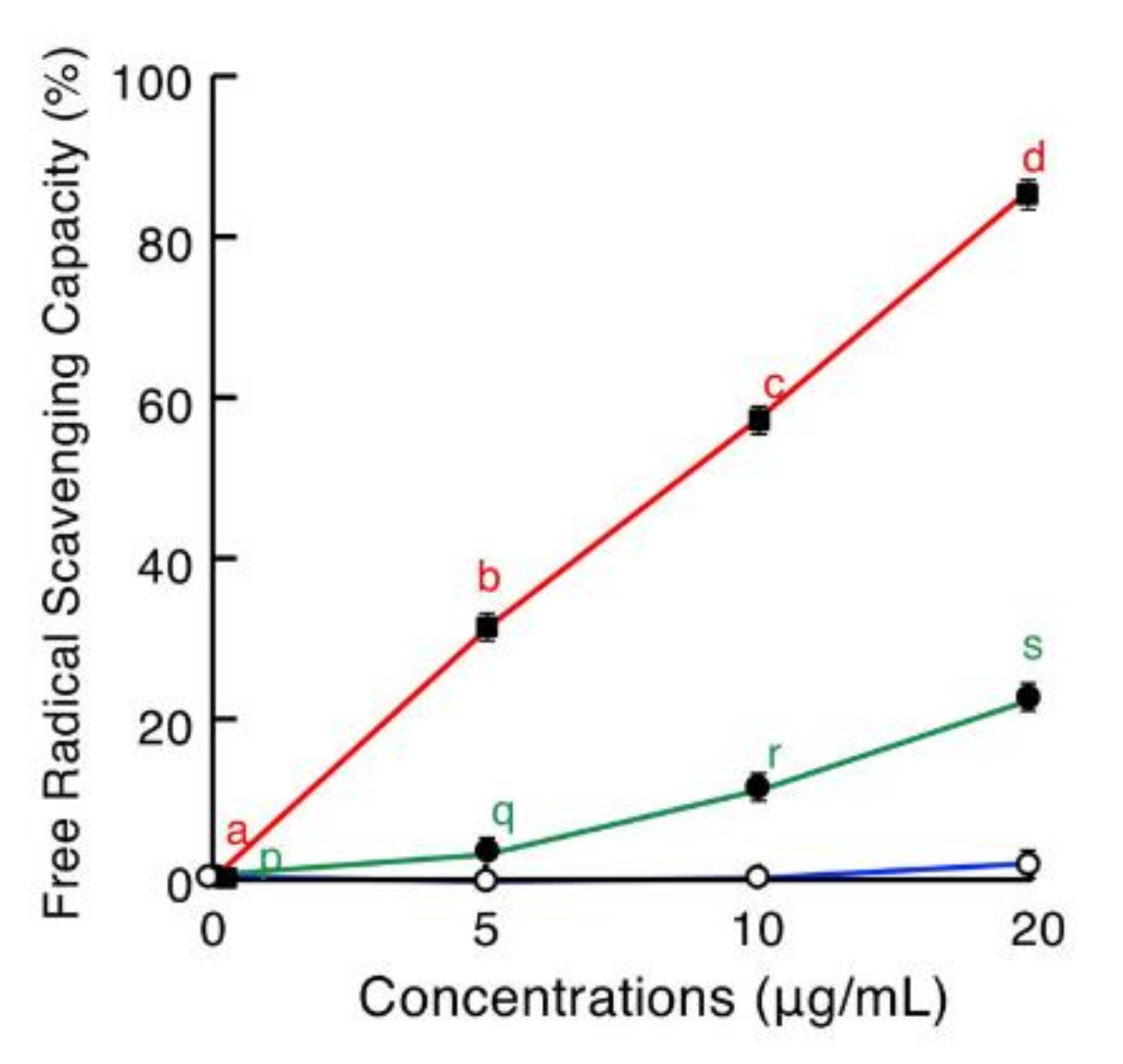
2.5. Antioxidative Assay
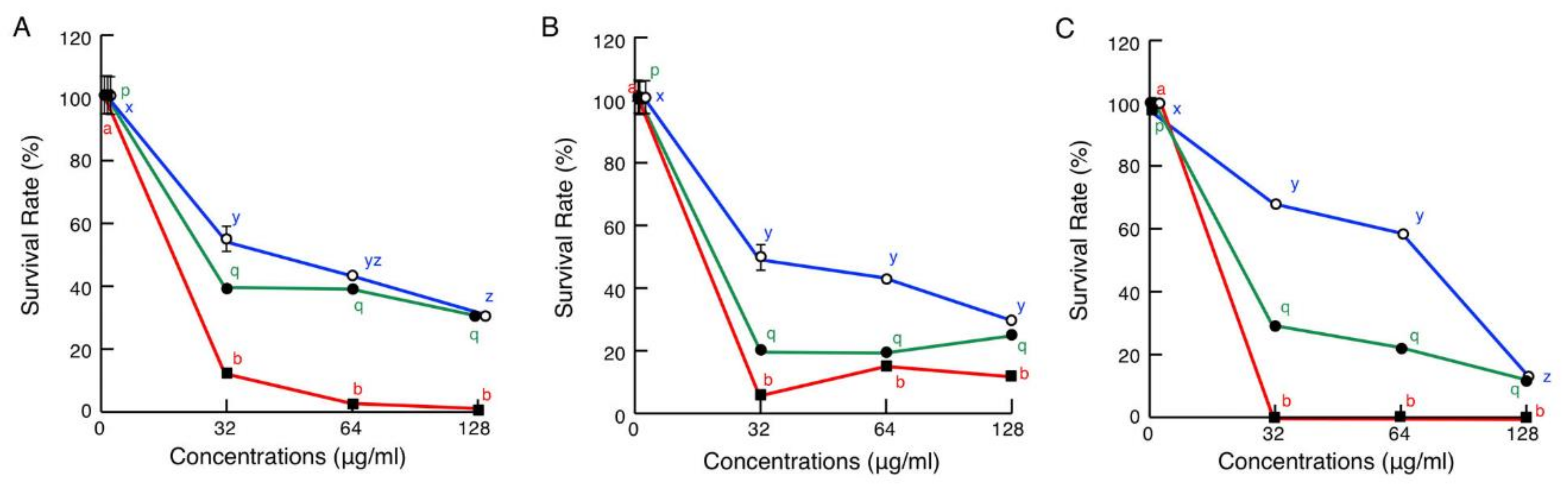
2.6. Cytotoxic Assays
3. Discussion
4. Materials and Methods
4.1. Bacterial and Fungal Cell Strains
4.2. Mammalian Cell Lines
4.3. Synthetic Peptides
4.4. Amplification of AMP Precursor cDNAs
4.5. Antimicrobial Assays
4.6. Scanning Electron Microscopy (SEM)
4.7. Enzyme-Linked Endotoxin Binding Assay (ELEBA)
4.8. Antioxidative Assay
4.9. Cytotoxicity Assays
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [PubMed]
- Auvynet, C.; Rosenstein, Y. Multifunctional host defense peptides: Antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J. 2009, 276, 6497–6508. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Pushpantathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial peptides: Versatile biological properties. Int. J. Pept. 2013, 675391. [Google Scholar]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, α-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Dobson, A.J.; Purves, J.; Rolff, J. Increased survival of experimentally evolved antimicrobial peptide-resistant Staphylococcus aureus in an animal host. Evol. App. 2014, 7, 905–912. [Google Scholar] [CrossRef]
- Conlon, J.M. Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides 2008, 29, 1815–1819. [Google Scholar] [CrossRef]
- Ladram, A.; Nicolas, P. Antimicrobial peptides from frog skin: Biodiversity and therapeutic promises. Front. Biosci. 2016, 21, 1341–1371. [Google Scholar] [CrossRef]
- Gissi, C.; San Mauro, D.; Pesole, G.; Zardoya, R. Mitochondrial phylogeny of Anura (Amphibia): A case study of congruent phylogenetic reconstruction using amino acid and nucleotide characters. Gene 2006, 366, 228–237. [Google Scholar] [CrossRef]
- Nicolas, P.; Vanhoye, D.; Amiche, M. Molecular strategies in biological evolution of antimicrobial peptides. Peptides 2003, 24, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, A.; Conlon, J.M.; Iwamuro, S. Differential expression of genes encoding preprobrevinin-2, prepropalustrin-2, and preproranatuerin-2 in developing larvae and adult tissues of the mountain brown frog Rana ornativentris. Comp. Biochem. Phys. C Toxicol Pharmacol. 2010, 151, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Mochitate, M.; Furukawa, M.; Hasunuma, I.; Kobayashi, T.; Kikuyama, S.; Iwamuro, S. Molecular cloning and functional characterization of antimicrobial peptides brevinin-1ULf and ulmin-1ULa in the skin of the newly classified Ryukyu brown frog Rana ulma. Zool. Sci. 2017, 34, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, K.; Ohtani, H.; Ogata, M.; Miura, I. Phyletic diversity in the frog Rana rugosa (Anura: Ranidae) with special reference to a unique morphotype gound from Sado Island, Japan. Curr. Herpetol. 2010, 29, 69–79. [Google Scholar]
- Sekiya, K.; Miura, I.; Ogata, M. A new frog species of the genus Rugosa from Sado Island, Japan (Anura, Ranidae). Zootaxa 2012, 3575, 49–62. [Google Scholar] [CrossRef]
- Shioda, T. A Comparison of iris color pattern between Glandirana susurra and G. rugosa (Amphibia, Anura, Ranidae). Curr. Hepetol. 2015, 34, 80–84. [Google Scholar]
- Suzuki, H.; Iwamuro, S.; Ohnuma, A.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; Taylor, C.K.; Abel, P.W.; Conlon, J.M. Expression of genes encoding antimicrobial and bradykinin-related peptides in skin of the stream brown frog Rana sakuraii. Peptides 2007, 28, 505–514. [Google Scholar] [CrossRef]
- Tazato, S.; Conlon, J.M.; Iwamuro, S. Cloning and expression of genes encoding antimicrobial peptides and bradykinin from the skin and brain of Oki Tago’s brown frog, Rana tagoi okiensis. Peptides 2010, 31, 1480–1487. [Google Scholar] [CrossRef]
- Nakajima, T.; Yasuhara, T. A new mast cell degranulating peptide, granuliberin-R, in the frog (Rana rugosa) skin. Chem. Pharm. Bull. 1977, 25, 2464–2465. [Google Scholar] [CrossRef]
- Nakao, S.; Komagoe, K.; Inoue, T.; Katsu, T. Comparative study of the membrane-permeabilizing activities of mastoparans and related histamine-releasing agents in bacteria, erythrocytes, and mast cells. Biochim. Biophys. Acta 2011, 1808, 490–497. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World 6.0, an Online Reference. Electronic Database Accessible at American Museum of Natural History, New York. 2017. Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 3 June 2020).
- Park, J.M.; Jung, J.E.; Lee, B.J. Antimicrobial peptides form the skin of a Korean frog, Rana rugosa. Biochem. Biophys. Res. Comuun. 1994, 205, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Jung, J.E.; Moon, H.M.; Lee, B.J. Molecular cloning of cDNAs encoding precursors of frog skin antimicrobial peptides from Rana rugosa. Biochim. Biophys. Acta 1995, 1264, 23–25. [Google Scholar] [CrossRef]
- Suzuki, S.; Ohe, Y.; Okubo, T.; Kakegawa, T.; Tatemoto, K. Isolation and characterization of novel antimicrobial peptides, rugosins A, B and C, from the skin of the frog, Rana rugosa. Biochem. Biophys. Res. Commun. 1995, 212, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Carlson, B.A.; Park, J.M.; Lee, B.J. Structural organization and expression of the gaegurin 4 gene of Rana rugosa. Biochim. Biophys. Acta 2000, 1492, 185–190. [Google Scholar] [CrossRef]
- Won, H.S.; Kang, S.J.; Lee, B.J. Action mechanism and structural requirements of the antimicrobial peptides, gaegurins. Biochim. Biophys. Acta 2009, 1788, 1620–1629. [Google Scholar] [CrossRef]
- Morikawa, N.; Hagiwara, K.; Nakajima, T. Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem. Biophys. Res. Comuun. 1992, 189, 184–190. [Google Scholar] [CrossRef]
- Clark, D.P.; Durell, S.; Maloy, W.L.; Zasloff, M. Ranalexin. A novel antimicrobial peptide from bullfrog (Rana catesbeiana) skin, structurally related to the bacterial antibiotic, polymyxin. J. Biol. Chem. 1994, 269, 10849–10855. [Google Scholar]
- Mai, X.T.; Huang, J.; Tan, J.; Huang, Y.; Chen, Y. Effects and mechanisms of the secondary structure on the antimicrobial activity and specificity of antimicrobial peptides. J. Pep. Sci. 2015, 21, 561–568. [Google Scholar] [CrossRef]
- Goraya, J.; Knoop, F.C.; Conlon, J.M. Ranatuerins: Antimicrobial peptides isolated from the skin of the American bullfrog, Rana catesbeiana. Biochem. Biophys. Res. Comuun. 1998, 250, 589–592. [Google Scholar] [CrossRef]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N. Antimicrobial peptides from the skins of North American frogs. Biochim. Biophys. Acta 2009, 1788, 1556–1563. [Google Scholar] [CrossRef]
- Conlon, J.M.; Sonnevend, A.; Jouenne, T.; Coquet, L.; Cosquer, D.; Vaudry, H.; Iwamuro, S. A family of acyclic brevinin-1 peptides from the skin of the Ryukyu brown frog Rana okinavana. Peptides 2005, 26, 185–190. [Google Scholar] [CrossRef]
- Roscetto, E.; Contursi, P.; Vollaro, A.; Fusco, S.; Notomista, E.; Catatani, M.S. Antifungal and anti-biofilm activity of the first cryptic antimicrobial peptide from an archaeal protein against Candida spp. Clinical isolates. Sci. Rep. 2018, 8, 17570. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic resistance in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Shi, W.; Li, C.; Zong, X.; Han, D.; Chen, Y. Antimicrobial peptide melittin against Xanthomonas oryzae pv. oryzae, the bacterial leaf blight pathogen in rice. Appl. Microbiol. Biotechnol. 2016, 100, 5059–5067. [Google Scholar] [CrossRef]
- Martínez de Tejada, G.; Sánchez-Gómez, S.; Rázquin-Olazaran, I.; Kowalski, I.; Kacoins, Y.; Heinbockel, L.; Andrä, J.; Schürholz, T.; Hornef, M.; Dupont, A.; et al. Bacterial cell wall compounds as promising targets of antimicrobial agents I. Antimicrobial peptides and lipopolyamines. Curr. Drug Targets 2012, 13, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Tack, B.F.; Sawai, M.V.; Kearney, W.R.; Robertson, A.D.; Sherman, M.A.; Wang, W.; Hong, T.; Boo, L.M.; Wu, H.; Waring, A.J.; et al. SMAP-29 has two LPS-binding sites and a central hinge. Eur. J. Biochem. 2002, 269, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Tsubaki, T.; Sagae, N.; Onda, Y.; Inada, Y.; Mochizuki, T.; Okumura, K.; Kikuyama, S.; Kobayashi, T.; Iwamuro, S. Bacterial toxin-inducible gene expression of cathelicidin-B1 in the chicken bursal lymphoma-derived cell line DT40: Functional characterization of cathelicidin-B1. Peptides 2014, 59, 94–102. [Google Scholar] [CrossRef]
- Stinson, M.W.; Mcaughlin, R.; Choi, S.H.; Juarez, Z.E.; Barnard, J. Streptococcal histone-like protein: Primary structure of hlpA and protein binding to lipoteichoic acid and epithelial cells. Infect. Immun. 1998, 66, 259–265. [Google Scholar] [CrossRef]
- Morita, S.; Tagai, C.; Shiraishi, T.; Miyaji, K.; Iwamuro, S. Differential mode of antimicrobial actions of arginine-rich and lysine-rich histones against Gram-positive Staphylococcus aureus. Peptides 2013, 48, 75–82. [Google Scholar] [CrossRef]
- Dong, W.; Sun, Y.; Shang, D. Interactions between chensinin-1, a natural antimicrobial peptide derived from Rana chensinensis, and lipopolysaccharide. Biopolymers 2015, 103, 719–726. [Google Scholar] [CrossRef]
- Barbosa, E.A.; Oliveira, A.; Placido, A.; Socodato, R.; Portugal, C.C.; Leite, J. Structure and function of a novel antioxidant peptide from the skin of tropical frogs. Free Radic. Biol. Med. 2018, 115, 68–79. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Liu, X.; Wu, J.; Liu, C.; Gong, W.; Zhao, Z.; Hong, J.; Lin, D.; Wang, Y.; et al. Antioxidant peptidomics reveals novel skin antioxidant system. Mol. Cell. Proteom. 2009, 8, 571–583. [Google Scholar] [CrossRef]
- Guo, C.; Hu, Y.; Li, J.; Liu, Y.; Li, S.; Yan, K.; Wang, X.; Liu, J.; Wang, H. Identification of multiple peptides with antioxidant and antimicrobial activities from skin and its secretions of Hylarana taipehensis, Amolops lifanensis, and Amolops granulosus. Biochimie 2014, 105, 192–201. [Google Scholar] [CrossRef]
- Wang, X.; Ren, S.; Guo, C.; Zhang, W.; Zhang, X.; Zhang, B.; Li, S.; Ren, J.; Hu, Y.; Wang, H. Identification and functional analyses of novel antioxidant peptides and antimicrobial peptides from skin secretions of four East Asian frog species. Acta Biochim. Biophys. Sin. 2017, 49, 550–559. [Google Scholar] [CrossRef]
- Song, Y.M.; Yang, S.T.; Lim, S.S.; Kim, Y.; Hahm, K.S.; Kim, J.I.; Shin, S.Y. Effects of L- or D-Pro incorporation into hydrophobic or hydrophilic helix face of amphipathic α-helical model peptide on structure and cell selectivity. Biochem. Biophys. Res. Commun. 2004, 314, 615–621. [Google Scholar] [CrossRef]
- Manzo, G.; Scorciapino, M.A.; Srinivasan, D.; Attoub, S.; Mangoni, M.L.; Rinaldi, A.C.; Casu, M.; Flatt, P.R.; Conlon, J.M. Conformational analysis of the host-defense peptides pseudhymenochirin-1Pb and -2Pa and design of analogues with insulin-releasing activities and reduced toxicities. J. Nat. Prod. 2015, 78, 3041–3048. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Di Grazia, A.; Cappiello, F.; Casciaro, B.; Luca, V. Naturally occurring peptides from Rana temporaria: Antimicrobial properties and more. Curr. Top. Med. Chem. 2016, 16, 54–64. [Google Scholar] [CrossRef]
- Irazazabal, L.N.; Porto, W.F.; Ribeiro, S.M.; Casale, S.; Humblot, V.; Ladram, A.; Franco, O.L. Selective amino acid substitution reduces cytotoxicity of the antimicrobial peptide mastoparan. Biochim. Biophys. Acta 2016, 1858, 2699–2708. [Google Scholar] [CrossRef]
- Iwamuro, S.; Kobayashi, T. An efficient protocol for DNA amplification of multiple amphibian skin antimicrobial peptide cDNAs. In Peptidomics; Soloviev, M., Ed.; Springer: Berlin, Germany, 2010; Volume 615, pp. 159–176. [Google Scholar]
- Baik, J.E.; Choe, H.I.; Hong, S.W.; Kang, S.S.; Ahn, K.B.; Cho, K.; Yun, C.H.; Han, S.H. Human salivary proteins with affinity to lipoteichoic acid of Enterococcus faecalis. Mol Immunol. 2016, 77, 52–59. [Google Scholar] [CrossRef]
- Yamauchi, R.; Fukamizu, S.; Kohama, Y.; Shimamura, T.; Kashiwagi, T.; Ukeda, H.; Akiyama, H.; Mastui, T.; Ishikawa, H. Comparative DPPH and ABTS radical scavenging activity assays for evaluating natural antioxidants as food additives. Food Preserv. Sci. 2014, 40, 55–64. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, D.; Suzuki, M.; Inamura, Y.; Saito, K.; Hasunuma, I.; Kobayashi, T.; Kikuyama, S.; Iwamuro, S. Antimicrobial Property and Mode of Action of the Skin Peptides of the Sado Wrinkled Frog, Glandirana susurra, against Animal and Plant Pathogens. Antibiotics 2020, 9, 457. https://doi.org/10.3390/antibiotics9080457
Ogawa D, Suzuki M, Inamura Y, Saito K, Hasunuma I, Kobayashi T, Kikuyama S, Iwamuro S. Antimicrobial Property and Mode of Action of the Skin Peptides of the Sado Wrinkled Frog, Glandirana susurra, against Animal and Plant Pathogens. Antibiotics. 2020; 9(8):457. https://doi.org/10.3390/antibiotics9080457
Chicago/Turabian StyleOgawa, Daisuke, Manami Suzuki, Yuriko Inamura, Kaito Saito, Itaru Hasunuma, Tetsuya Kobayashi, Sakae Kikuyama, and Shawichi Iwamuro. 2020. "Antimicrobial Property and Mode of Action of the Skin Peptides of the Sado Wrinkled Frog, Glandirana susurra, against Animal and Plant Pathogens" Antibiotics 9, no. 8: 457. https://doi.org/10.3390/antibiotics9080457
APA StyleOgawa, D., Suzuki, M., Inamura, Y., Saito, K., Hasunuma, I., Kobayashi, T., Kikuyama, S., & Iwamuro, S. (2020). Antimicrobial Property and Mode of Action of the Skin Peptides of the Sado Wrinkled Frog, Glandirana susurra, against Animal and Plant Pathogens. Antibiotics, 9(8), 457. https://doi.org/10.3390/antibiotics9080457



