How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response
Abstract
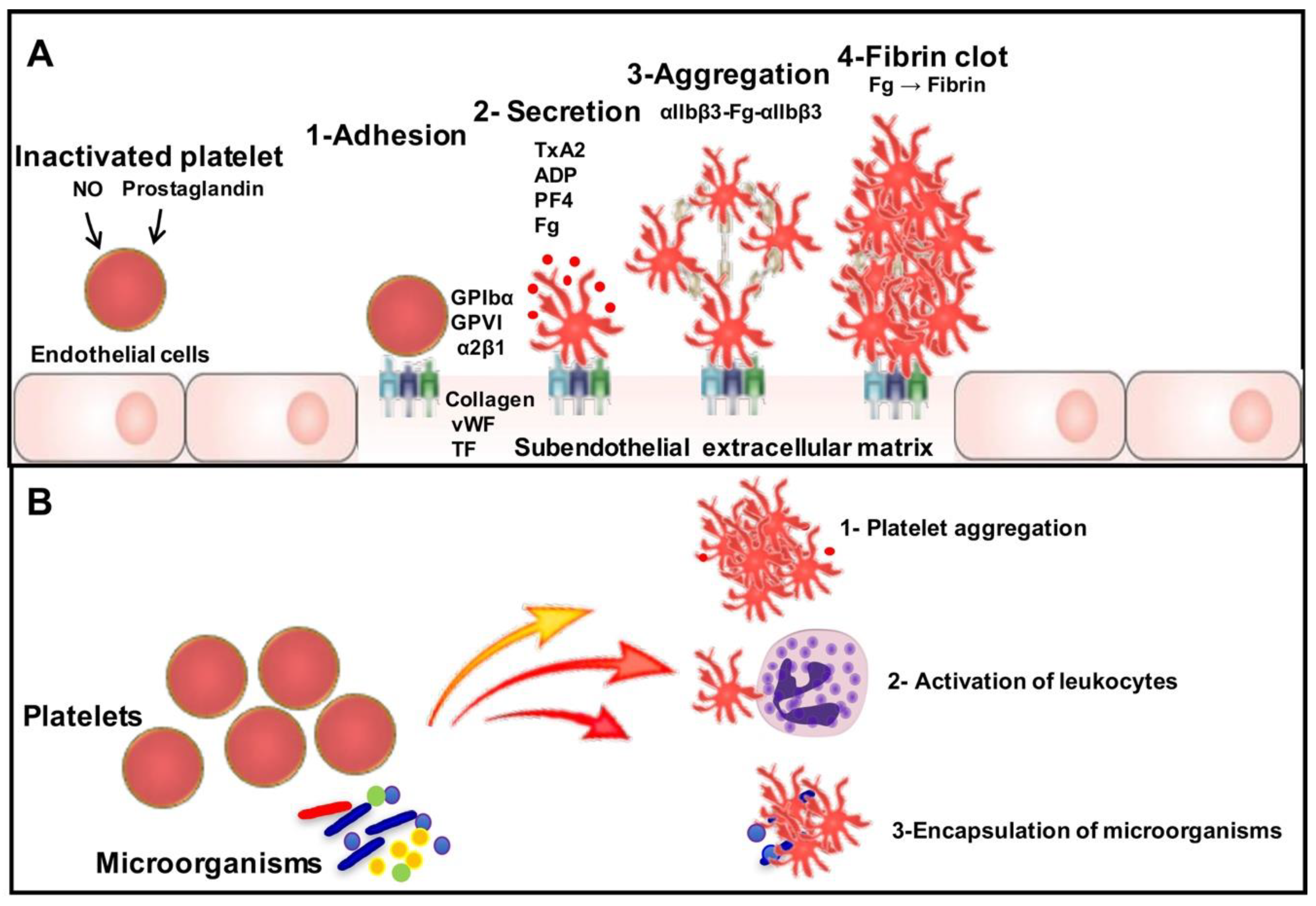
Conclusions
Funding
Conflicts of Interest
References
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Topol, E.J.; Byzova, T.V.; Plow, E.F. Platelet GPIIb-IIIa blockers. Lancet 1999, 353, 227–231. [Google Scholar] [CrossRef]
- Manon-Jensen, T.; Kjeld, N.G.; Karsdal, M.A. Collagen-mediated hemostasis. J. Thromb. Haemost. 2016, 14, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; Qin, J.; Plow, E.F. Platelet integrin alpha(IIb)beta(3): Activation mechanisms. J. Thromb. Haemost. 2007, 5, 1345–1352. [Google Scholar] [CrossRef]
- Gawaz, M.; Fateh-Moghadam, S.; Pilz, G.; Gurland, H.J.; Werdan, K. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur. J. Clin. Investig. 1995, 25, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Simon, D.I.; Chen, Z.; Xu, H.; Li, C.Q.; Dong, J.; McIntire, L.V.; Ballantyne, C.M.; Zhang, L.; Furman, M.I.; Berndt, M.C.; et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J. Exp. Med. 2000, 192, 193–204. [Google Scholar] [CrossRef]
- Hamburger, S.A.; McEver, R.P. GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood 1990, 75, 550–554. [Google Scholar] [CrossRef]
- Wrigley, B.J.; Shantsila, E.; Tapp, L.D.; Lip, G.Y. Increased formation of monocyte-platelet aggregates in ischemic heart failure. Circ. Heart Fail. 2013, 6, 127–135. [Google Scholar] [CrossRef]
- Pervushina, O.; Scheuerer, B.; Reiling, N.; Behnke, L.; Schroder, J.M.; Kasper, B.; Brandt, E.; Bulfone-Paus, S.; Petersen, F. Platelet factor 4/CXCL4 induces phagocytosis and the generation of reactive oxygen metabolites in mononuclear phagocytes independently of Gi protein activation or intracellular calcium transients. J. Immunol. 2004, 173, 2060–2067. [Google Scholar] [CrossRef]
- Cattaneo, M.; Gachet, C. ADP receptors and clinical bleeding disorders. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2281–2285. [Google Scholar] [CrossRef]
- Erhardt, J.A.; Toomey, J.R.; Douglas, S.A.; Johns, D.G. P2X1 stimulation promotes thrombin receptor-mediated platelet aggregation. J. Thromb. Haemost. 2006, 4, 882–890. [Google Scholar] [CrossRef]
- Cattaneo, M. Platelet P2 receptors: Old and new targets for antithrombotic drugs. Exp. Rev. Cardiovasc. Ther. 2007, 5, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Hechler, B.; Gachet, C. P2 receptors and platelet function. Purinergic Signal. 2011, 7, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Li, W.; Holinstat, M. Platelet Signaling and Disease: Targeted Therapy for Thrombosis and Other Related Diseases. Pharmacol. Rev. 2018, 70, 526–548. [Google Scholar] [CrossRef]
- Garraud, O.; Cognasse, F. Platelet Toll-like receptor expression: The link between “danger” ligands and inflammation. Inflamm. Allergy Drug Targ. 2010, 9, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, R.; Inoue, N.; Kawasaki, S.; Takei, A.; Kadotani, M.; Ohnishi, Y.; Ejiri, J.; Kobayashi, S.; Hirata, K.; Kawashima, S.; et al. Expression of Toll-like receptors on human platelets. Thromb. Res. 2004, 113, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Andonegui, G.; Kerfoot, S.M.; McNagny, K.; Ebbert, K.V.; Patel, K.D.; Kubes, P. Platelets express functional Toll-like receptor-4. Blood 2005, 106, 2417–2423. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh, H.; Chavarin, P.; Acquart, S.; Genin, C.; Garraud, O. Evidence of Toll-like receptor molecules on human platelets. Immunol. Cell Biol. 2005, 83, 196–198. [Google Scholar] [CrossRef]
- Koupenova, M.; Vitseva, O.; MacKay, C.R.; Beaulieu, L.M.; Benjamin, E.J.; Mick, E.; Kurt-Jones, E.A.; Ravid, K.; Freedman, J.E. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood 2014, 124, 791–802. [Google Scholar] [CrossRef]
- Thon, J.N.; Peters, C.G.; Machlus, K.R.; Aslam, R.; Rowley, J.; Macleod, H.; Devine, M.T.; Fuchs, T.A.; Weyrich, A.S.; Semple, J.W.; et al. T granules in human platelets function in TLR9 organization and signaling. J. Cell Biol. 2012, 198, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Damien, P.; Cognasse, F.; Payrastre, B.; Spinelli, S.L.; Blumberg, N.; Arthaud, C.A.; Eyraud, M.A.; Phipps, R.P.; McNicol, A.; Pozzetto, B.; et al. NF-kappaB Links TLR2 and PAR1 to Soluble Immunomodulator Factor Secretion in Human Platelets. Front. Immunol. 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Rex, S.; Vitseva, O.; Beaulieu, L.; Tanriverdi, K.; Chakrabarti, S.; Hayashi, C.; Genco, C.A.; Iafrati, M.; Freedman, J.E. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ. Res. 2009, 104, 346–354. [Google Scholar] [CrossRef]
- Zhang, G.; Han, J.; Welch, E.J.; Ye, R.D.; Voyno-Yasenetskaya, T.A.; Malik, A.B.; Du, X.; Li, Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J. Immunol. 2009, 182, 7997–8004. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, B.; Liang, Y.; Cao, S.H.; Liu, L.; Xu, X.N. Role of platelet TLR4 expression in pathogensis of septic thrombocytopenia. World J. Emerg. Med. 2011, 2, 13–17. [Google Scholar] [CrossRef]
- Williams, B.; Neder, J.; Cui, P.; Suen, A.; Tanaka, K.; Zou, L.; Chao, W. Toll-like receptors 2 and 7 mediate coagulation activation and coagulopathy in murine sepsis. J. Thromb. Haemost. 2019, 17, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Ma, Y.; Hong, L.; Gao, D.; West, X.Z.; Salomon, R.G.; Byzova, T.V.; Podrez, E.A. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ. Res. 2013, 112, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Czuprynski, C.J.; Balish, E. Interaction of rat platelets with Listeria monocytogenes. Infect. Immun. 1981, 33, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Muhly, M.; Mannhardt, U.; Hugo, F.; Klapettek, K.; Mueller-Eckhardt, C.; Roka, L. Staphylococcal alpha toxin promotes blood coagulation via attack on human platelets. J. Exp. Med. 1988, 168, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Speth, C.; Hagleitner, M.; Ott, H.W.; Wurzner, R.; Lass-Florl, C.; Rambach, G. Aspergillus fumigatus activates thrombocytes by secretion of soluble compounds. J. Inf. Dis. 2013, 207, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y.; Ohshima, Y.; Ichiman, Y.; Ohtomo, T.; Suganuma, M.; Yoshida, K. Platelet aggregation induced by strains of various species of coagulase-negative staphylococci. Microbiol. Immunol. 1991, 35, 15–26. [Google Scholar] [CrossRef]
- Berthet, J.; Damien, P.; Hamzeh-Cognasse, H.; Arthaud, C.A.; Eyraud, M.A.; Zeni, F.; Pozzetto, B.; McNicol, A.; Garraud, O.; Cognasse, F. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin. Immunol. 2012, 145, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chen, Y.C.; Chang, S.C.; Luh, K.T.; Hsieh, W.C. Nosocomial candidemia in a university hospital in Taiwan. J. Formos. Med. Assoc. 1996, 95, 19–28. [Google Scholar]
- Holder, I.A.; Nathan, P. Effect in mice of injection of viable Candida albicans and a cell-free sonic extract on circulating platelets. Inf. Immun. 1973, 7, 468–472. [Google Scholar] [CrossRef]
- Robert, R.; Nail, S.; Marot-Leblond, A.; Cottin, J.; Miegeville, M.; Quenouillere, S.; Mahaza, C.; Senet, J.M. Adherence of platelets to Candida species in vivo. Infect. Immun. 2000, 68, 570–576. [Google Scholar] [CrossRef]
- Robert, R.; Mahaza, C.; Miegeville, M.; Ponton, J.; Marot-Leblond, A.; Senet, J.M. Binding of resting platelets to Candida albicans germ tubes. Inf. Immun. 1996, 64, 3752–3757. [Google Scholar] [CrossRef]
- Calderone, R.A.; Rotondo, M.F.; Sande, M.A. Candida albicans endocarditis: Ultrastructural studies of vegetation formation. Inf. Immun. 1978, 20, 279–289. [Google Scholar] [CrossRef]
- Maisch, P.A.; Calderone, R.A. Role of surface mannan in the adherence of Candida albicans to fibrin-platelet clots formed in vitro. Inf. Immun. 1981, 32, 92–97. [Google Scholar] [CrossRef]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Poulain, D.; Sendid, B.; Standaert-Vitse, A.; Fradin, C.; Jouault, T.; Jawhara, S.; Colombel, J.F. Yeasts: Neglected pathogens. Dig. Dis. 2009, 27, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.A.; Westwater, C.; Warner, T.; Balish, E. Differential Candida albicans lipase gene expression during alimentary tract colonization and infection. FEMS Microbiol. Lett. 2005, 244, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hube, B. Extracellular proteinases of human pathogenic fungi. Contrib. Microbiol. 2000, 5, 126–137. [Google Scholar]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Gropp, K.; Schild, L.; Schindler, S.; Hube, B.; Zipfel, P.F.; Skerka, C. The yeast Candida albicans evades human complement attack by secretion of aspartic proteases. Mol. Immunol. 2009, 47, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, E.; Schneider, A.E.; Sandor, N.; Lermann, U.; Staib, P.; Kremlitzka, M.; Bajtay, Z.; Barz, D.; Erdei, A.; Jozsi, M. Secreted aspartic protease 2 of Candida albicans inactivates factor H and the macrophage factor H-receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18). Immunol. Lett. 2015, 168, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Hofs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef]
- Soloviev, D.A.; Fonzi, W.A.; Sentandreu, R.; Pluskota, E.; Forsyth, C.B.; Yadav, S.; Plow, E.F. Identification of pH-regulated antigen 1 released from Candida albicans as the major ligand for leukocyte integrin alphaMbeta2. J. Immunol. 2007, 178, 2038–2046. [Google Scholar] [CrossRef]
- Soloviev, D.A.; Jawhara, S.; Fonzi, W.A. Regulation of innate immune response to Candida albicans infections by alphaMbeta2-Pra1p interaction. Inf. Immun. 2011, 79, 1546–1558. [Google Scholar] [CrossRef]
- Jawhara, S.; Pluskota, E.; Verbovetskiy, D.; Skomorovska-Prokvolit, O.; Plow, E.F.; Soloviev, D.A. Integrin alphaXbeta(2) is a leukocyte receptor for Candida albicans and is essential for protection against fungal infections. J. Immunol. 2012, 189, 2468–2477. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, S.L.; Sorsby, E.; Mahtey, N.; Kumwenda, P.; Lenardon, M.D.; Brown, I.; Ballou, E.R.; MacCallum, D.M.; Hall, R.A. Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLoS Pathog. 2017, 13, e1006403. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Munro, C.A.; de Bruijn, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef] [PubMed]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; Netea, M.G.; Munro, C.A.; Ferwerda, G.; Bates, S.; Mora-Montes, H.M.; Walker, L.; Jansen, T.; Jacobs, L.; Tsoni, V.; et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J. Infect. Dis. 2007, 196, 1565–1571. [Google Scholar] [CrossRef]
- Esteban, A.; Popp, M.W.; Vyas, V.K.; Strijbis, K.; Ploegh, H.L.; Fink, G.R. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. USA 2011, 108, 14270–14275. [Google Scholar] [CrossRef]
- Charlet, R.; Bortolus, C.; Barbet, M.; Sendid, B.; Jawhara, S. A decrease in anaerobic bacteria promotes Candida glabrata overgrowth while beta-glucan treatment restores the gut microbiota and attenuates colitis. Gut. Pathog. 2018, 10, 50. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005, 24, 1277–1286. [Google Scholar] [CrossRef]
- Van der Graaf, C.A.; Netea, M.G.; Verschueren, I.; van der Meer, J.W.; Kullberg, B.J. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Inf. Immun. 2005, 73, 7458–7464. [Google Scholar] [CrossRef]
- Schlosser, A.; Thomsen, T.; Moeller, J.B.; Nielsen, O.; Tornoe, I.; Mollenhauer, J.; Moestrup, S.K.; Holmskov, U. Characterization of FIBCD1 as an acetyl group-binding receptor that binds chitin. J. Immunol. 2009, 183, 3800–3809. [Google Scholar] [CrossRef]
- Wagener, J.; Malireddi, R.K.; Lenardon, M.D.; Koberle, M.; Vautier, S.; MacCallum, D.M.; Biedermann, T.; Schaller, M.; Netea, M.G.; Kanneganti, T.D.; et al. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 2014, 10, e1004050. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, C.S.; Dubey, L.K.; Colmorten, K.B.; Moeller, J.B.; Hammond, M.A.; Nielsen, O.; Schlosser, A.; Templeton, S.P.; Sorensen, G.L.; Holmskov, U. FIBCD1 Binds Aspergillus fumigatus and Regulates Lung Epithelial Response to Cell Wall Components. Front. Immunol. 2018, 9, 1967. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; MacCallum, D.M.; Brown, G.D.; Gow, N.A. Candida albicans Chitin Increases Arginase-1 Activity in Human Macrophages, with an Impact on Macrophage Antimicrobial Functions. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Mora-Montes, H.M.; Netea, M.G.; Ferwerda, G.; Lenardon, M.D.; Brown, G.D.; Mistry, A.R.; Kullberg, B.J.; O’Callaghan, C.A.; Sheth, C.C.; Odds, F.C.; et al. Recognition and blocking of innate immunity cells by Candida albicans chitin. Inf. Immun. 2011, 79, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Charlet, R.; Pruvost, Y.; Tumba, G.; Istel, F.; Poulain, D.; Kuchler, K.; Sendid, B.; Jawhara, S. Remodeling of the Candida glabrata cell wall in the gastrointestinal tract affects the gut microbiota and the immune response. Sci. Rep. 2018, 8, 3316. [Google Scholar] [CrossRef]
- Sendid, B.; Dotan, N.; Nseir, S.; Savaux, C.; Vandewalle, P.; Standaert, A.; Zerimech, F.; Guery, B.P.; Dukler, A.; Colombel, J.F.; et al. Antibodies against glucan, chitin, and Saccharomyces cerevisiae mannan as new biomarkers of Candida albicans infection that complement tests based on C. albicans mannan. Clin. Vaccine Immunol. 2008, 15, 1868–1877. [Google Scholar] [CrossRef]
- Obayashi, T.; Negishi, K.; Suzuki, T.; Funata, N. Reappraisal of the serum (1-->3)-beta-D-glucan assay for the diagnosis of invasive fungal infections—A study based on autopsy cases from 6 years. Clin. Infect. Dis. 2008, 46, 1864–1870. [Google Scholar] [CrossRef]
- Sims, C.R.; Jaijakul, S.; Mohr, J.; Rodriguez, J.; Finkelman, M.; Ostrosky-Zeichner, L. Correlation of clinical outcomes with beta-glucan levels in patients with invasive candidiasis. J. Clin. Microbiol. 2012, 50, 2104–2106. [Google Scholar] [CrossRef]
- Moore, K.L.; Patel, K.D.; Bruehl, R.E.; Li, F.; Johnson, D.A.; Lichenstein, H.S.; Cummings, R.D.; Bainton, D.F.; McEver, R.P. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J. Cell Biol. 1995, 128, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, P.H.; Gallardo Torres, H.I.; van der Linden, J.A.; Lammers, J.W.; Sixma, J.J.; Koenderman, L.; Zwaginga, J.J. Platelet-dependent primary hemostasis promotes selectin- and integrin-mediated neutrophil adhesion to damaged endothelium under flow conditions. Blood 1996, 87, 3271–3281. [Google Scholar] [CrossRef] [PubMed]
- Sayeh, E.; Aslam, R.; Speck, E.R.; Le-Tien, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Immune responsiveness against allogeneic platelet transfusions is determined by the recipient’s major histocompatibility complex class II phenotype. Transfusion 2004, 44, 1572–1578. [Google Scholar] [CrossRef]
- Miedzobrodzki, J.; Panz, T.; Plonka, P.M.; Zajac, K.; Dracz, J.; Pytel, K.; Mateuszuk, L.; Chlopicki, S. Platelets augment respiratory burst in neutrophils activated by selected species of gram-positive or gram-negative bacteria. Folia Histochem. Cytobiol. Pol. Acad. Sci. Pol. Histochem. Cytochem. Soc. 2008, 46, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Vancraeyneste, H.; Charlet, R.; Guerardel, Y.; Choteau, L.; Bauters, A.; Tardivel, M.; Francois, N.; Dubuquoy, L.; Soloviev, D.; Poulain, D.; et al. Short fungal fractions of beta-1,3 glucans affect platelet activation. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H725–H734. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.L.; Zheng, Y.W.; Huang, W.; Bigornia, V.; Zeng, D.; Moff, S.; Farese, R.V., Jr.; Tam, C.; Coughlin, S.R. A dual thrombin receptor system for platelet activation. Nature 1998, 394, 690–694. [Google Scholar] [CrossRef]
- Wei, A.H.; Schoenwaelder, S.M.; Andrews, R.K.; Jackson, S.P. New insights into the haemostatic function of platelets. Br. J. Haematol. 2009, 147, 415–430. [Google Scholar] [CrossRef]
- Saluk-Juszczak, J.; Krolewska, K.; Wachowicz, B. Response of blood platelets to beta-glucan from Saccharomyces cerevisiae. Platelets 2010, 21, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Saluk-Juszczak, J.; Krolewska, K.; Wachowicz, B. Beta-glucan from Saccharomyces cerevisiae as a blood platelet antioxidant. Platelets 2010, 21, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Miura, N.N.; Adachi, Y.; Ohno, N.; Yadomae, T. Relationship between solubility of grifolan, a fungal 1,3-beta-D-glucan, and production of tumor necrosis factor by macrophages in vitro. Biosci. Biotechnol. Biochem. 2001, 65, 1993–2000. [Google Scholar] [CrossRef]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef]
- Chai, L.Y.; Vonk, A.G.; Kullberg, B.J.; Verweij, P.E.; Verschueren, I.; van der Meer, J.W.; Joosten, L.A.; Latge, J.P.; Netea, M.G. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect. Inst. Pasteur 2011, 13, 151–159. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ohno, N.; Murai, T. Suppression by Candida albicans beta-glucan of cytokine release from activated human monocytes and from T cells in the presence of monocytes. J. Infect. Dis. 2003, 187, 710–713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, V.B.; Williams, D.L.; Keshvara, L. Beta-Glucan attenuates TLR2- and TLR4-mediated cytokine production by microglia. Neurosci. Lett. 2009, 458, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2012, 10, 112–122. [Google Scholar] [CrossRef]
- Leroy, J.; Bortolus, C.; Lecointe, K.; Parny, M.; Charlet, R.; Sendid, B.; Jawhara, S. Fungal Chitin Reduces Platelet Activation Mediated via TLR8 Stimulation. Front. Cell Infect. Microbiol. 2019, 9, 383. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawhara, S. How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics 2020, 9, 385. https://doi.org/10.3390/antibiotics9070385
Jawhara S. How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics. 2020; 9(7):385. https://doi.org/10.3390/antibiotics9070385
Chicago/Turabian StyleJawhara, Samir. 2020. "How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response" Antibiotics 9, no. 7: 385. https://doi.org/10.3390/antibiotics9070385
APA StyleJawhara, S. (2020). How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics, 9(7), 385. https://doi.org/10.3390/antibiotics9070385




