Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery
Abstract
1. Introduction
2. Results
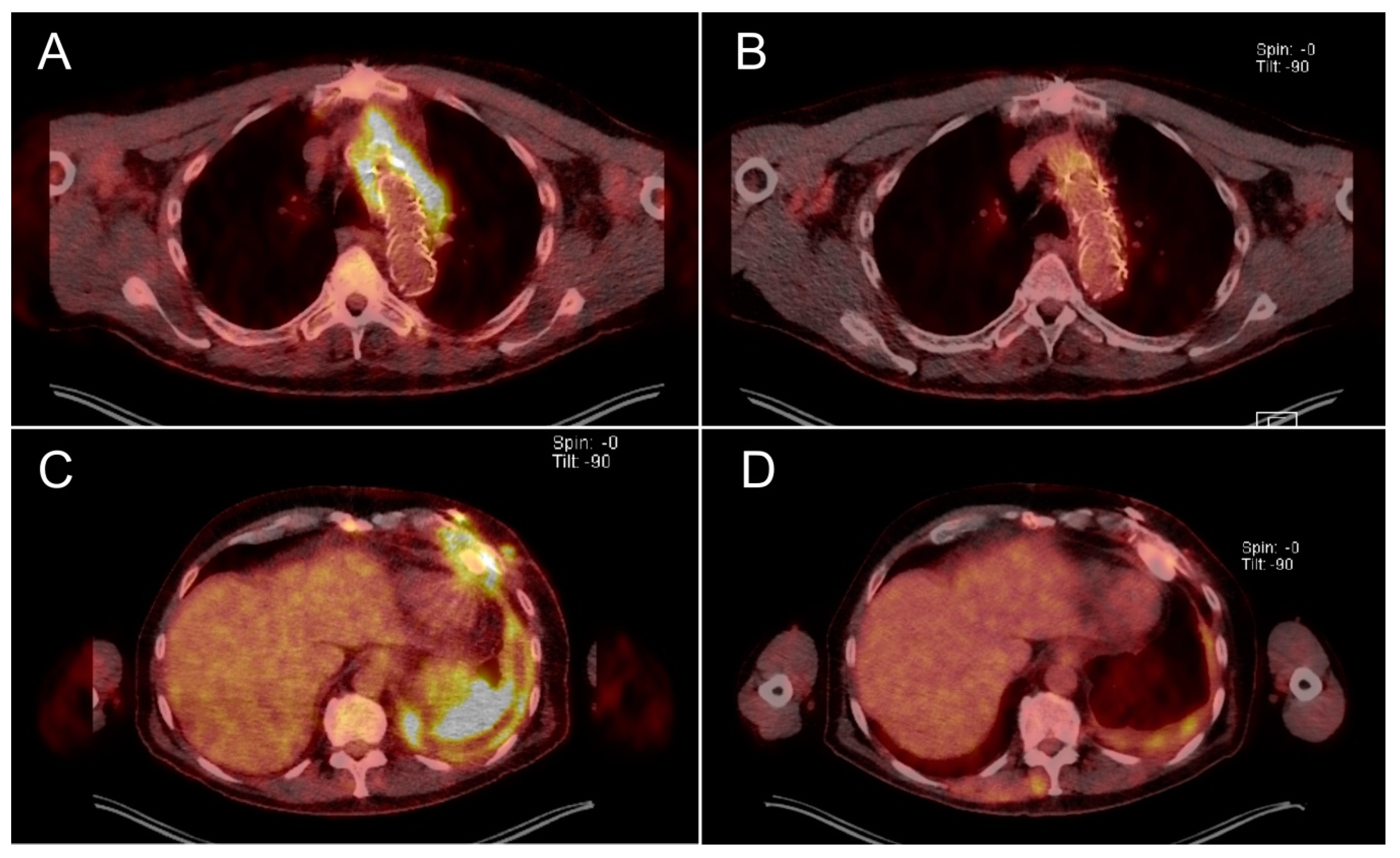
2.1. Clinical Outcome
2.2. Safety and Adverse Events
3. Discussion
4. Materials and Methods
4.1. Phage Preparation
4.2. Pre-Study Evaluation
- pan-resistance of the bacterial agent to all available antibiotics;
- complication of the clinical picture despite continuous therapy with antibiotics deemed appropriate by an antibiogram; or
- repetitive medical device infection despite appropriate antibiotic and surgical therapy.
4.3. Patients with Infected Vascular Grafts
4.4. Patients with Infected, Implanted, Metallic Medical Devices
4.5. Patients Infected During Drug-Induced Immunosuppression After Organ Transplantation
4.6. Patient with a Deep Wound Infection
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.C. Bacteriophage therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Fish, R.; Kutter, E.; Wheat, G.; Blasdel, B.; Kutateladze, M.; Kuhl, S. Bacteriophage treatment of intransigent diabetic toe ulcers: A case series. J. Wound Care 2016, 25, S27–S33. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Aleshkin, A.; Bochkareva, S.; Modin, E.; Mashaqi, B.; Boyle, E.C.; Boethig, D.; Rubalsky, M.; et al. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 2019, 9, 2091. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.C. Félix d’Herelle and the Origins of Molecular Biology; Yale University Press: New Haven, CT, USA, 1999. [Google Scholar]
- Merril, C.R.; Scholl, D.; Adhya, S.L. The prospect for bacteriophage therapy in Western medicine. Nat. Rev. Drug Discov. 2003, 2, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.K.; Collins, J.J. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. USA 2007, 104, 11197–11202. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Zaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Kłak, M.; Fortuna, W.; Letkiewicz, S.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 2014, 27, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Kłak, M.; Bubak, B.; Międzybrodzki, R.; Weber-Dąbrowska, B.; Żaczek, M.; Fortuna, W.; Rogóż, P.; Letkiewicz, S.; et al. The effect of bacteriophage preparations on intracellular killing of bacteria by phagocytes. J. Immunol. Res. 2015, 2015, 482863. [Google Scholar] [CrossRef] [PubMed]
- Samokhin, A.G.; Fedorov, E.A.; Kozlova, Y.N.; Tikunova, N.V.; Pavlov, V.V.; Morozova, V.V.; Kretien, S.O. Application of the lytic bacteriophages during surgical treatment of the periprosthetic infection of the hip joint endoprosthesis (pilot study). Sovrem. Probl. Nauk. Obraz. 2016, 6. (in Russian) [Google Scholar] [CrossRef][Green Version]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar] [PubMed]
- Kutter, E. Phage host range and efficiency of plating. Methods Mol. Biol. 2009, 501, 141–149. [Google Scholar] [PubMed]
- Aleshkin, A.V.; Volozhantsev, N.V.; Svetoch, E.A.; Kiseleva, I.A.; Rubal’sky, E.O.; Afanas’ev, S.S.; Borzilov, A.I.; Zatevalov, A.M.; Vasil’ev, D.A.; Zolotukhin, S.N.; et al. Bacteriophages as probiotics: Phage-based probiotic dietary supplement in prophylaxis against foodborne infections. Infect. Dis. Infekt. Bolezn. 2016, 14, 31–40. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Blasdel, B.G.; Bretaudeau, L.; Buckling, A.; Chanishvili, N.; Clark, J.R.; Corte-Real, S.; Debarbieux, L.; Dublanchet, A.; De Vos, D.; et al. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 2015, 32, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Aleshkin, A.V.; Rubalskii, E.O.; Volozhantsev, N.V.; Verevkin, V.V.; Svetoch, E.A.; Kiseleva, I.A.; Bochkareva, S.S.; Borisova, O.Y.; Popova, A.V.; Bogun, A.G.; et al. A small-scale experiment of using phage-based probiotic dietary supplement for prevention of E. coli traveler’s diarrhea. Bacteriophage 2015, 5, e1074329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aleshkin, A.V.; Ershova, O.N.; Volozhantsev, N.V.; Svetoch, E.A.; Popova, A.V.; Rubalskii, E.O.; Borzilov, A.I.; Aleshkin, V.A.; Afanas’ev, S.S.; Karaulov, A.V.; et al. Phagebiotics in treatment and prophylaxis of healthcare-associated infections. Bacteriophage 2016, 6, e1251379. [Google Scholar] [CrossRef] [PubMed]
| Patient Data, Infection Site, and Date of Surgery 1 | Source and Date of Isolated Bacteria | Basis for Phage Therapy | Titer and Bacteriophage(s) | Date, Dosage, and Route of Phage Administration | Antibiotic Therapy Before & During Phage Application | Microbiological Control After Phage Therapy | Survival After Phage Therapy |
|---|---|---|---|---|---|---|---|
| Patient 1, 52 y.o., m. Prosthetic infection after aortic arch replacement. Replacement: 03.12.2013. | Implant drainage Since 20.08.2015: S. aureus, E. faecium. Since 09.09.2015: P. aeruginosa. Bronchial lavage Since 27.08.2015: E. faecium, P. aeruginosa. | Continuous isolation of S. aureus, E. faecium, P. aeruginosa despite conventional antibiotic therapy | 1 × 108 pfu/mL Staphylococcus phage CH1 Enterococcus phage Enf1 Pseudomonas phage PA5 Pseudomonas phage PA10 | 10.09.2015:
| 2000 mg cefepime, 500 mg daptomycin, 600 mg linezolid, tobramycin depending on drug concentration in blood (target concentration 2 mg/L). All antibiotics intravenously once per day. | S. aureus, E. faecium, and P. aeruginosa not detected | Died 2 months after phage therapy due to a new bacterial infection caused by E. coli and P. aeruginosa |
| Patient 2, 40 y.o., m. Lung infection during drug-induced immuno-suppression after heart transplantation. Transplantation: 23.07.2016. | Bronchial lavage Since 16.08.2016: pan-resistant K. pneumoniae. Rectal swab Since 27.06.2016: pan-resistant K. pneumoniae. | Infection with the pan-resistant bacteria | 1 × 108 pfu/mL Klebsiella phage KPV811 Klebsiella phage KPV15 | 29.08.2016–30.08.2016:
| 2000 mg ceftazidime, 600 mg linezolid, 500 mg avibactam intravenously twice per day. Inhalation of 1 MIU colistin three times per day. 2000 mg meropenem intravenously three times per day. 960 mg cotrimoxazole per os once per day. Tobramycin depending on drug concentration in blood (target concentration 2 mg/L). | K. pneumoniae not detected in bronchial lavage | Until present |
| Patient 3, 59 y.o., m. Chronic vascular graft infection after aortic arch replacement. Replacement: 22.10.2014. | Blood culture Since 19.12.2016: S. aureus | Continuous isolation of S. aureus and high inflammation parameters despite conventional antibiotic therapy | 1 × 109 pfu/mL Staphylococcus phage CH1 | 06.01.2017–08.01.2017:
| 600 mg rifampicin intravenously twice per day. 2000 mg flucloxacillin intravenously four times per day. | S. aureus not detected | Until present |
| Patient 4, 62 y.o., m. Fulminant pleural empyema after LVAD implantation. Implantation: 21.04.2017. | Wound swab Since 19.06.2017: S. aureus | Continuous isolation of S. aureus and high inflammation parameters despite conventional antibiotic therapy | 1 × 109 pfu/mL Staphylococcus phage CH1 | 30.06.2017–06.07.2017:
| 500 mg daptomycin intravenously once per day. | S. aureus not detected | Died 20 months after heart transplantation due transplant failure |
| Patient 5, 51 y.o., m. Chronic LVAD infection. Implantation: 28.03.2017. | Implant drainage Since 25.07.2017: S. aureus Nasal swab Since 28.05.2014: S. aureus Throat swab Since 27.01.2015: S. aureus | Continuous isolation of S. aureus and high inflammation parameters despite conventional antibiotic therapy | 1 × 109 pfu/mL Staphylococcus phage Sa30 Staphylococcus phage CH1 Staphylococcus phage SCH1 Staphylococcus phage SCH111 | 09.08.2017–17.08.2017:
| 500 mg daptomycin intravenously once per day. | 100× reduction of S. aureus in the drainage fluid. Complete eradication of S. aureus from nose and throat | Died 1.5 months after beginning phage therapy due to S. aureus sepsis |
| Patient 6, 45 y.o., m. Repetitive treprostinil pump infection. First implantation: 08.08.2017. Second implantation: 12.09.2017. | Catheter Since 16.11.2017: S. aureus Blood culture Since 16.11.2017: S. aureus | Continuous isolation of S. aureus and pump reinfection despite conventional antibiotic and surgical therapy | 4 × 1010 pfu/mL Staphylococcus phage Sa30 | 29.11.2017:
| 375 mg sultamicillin two times per day per os. | Not tested | Until present |
| Patient 7, 66 y.o., f. Sternal wall healing disorder after mitral valve replacement and aortocoronary bypass surgery. Surgery: 23.03.2018. | Wound swab Since 20.04.2018: E. coli | Continuous isolation of E. coli and high inflammation parameters despite conventional antibiotic therapy | 4 × 1010 pfu/mL Escherichia phage ECD7 Escherichia phage V18 | 09.05.2018:
| 600 mg clindamycin three times per day per os. | E. coli not detected | Until present |
| Patient 8, 13 y.o., m. Sternal wound abscesses after double lung transplantation. Transplantation: 10.03.2018. | Wound swab Since 27.05.2018: P. aeruginosa | Continuous isolation of P. aeruginosa and high inflammation parameters despite conventional antibiotic therapy | 4 × 1010 pfu/mL Pseudomonas phage PA5 Pseudomonas phage PA10 | 13.06.2018:
| 2 MIU colistin intravenously twice per day. 750 mg ceftazidime, 187.5 mg avibactam intravenously three times per day. | P. aeruginosa not detected | Until present |
| Bacteriophage Name | Taxonomy | GenBank Accession Number | Isolation Source | Source |
|---|---|---|---|---|
| Enterococcus phage Enf1 | Order Caudovirales; family Siphoviridae; genus Sap6virus | MK800154.1 | Wastewater, Moscow, Russia | This study |
| Escherichia phage ECD7 | Order Caudovirales; family Myoviridae; subfamily Tevenvirinae; genus Rb49virus | KY683735.1 | Chicken feces, Moscow Region, Russia | [17,19] |
| Escherichia phage V18 | Order Caudovirales; family Myoviridae; subfamily Vequintavirinae; genus V5virus | KY683736.1 | Cowshed sewage, Moscow Region, Russia | [17,19] |
| Pseudomonas phage PA5 | Order Caudovirales; family Myoviridae; genus Pbunavirus | KY000082.1 | Wastewater, Moscow region, Russia | [6,20] |
| Pseudomonas phage PA10 | Order Caudovirales; family Myoviridae; genus Pakpunavirus | KY000083.1 | Wastewater, Moscow region, Russia | [20] |
| Staphylococcus phage Sa30 | Order Caudovirales; family Myoviridae; subfamily Spounavirinae; genus Kayvirus | MK331931.1 | Clinical material, Astrakhan, Russia | This study |
| Staphylococcus phage CH1 | Order Caudovirales; family Myoviridae; subfamily Spounavirinae; genus Kayvirus | MK331930.1 | Patient’s wound, Chelyabinsk, Russia | [17,19] |
| Staphylococcus phage SCH1 | Order Caudovirales; family Podoviridae; subfamily Picovirinae; genus P68virus | KY000084.1 | Clinical material, Chelyabinsk, Russia | [20] |
| Staphylococcus phage SCH111 | Order Caudovirales; family Podoviridae; subfamily Picovirinae; genus P68virus | KY000085.1 | Clinical material, Moscow, Russia | [20] |
| Klebsiella phage KPV811 | Order Caudovirales; family Podoviridae; subfamily Autographivirinae; genus Drulisvirus | KY000081.1 | Wastewater, Moscow region, Russia | [20] |
| Klebsiella phage KPV15 | Order Caudovirales; family Myoviridae; subfamily Tevenvirinae; genus Jiaodavirus | KY000080.1 | Wastewater, Moscow region, Russia | [20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. https://doi.org/10.3390/antibiotics9050232
Rubalskii E, Ruemke S, Salmoukas C, Boyle EC, Warnecke G, Tudorache I, Shrestha M, Schmitto JD, Martens A, Rojas SV, et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics. 2020; 9(5):232. https://doi.org/10.3390/antibiotics9050232
Chicago/Turabian StyleRubalskii, Evgenii, Stefan Ruemke, Christina Salmoukas, Erin C. Boyle, Gregor Warnecke, Igor Tudorache, Malakh Shrestha, Jan D. Schmitto, Andreas Martens, Sebastian V. Rojas, and et al. 2020. "Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery" Antibiotics 9, no. 5: 232. https://doi.org/10.3390/antibiotics9050232
APA StyleRubalskii, E., Ruemke, S., Salmoukas, C., Boyle, E. C., Warnecke, G., Tudorache, I., Shrestha, M., Schmitto, J. D., Martens, A., Rojas, S. V., Ziesing, S., Bochkareva, S., Kuehn, C., & Haverich, A. (2020). Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics, 9(5), 232. https://doi.org/10.3390/antibiotics9050232







