Tigecycline Therapy for Infections Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Critically Ill Patients
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Patients
2.3. Study Centres
2.4. Assessment of Outcomes
2.4.1. Primary Clinical Outcomes at the End of Therapy (EOT)
2.4.2. Secondary Clinical Outcomes by 30 Days after EOT
2.4.3. Microbiological Outcomes
2.4.4. Data Collection
2.5. Statistical Analyses
2.6. Ethics Approval
3. Results
3.1. Patient Characteristics
3.2. Infection Sites and Characteristics
3.3. Pathogens
3.4. Risk Factors for Poor Clinical Outcome
3.5. Hazard Ratio for Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolau, D.-P. Management of complicated infections in the era of antimicrobial resistance: The role of tigecycline. Expert Opin. Pharmacother. 2009, 10, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.-S.; Castanheira, M.; Flamm, R.-K.; Mendes, R.-E.; Farrell, D.-J.; Jones, R.-N. Tigecycline activity tested against carbapenem-resistant Enterobacteriaceae from 18 European nations: Results from the SENTRY surveillance program (2010–2013). Diagn. Microbiol. Infect. Dis. 2015, 83, 183–186. [Google Scholar] [CrossRef]
- Sader, H.-S.; Farrell, D.-J.; Flamm, R.-K.; Jones, R.-N. Variation in potency and spectrum of tigecycline activity against bacterial strains from U.S. medical centers since its approval for clinical use (2006 to 2012). Antimicrob. Agents Chemother. 2014, 58, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing, 26th (M100-S26); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Heizmann, W.-R.; Loschmann, P.-A.; Eckmann, C.; Von Eiff, C.; Bodmann, K.-F.; Petrik, C. Clinical efficacy of tigecycline used as monotherapy or in combination regimens for complicated infections with documented involvement of multiresistant bacteria. Infection 2015, 43, 37–43. [Google Scholar] [CrossRef]
- Vasilev, K.; Reshedko, G.; Orasan, R.; Sanchez, M.; Teras, J.; Babinchak, T.; Dukart, G.; Cooper, A.; Dartois, N.; Gandjini, H.; et al. A Phase 3, open-label, non-comparative study of tigecycline in the treatment of patients with selected serious infections due to resistant Gram-negative organisms including Enterobacter species, Acinetobacter baumannii and Klebsiella pneumoniae. J. Antimicrob. Chemother. 2008, 62, i29–i40. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L.; Chen, Y.-S.; Toh, H.-S.; Huang, C.-C.; Liu, Y.-M.; Ho, C.-M.; Lu, P.-L.; Ko, W.-C.; Chen, Y.-H.; Wang, J.-H.; et al. Antimicrobial susceptibility of pathogens isolated from patients with complicated intra-abdominal infections at five medical centers in Taiwan that continuously participated in the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2006 to 2010. Int. J. Antimicrob. Agents 2012, 40, S29–S36. [Google Scholar]
- Wang, J.-T.; Chang, S.-C.; Chang, F.-Y.; Fung, C.-P.; Chuang, Y.-C.; Chen, Y.-S.; Shiau, Y.-R.; Tan, M.-C.; Wang, H.-Y.; Lai, J.-F.; et al. Antimicrobial non-susceptibility of Escherichia coli from outpatients and patients visiting emergency rooms in Taiwan. PLoS ONE 2015, 10, e0144103. [Google Scholar] [CrossRef]
- Tsai, W.-L.; Hung, C.-H.; Chen, H.-A.; Wang, J.-L.; Huang, I.-F.; Chiou, Y.-H.; Chen, Y.-S.; Lee, S.-S.; Hung, W.-Y.; Cheng, M.-F. Extended-spectrum β-lactamase-producing Escherichia coli bacteremia: Comparison of pediatric and adult populations. J. Microbiol. Immunol. Infect. 2018, 51, 723–731. [Google Scholar] [CrossRef]
- Apisarnthanarak, A.; Hsu, L.-Y.; Khawcharoenporn, T.; Mundy, L.-M. Carbapenem-resistant Gram-negative bacteria: How to prioritize infection prevention and control interventions in resource-limited settings? Expert Rev. Anti-Infect. Ther. 2013, 11, 147–157. [Google Scholar] [CrossRef]
- Sheng, W.-H.; Liao, C.-H.; Lauderdale, T.-L.; Ko, W.-C.; Chen, Y.-S.; Liu, J.-W.; Lau, Y.-J.; Wang, L.-S.; Liu, K.-S.; Tsai, T.-Y.; et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int. J. Infect. Dis. 2010, 14, e764–e769. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Pérez-Galera, S.; Salamanca, E.; De Cueto, M.; Calbo, E.; Almirante, B.; Viale, P.; Oliver, A.; Pintado, V.; Gasch, O.; et al. A multinational, preregistered cohort study of β-lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-β-lactamase-producing Enterobacteriaceae. Antimicrob. Agent Chemother. 2016, 60, 4159–4169. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-L.; Lee, C.-C.; Li, C.-W.; Li, M.-C.; Hsueh, P.-R.; Lee, N.-Y.; Ko, W.-C. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J. Microbiol. Immunol. Infect. 2017, 50, 355–361. [Google Scholar] [CrossRef]
- Hsueh, P.-R.; Chen, W.-H.; Luh, K.-T. Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan. Int. J. Antimicrob. Agents 2005, 26, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.-W.; Lee, C.-H.; Liu, J.-W. Risk factors and outcomes for the acquisition of carbapenem-resistant Gram-negative bacillus bacteremia: A retrospective propensity-matched case control study. J. Microbiol. Immunol. Infect. 2018, 51, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.-H.; Lee, C.-M.; Lin, T.-Y.; Chang, S.-C.; Chuang, Y.-C.; Yen, M.-Y.; Hwang, K.-P.; Leu, H.-S.; Yen, C.-C.; Chang, F.-Y. Combating antimicrobial resistance: Antimicrobial stewardship program in Taiwan. J. Microbiol. Immunol. Infect. 2012, 45, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Lin, I.-F.; Yen, Y.-F.; Lin, P.-C.; Shiu, Y.-C.; Hu, H.-Y.; Yang, Y.-P. Impact of an antimicrobial stewardship program with multidisciplinary cooperation in a community public teaching hospital in Taiwan. Am. J. Infect. Control 2013, 41, 1069–1072. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Chiu, C.-H.; Huang, C.-T.; Cheng, C.-W.; Lin, Y.-J.; Hsu, Y.-J.; Chen, C.-H.; Deng, S.-T.; Leu, H.-S. Blood culture-guided de-escalation of empirical antimicrobial regimen for critical patients in an online antimicrobial stewardship programme. Int. J. Antimicrob. Agents 2014, 44, 520–527. [Google Scholar] [CrossRef]
- Lai, C.-C.; Shi, Z.-Y.; Chen, Y.-H.; Wang, F.-D. Effects of various antimicrobial stewardship programs on antimicrobial usage and resistance among common gram-negative bacilli causing health care-associated infections: A multicenter comparison. J. Microbiol. Immunol. Infect. 2016, 49, 74–82. [Google Scholar] [CrossRef]
- Wu, C.-T.; Chen, C.-L.; Lee, H.-Y.; Chang, C.-J.; Liu, P.-Y.; Li, C.-Y.; Liu, M.-Y.; Liu, C.-H. Decreased antimicrobial resistance and defined daily doses after implementation of a clinical culture-guided antimicrobial stewardship program in a local hospital. J. Microbiol. Immunol. Infect. 2017, 50, 846–856. [Google Scholar] [CrossRef]
- Chen, I.-L.; Lee, C.-H.; Su, L.-H.; Wang, Y.-L.; Liu, J.-W. Effects of implementation of an online comprehensive antimicrobial-stewardship program in ICUs: A longitudinal study. J. Microbiol. Immunol. Infect. 2018, 51, 55–63. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Hung, C.-H.; Lai, L.-J.; Chuang, H.-J.; Wang, C.-C.; Lin, P.-T.; Hsu, W.-H. Implementation and outcomes of hospital-wide computerized antimicrobial approval system and on-the-spot education in a traumatic intensive care unit in Taiwan. J. Microbiol. Immunol. Infect. 2018, 51, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Lee, C.-H.; Hong, M.-Y.; Hsieh, C.-C.; Tang, H.-J.; Ko, W.-C. Propensity-matched analysis of the impact of extended-spectrum β-lactamase production on adults with community-onset Escherichia coli, Klebsiella species, and Proteus mirabilis bacteremia. J. Microbiol. Immunol. Infect. 2018, 51, 519–526. [Google Scholar] [CrossRef]
- Chan, Y.-Y.; Lin, T.-Y.; Huang, C.-T.; Deng, S.-T.; Wu, T.-L.; Leu, H.-S.; Chiu, C.-H. Implementation and outcomes of a hospital-wide computerised antimicrobial stewardship programme in a large medical centre in Taiwan. Int. J. Antimicrob. Agents 2011, 38, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Roumbelaki, M.; Christidou, A.; Gikas, A. Antibiotic use and the risk of carbapenem-resistant extended-spectrum-β-lactamase-producing Klebsiella pneumoniae infection in hospitalized patients: Results of a double case–control study. J. Antimicrob. Chemother. 2011, 66, 1383–1391. [Google Scholar]
- Huang, P.-Y.; Shie, S.-S.; Ye, J.-J.; Lin, S.-P.; Liu, T.-P.; Wu, T.-S.; Wu, T.-L.; Cheng, M.-H.; Hsieh, Y.-C.; Chuang, S.-S.; et al. Acquisition and clearance of multidrug resistant Acinetobacter baumannii on healthy young adults concurrently burned in a dust explosion in Taiwan: The implication for antimicrobial stewardship. BMC Infect. Dis. 2017, 17, 598. [Google Scholar] [CrossRef]
- Eckmann, C.; Montravers, P.; Bassetti, M.; Bodmann, K.F.; Heizmann, W.R.; García, M.S.; Guirao, X.; Capparella, M.R.; Simoneau, D.; Dupont, H. Efficacy of tigecycline for the treatment of complicated intra-abdominal infections in real-life clinical practice from five European observational studies. J. Antimicrob. Chemother. 2013, 68, S25–S35. [Google Scholar] [CrossRef][Green Version]
- Solomkin, J.; Mullins, C.-D.; Quintana, A.; Eckmann, C.; Shelbaya, A.; Ernst, F.-R.; Krukas, M.-R.; Reisman, A. Evaluation of tigecycline efficacy and post-discharge outcomes in a clinical practice population with complicated intra-abdominal infection: A propensity score-matched analysis. Surg. Infect. 2016, 17, 402–411. [Google Scholar] [CrossRef]
- Kahlon, S.; Pederson, J.; Majumdar, S.-R.; Belga, S.; Lau, D.; Fradette, M.; Boyko, D.; Bakal, J.-A.; Johnston, C.; Padwal, R.; et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ 2015, 187, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Scheuerman, O.; Schechner, V.; Carmeli, Y.; Gutiérrez-Gutiérrez, B.; Calbo, E.; Almirante, B.; Viale, P.-L.; Oliver, A.; Ruíz-Garbajosa, P.; Gasch, O.; et al. Comparison of predictors and mortality between bloodstream infections caused by ESBL-producing Escherichia coli and ESBL-producing Klebsiella pneumoniae. Infect. Control. Hosp. Epidemiol. 2018, 39, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Fahrbach, K.; Zhao, Q.; Lodise, T. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: Results of a systematic literature review and meta-analysis. Open Forum Infect. Dis. 2018, 5, ofy150. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.-H.; Chuang, Y.-C.; Yu, W.-L. In vitro activity of tigecycline against clinical isolates of extended-spectrum β-lactamase-producing Klebsiella pneumoniae, Serratia marcescens and Enterobacter cloacae. J. Microbiol. Immunol. Infect. 2008, 41, 332–336. [Google Scholar] [PubMed]
- Taneja, J.; Mishra, B.; Thakur, A.; Dogra, V.; Loomba, P. Nosocomial blood-stream infections from extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae from GB Pant Hospital, New Delhi. J. Infect. Dev. Ctries 2010, 4, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Schwaber, M.-J.; Carmeli, Y. Mortality and delay in effective therapy associated with extended-spectrum β-lactamase production in Enterobacteriaceae bacteraemia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2007, 60, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Dartois, N.; Gandjini, H.; Yan, J.-L.; Korth-Bradley, J.; McGovern, P.-C. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob. Agents Chemother. 2013, 57, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Goessens, W.-H.; Mouton, J.-W.; Ten Kate, M.-T.; Sörgel, F.; Kinzig, M.; Bakker-Woudenberg, I.-A. The therapeutic effect of tigecycline, unlike that of Ceftazidime, is not influenced by whether the Klebsiella pneumoniae strain produces extended-spectrum β-lactamases in experimental pneumonia in rats. Antimicrob. Agents Chemother. 2013, 57, 643–646. [Google Scholar] [CrossRef]
- Ku, Y.-H.; Chen, C.-C.; Lee, M.-F.; Chuang, Y.-C.; Tang, H.-J.; Yu, W.-L. Comparison of synergism between colistin, fosfomycin and tigecycline against extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates or with carbapenem resistance. J. Microbiol. Immunol. Infect. 2017, 50, 931–939. [Google Scholar] [CrossRef]
- Su, C.-F.; Chuang, C.; Lin, Y.-T.; Chan, Y.-J.; Lin, J.-C.; Lu, P.-L.; Wang, J.-T.; Siu, L.-K.; Fung, C.-P. Treatment outcome of non-carbapenemase-producing carbapenem-resistant Klebsiella pneumoniae infections: A multicenter study in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 651–659. [Google Scholar] [CrossRef]
- Tang, H.-J.; Lai, C.-C.; Chen, C.-C.; Zhang, C.-C.; Weng, T.-C.; Chiu, Y.-H.; Toh, H.-S.; Chiang, S.-R.; Yu, W.-L.; Ko, W.-C.; et al. Colistin-sparing regimens against Klebsiella pneumoniae carbapenemase-producing K. pneumoniae isolates: Combination of tigecycline or doxycycline and gentamicin or amikacin. J. Microbiol. Immunol. Infect. 2019, 52, 273–281. [Google Scholar] [CrossRef]
- Ferreira, F.-L.; Bota, D.-P.; Bross, A.; Mélot, C.; Vincent, J.-L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef]
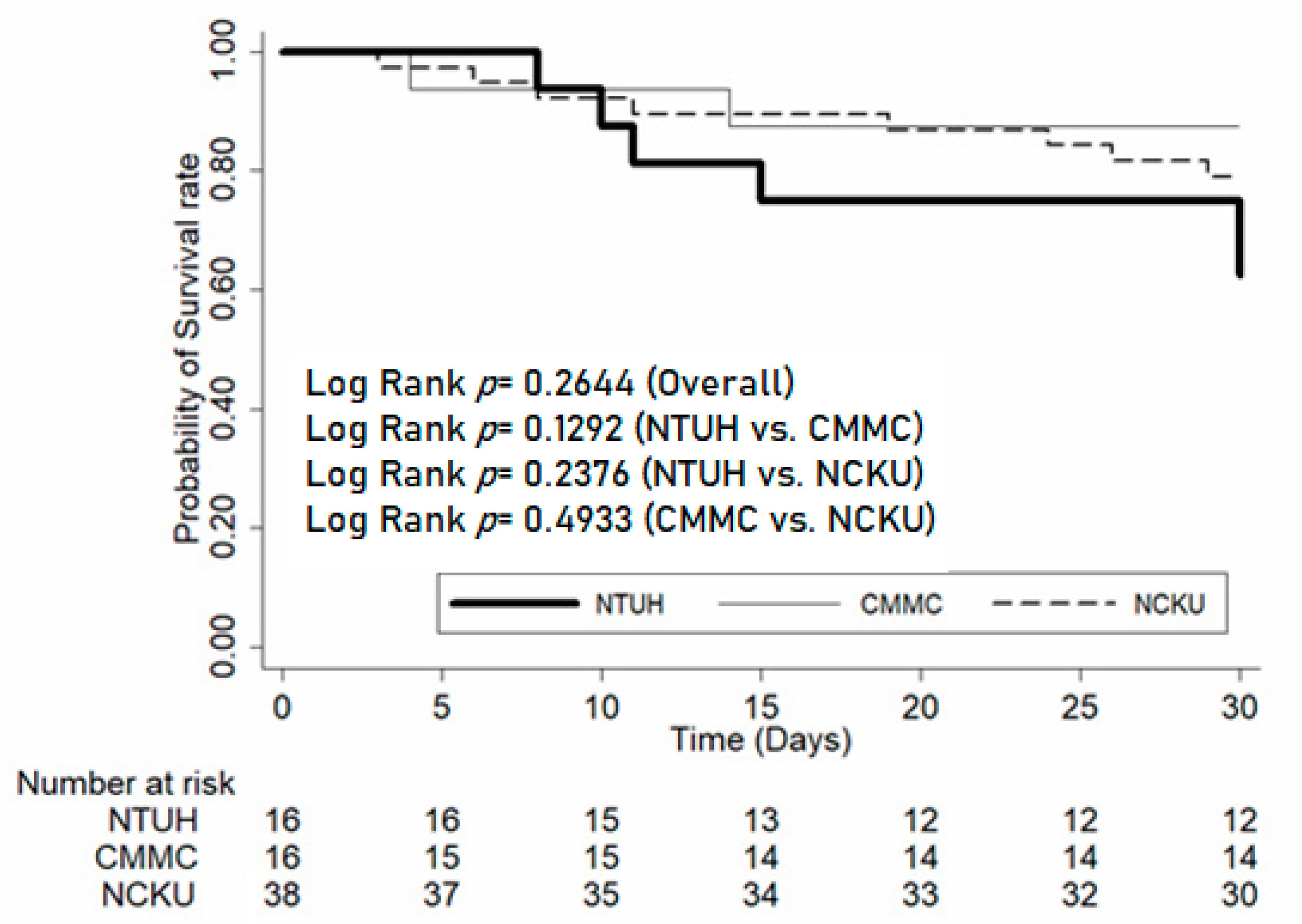
| Variables | NTUH (n = 16) | CMMC (n = 17) | NCKU (n = 38) | p | Total (n = 71) |
|---|---|---|---|---|---|
| Sex, no (%) | |||||
| Female | 5 (31.3) | 3 (17.7) | 20 (52.6) | 0.04 | 28 (39.4) |
| Male | 11 (68.8) | 14 (82.4) | 18 (47.4) | ||
| Age (mean ± SD) | 72.1 ± 15.4 | 66.3 ± 19.7 | 59.9 ± 13.8 | 0.02 | 64.2 ± 16.3 |
| Underlying diseases, no (%) | |||||
| Cardiovascular disease | 10 (62.5) | 12 (70.6) | 24 (63.2) | 0.894 | 46 (64.8) |
| Respiratory disease | 10 (62.5) | 5 (29.4) | 1 (2.7) | <0001 | 16 (22.5) |
| Neurological disease | 6 (37.5) | 5 (29.4) | 5 (13.2) | 0.09 | 16 (22.5) |
| Hepatobiliary disease | 6 (37.5) | 7 (41.2) | 10 (26.3) | 0.50 | 23 (32.4) |
| Metabolic disease | 11 (68.8) | 7 (41.2) | 23 (60.5) | 0.27 | 41 (57.8) |
| Autoimmune disease | 2 (12.5) | 0 (0.0) | 1 (2.6) | 0.18 | 3 (4.2) |
| Solid cancer | 9 (56.3) | 2 (11.8) | 19 (50.0) | 0.01 | 30 (42.3) |
| Charlson score (mean ± SD) | 9.5 ± 3.5 | 2.8 ± 2.0 | 4.3 ± 2.2 | <0001 | 5.1 ± 3.5 |
| Community-acquired, no (%) | 1 (6.3) | 6 (35.3) | 0 (0.0) | <0001 | 7 (9.9) |
| Symptoms on onset, no (%) | |||||
| Fever | 7 (43.8) | 11 (64.7) | 14 (36.8) | 0.16 | 32 (45.1) |
| Chest pain | 0 (0.0) | 1 (5.9) | 1 (2.6) | 0.72 | 2 (2.8) |
| Diarrhea | 4 (25.0) | 2 (11.8) | 2 (5.3) | 0.07 | 8 (11.3) |
| Dyspnea | 13 (81.3) | 7 (41.2) | 2 (5.3) | <0001 | 22 (31.0) |
| Cough | 6 (37.5) | 2 (11.8) | 0 (0.0) | 0.0003 | 8 (11.3) |
| Infection site, no (%) | |||||
| cSSTI | 9 (56.3) | 3 (17.7) | 14 (36.8) | 0.08 | 26 (36.6) |
| cIAI | 6 (37.5) | 4 (23.5) | 18 (47.4) | 0.23 | 28 (39.4) |
| Pneumonia | 10 (62.5) | 10 (58.8) | 3 (7. 9) | <0001 | 23 (32.4) |
| UTI | 8 (50.0) | 3 (17.6) | 3 (7.9) | 0.002 | 14 (19.7) |
| Severity status, no (%) | |||||
| Secondary bacteremia | 5 (31.3) | 2 (11.8) | 3 (7.9) | 0.07 | 10 (14.1) |
| Stay in ICU | 12 (75.0) | 14 (82.4) | 29 (76.3) | 0.87 | 55 (77.5) |
| Post operation status | 9 (56.3) | 8 (47.1) | 2 (5.3) | <0001 | 19 (26.8) |
| Ventilator use | 12 (75.0) | 13 (76.5) | 19 (50.0) | 0.10 | 44 (62.0) |
| SOFA score (mean ± SD) | 7.8 ± 2.8 | 6.2 ± 5.1 | 9.3 ± 4.2 | 0.03 | 8.2 ± 4.3 |
| APACHE II score (mean ± SD) | 20.3 ± 9.9 | 23.5 ± 9.9 | - a | 0.46 | - a |
| Complications, no (%) | |||||
| Septic shock | 13 (81.3) | 7 (41.2) | 18 (47.4) | 0.04 | 38 (53.5) |
| Acute renal failure | 10 (62.5) | 7 (41.2) | 18 (47.4) | 0.46 | 35 (49.3) |
| Liver function impairment | 4 (25.0) | 6 (35.3) | 25 (65.8) | 0.01 | 35 (49.3) |
| Platelet count <100,000/μL | 6 (37.5) | 5 (29.4) | 15 (39.5) | 0.80 | 26 (36.6) |
| Prolonged prothrombin time | 8 (50.0) | 3 (17.7) | 2 (5.3) | 0.0006 | 13 (18.3) |
| ARDS | 10 (62.5) | 0 (0.0) | 0 (0.0) | <0001 | 10 (14.1) |
| Neurological complication | 3 (18.8) | 0 (0.0) | 13 (34.2) | 0.009 | 16 (22.5) |
| Pathogen, no (%) | |||||
| Escherichia coli | 2 | 14 | 14 | 30 | |
| With carbapenem resistance | 2 (100.0) | 0 (0.0) | 1 (7.1) | 0.007 | 3 (10.0) |
| Klebsiella pneumoniae | 14 | 3 | 22 | 39 | |
| With carbapenem resistance | 14 (100.0) | 0 (0.0) | 14 (63.6) | <0001 | 28 (71.8) |
| Empirical Antibiotic therapy | |||||
| Inappropriate | 16 (100.0) | 13 (76.5) | 31 (81.6) | 0.1 | 60 (84.5) |
| Therapy for infection, no (%) | |||||
| Antibiotic only | 5 (31.3) | 12 (70.6) | 31 (81.6) | 0.001 | 48 (67.6) |
| Antibiotic + source control | 11 (68.8) | 5 (29.4) | 7 (18.4) | 23 (32.4) | |
| Clinical outcome on EOT | |||||
| Success (cure/improvement) | 7 (43.8) | 14 (82.4) | 24 (63.2) | 0.02 | 45 (63.4) |
| Failure | 6 (37.5) | 3 (17.7) | 14 (36.8) | 23 (32.4) | |
| Undetermined | 3 (18.8) | 0 (0.0) | 0 (0.0) | 3 (4.2) | |
| Microbiological outcome on EOT | |||||
| Eradication | 0 (0.0) | 12 (70.6) | 24 (63.2) | <0001 | 36 (50.7) |
| Clinical outcome on 30 days after EOT b | |||||
| Success (survival, no readmission) | 6 (37.5) | 12 (75.0) | 23 (60.5) | 0.04 | 41 (57.8) |
| Overall death | 8 (50.0) | 3 (18.8) | 15 (39.5) | 26 (36.6) | |
| Readmission | 2 (12.5) | 1 (6.3) | 0 (0.0) | 3 (4.2) | |
| Days on discharge after infection (mean ± SD) | 44.7 ± 39.7 | 35.7 ± 25.6 | 48.7 ± 46.9 | 0.86 | 44.7 ± 40.9 |
| Bacteriology | Success at EOT (Cure/Improvement) | p | Microbiological Eradication at EOT | p | Success by 30 Days after EOT | p |
|---|---|---|---|---|---|---|
| cSSTI (n = 26) a | 16 (61.5%) | 11 (42.3%) | 15 (57.7%) | |||
| ESBL-CS (n = 13) | 9 (69.2%) | 0.69 | 8 (61.5%) | 0.11 | 10 (76.9%) | 0.11 |
| ESBL-CR (n = 13) | 7 (53.8%) | 3 (23.1%) | 5 (38.5%) | |||
| Escherichia coli (n = 10) | 9 (90%) | 7 (70%) | 9 (90%) b | |||
| ESBL-CS (n = 9) | 8 (88.9%) | 7 (77.8%) | 8 (88.9%) | |||
| ESBL-CR (n = 1) | 1 (100%) | 0 (0%) | 1 (100%) | |||
| Klebsiella pneumoniae (n = 14) | 7 (50%) | 4 (28.6%) | 5 (35.7%) b | |||
| ESBL-CS (n = 2) | 1 (50%) | 1 (50%) | 1 (50%) | |||
| ESBL-CR (n = 12) | 6 (50%) | 3 (25%) | 4 (33.3%) | |||
| cIAI (n = 28) c | 17 (60.7%) | 13 (46.4%) | 16 (57.1%) | |||
| ESBL-CS (n = 13) | 7 (53.8%) | 0.22 | 6 (46.2%) | 0.90 | 6 (46.2%) | 0.38 |
| ESBL-CR (n = 16) | 10 (62.5%) | 7 43.8%) | 10 (62.5%) | |||
| Escherichia coli (n = 10) | 7 (60%) | 5 (50%) | 6 (60%) | |||
| ESBL-CS (n = 8) | 5 (62.5%) | 4 (50%) | 4 (50%) | |||
| ESBL-CR (n = 2) | 2 (100%) | 1 (50%) | 2 (100%) | |||
| Klebsiella pneumoniae (n = 19) | 9 (47.4%) | 7 (36.8%) | 9 (47.4%) | |||
| ESBL-CS (n = 5) | 2 (40%) | 2 (40%) | 2 (40%) | |||
| ESBL-CR (n = 14) | 7 (50%) | 5 (35.7%) | 7 (50%) | |||
| Pneumonia (n = 23) d | 12 (52.2%) | 9 (39.1%) | 10 (43.5%) | |||
| ESBL-CS (n = 13) | 9 (69.2%) | 0.22 | 9 (69.2%) | 0.0006 | 7 (53.8%) | 0.24 |
| ESBL-CR (n = 11) | 4 (36.4%) | 0 (0%) | 3 (27.3%) | |||
| Escherichia coli (n = 10) | 8 (80%) e | 6 (60%) | 6 (60%) | |||
| ESBL-CS (n = 9) | 7 (77.8%) | 6 (66.7%) | 0.40 | 5 (55.6%) | ||
| ESBL-CR (n = 1) | 1 (100%) | 0 (0%) | 1 (100%) | |||
| Klebsiella pneumoniae (n = 14) | 5 (35.7%) e | 3 (21.4%) | 4 (28.6%) | |||
| ESBL-CS (n = 4) | 2 (50%) | 3 (75%) | 0.011 | 2 (50%) | ||
| ESBL-CR (n = 10) | 3 (30%) | 0 (0%) | 2 (20%) | |||
| Multiple infections (n = 18) | 8 (44.4%) | 4 (22.2%) | 6 (33.3%) |
| Variables, no. (%) | Success (Survival without Readmission) (n = 41) | All-Cause Death (n = 26) | p |
|---|---|---|---|
| Sex, no (%) | |||
| Female | 13 (31.7) | 14 (53.8) | 0.08 |
| Male | 28 (68.3) | 12 (46.2) | |
| Age | 63.1 ± 16.4 | 64.7 ± 16.6 | 0.93 |
| Acquired source of infection | |||
| Community | 5 (12.2) | 1 (3.9) | 0.15 |
| Hospital and healthcare institute | 36 (87.8) | 25(96.2) | |
| Symptoms on onset | |||
| Fever | 21 (51.2) | 8 (30.8) | 0.13 |
| Dyspnea | 7 (17.1) | 12 (46.2) | 0.01 |
| Cough | 3 (7.3) | 5 (19.2) | 0.25 |
| Chest pain | 2 (4.9) | 0 (0.0) | 0.52 |
| Diarrhea | 5 (12.2) | 2 (7.7) | 0.70 |
| Infection site | |||
| cSSTI | 15 (36.6) | 9 (34.6) | >999 |
| cIAI | 16 (39.0) | 12 (46.2) | 0.62 |
| Pneumonia | 10 (24.4) | 10 (38.5) | 0.28 |
| UTI | 5 (12.2) | 6 (23.1) | 0.32 |
| Severity status | |||
| Secondary bacteremia | 6 (14.6) | 4 (15.4) | >999 |
| Stay in ICU | 28 (68.3) | 24 (92.3) | 0.03 |
| Post operation status | 12 (29.3) | 4 (15.4) | 0.25 |
| Ventilator use | 21 (51.2) | 20 (76.9) | 0.04 |
| SOFA score | 6.71 ± 3.7 | 10.77 ± 4.3 | 0.0004 |
| APACHE II score | 21.4 ± 10.0 | 20.0 ± 8.8 | 0.77 b |
| Underlying diseases | |||
| Cardiovascular disease | 28 (68.3) | 16 (61.5) | 0.61 |
| Respiratory disease | 9 (22.5) | 6 (23.1) | >999 |
| Neurological disease | 8 (19.5) | 5 (19.2) | >999 |
| Hepatobiliary disease | 16 (39.0) | 6 (23.1) | 0.20 |
| Metabolic disease | 27 (65.9) | 12 (46.2) | 0.13 |
| Autoimmune disease | 1 (2.4) | 2 (7.7) | 0.56 |
| Solid cancer | 17 (41.5) | 11 (42.3) | >999 |
| Charlson score (mean ± SD) | 4.6 ± 2.8 | 5.6 ± 4.1 | 0.68 |
| Complications | |||
| Septic shock | 18 (43.9) | 19 (73.1) | 0.03 |
| Acute renal failure | 16 (39.0) | 17 (65.4) | 0.047 |
| Liver function impairment | 19 (46.3) | 16 (61.5) | 0.32 |
| Platelet count < 100,000/μL | 8 (19.5) | 17 (65.4) | 0.0002 |
| Prolonged prothrombin time | 4 (9.8) | 8 (30.8) | 0.048 |
| ARDS | 4 (9.8) | 5 (19.2) | 0.29 |
| Neurological complication | 6 (14.6) | 10 (38.5) | 0.04 |
| Pathogen, no (%) | |||
| Escherichia coli (ESBL-CR) | 3 (14.3) | 0 (0.0) | 0.55 |
| Klebsiella pneumoniae (ESBL-CR) | 12 (66.7) | 14 (73.7) | 0.73 |
| Therapy for infection, no (%) | |||
| Appropriate empirical antibiotic therapy | 6 (14.6) | 5 (19.2) | 0.74 |
| Antibiotic + source control | 15 (36,6) | 6 (23.1) | 0.25 |
| Clinical outcome on EOT | |||
| Success (cure/improvement) | 38 (92.7) | 3 (11.5) | <0001 |
| Failure | 3 (7.32) | 20 (76.92) | |
| Undetermined | 0 (0.00) | 3 (11.54) | |
| Microbiological outcome on EOT | |||
| Eradication | 29 (70.7) | 5 (19.2) | <0001 |
| Days on discharge after infection (mean ± SD) | 52.1 ± 46.79 | 32.9 ± 29.3 | 0.03 |
| Variables | Heading | Heading | Model 1 (p < 0.05 with Categorical) | Model 2 (Collett’s Model Selection Approach) | ||
|---|---|---|---|---|---|---|
| Univariate HR | p | Multivariable HR | p | Multivariable HR | p | |
| (95% C.I.) | (95% C.I.) | (95% C.I.) | ||||
| Hospital | ||||||
| NTUH | reference | |||||
| CMMC | 0.31 (0.06–1.53) | 0.150 | ||||
| NCKU | 0.53 (0.19–1.53) | 0.243 | ||||
| Dyspnea symptom | 4.71 (1.71–13.00) | 0.003 | 14.49 (2.01–104.48) | 0.008 | 7.33 (2.58–20.78) | 0.0002 |
| Stay in ICU | 2.27 (0.52–10.00) | 0.278 | ||||
| Ventilator use | 2.17 (0.70–6.73) | 0.180 | ||||
| SOFA score | 1.22 (1.09–1.36) | 0.0004 | ||||
| SOFA score (categorical) | ||||||
| ≤8 | reference | reference | reference | |||
| >8 | 7.95 (2.26–27.96) | 0.001 | 10.04 (1.10–92.00) | 0.041 | 4.58 (1.64–12.79) | 0.004 |
| APACHE II score | 1.01 (0.94–1.08) | 0.812 | ||||
| APACHE II score (categorical) | ||||||
| ≤20 | reference | |||||
| >20 | 1.32 (0.46–3.79) | 0.612 | ||||
| Charlson score | 1.09 (0.96–1.24) | 0.187 | ||||
| Charlson (categorical) | ||||||
| 0 | reference | |||||
| 1 | 0.88 (0.06–14.10) | 0.929 | ||||
| ≥2 | 0.78 (0.10–5.97) | 0.815 | ||||
| Complications | ||||||
| Septic shock | 4.35 (1.24–15.29) | 0.022 | 1.68 (0.23–12.38) | 0.612 | ||
| Acute renal failure | 2.52 (0.87–7.25) | 0.088 | ||||
| Thrombocytopenia | 9.70 (2.75–34.15) | 0.0004 | 0.47 (0.04–5.82) | 0.558 | ||
| Prolonged prothrombin time | 3.39 (1.23–9.35) | 0.018 | 0.28 (0.04–2.06) | 0.210 | ||
| ARDS | 2.21 (0.71–6.85) | 0.170 | ||||
| Neurological complication | 1.69 (0.59–4.87) | 0.331 | ||||
| Microbiological eradication | 0.11 (0.03–0.50) | 0.004 | 0.16 (0.03–0.93) | 0.042 | ||
| Klebsiella pneumoniae | ||||||
| ESBL-CS | reference | |||||
| ESBL-CR | 0.71 (0.21–2.35) | 0.574 | ||||
| Therapy for infections | ||||||
| Antibiotic only | reference | |||||
| Antibiotic + source control | 0.43 (0.10–1.91) | 0.269 | ||||
| Empirical antibiotic therapy | ||||||
| Appropriate | reference | |||||
| Inappropriate | 0.52 (0.17–1.61) | 0.255 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.-L.; Lee, N.-Y.; Wang, J.-T.; Ko, W.-C.; Ho, C.-H.; Chuang, Y.-C. Tigecycline Therapy for Infections Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Critically Ill Patients. Antibiotics 2020, 9, 231. https://doi.org/10.3390/antibiotics9050231
Yu W-L, Lee N-Y, Wang J-T, Ko W-C, Ho C-H, Chuang Y-C. Tigecycline Therapy for Infections Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Critically Ill Patients. Antibiotics. 2020; 9(5):231. https://doi.org/10.3390/antibiotics9050231
Chicago/Turabian StyleYu, Wen-Liang, Nan-Yao Lee, Jann-Tay Wang, Wen-Chien Ko, Chung-Han Ho, and Yin-Ching Chuang. 2020. "Tigecycline Therapy for Infections Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Critically Ill Patients" Antibiotics 9, no. 5: 231. https://doi.org/10.3390/antibiotics9050231
APA StyleYu, W.-L., Lee, N.-Y., Wang, J.-T., Ko, W.-C., Ho, C.-H., & Chuang, Y.-C. (2020). Tigecycline Therapy for Infections Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Critically Ill Patients. Antibiotics, 9(5), 231. https://doi.org/10.3390/antibiotics9050231





