Bacteriology and Antimicrobial Resistance in Vanuatu: January 2017 to December 2019
Abstract
1. Introduction
2. Results
2.1. Overview of Specimens Cultured
2.1.1. Number Cultured
2.1.2. Culture Results
2.1.3. Gender and Age Group
2.2. Pathogens
2.2.1. Pathogens Isolated from Blood Specimens
2.2.2. Pathogens Isolated from Pus and Wound Specimens
2.2.3. Pathogens Isolated from Urine Specimens
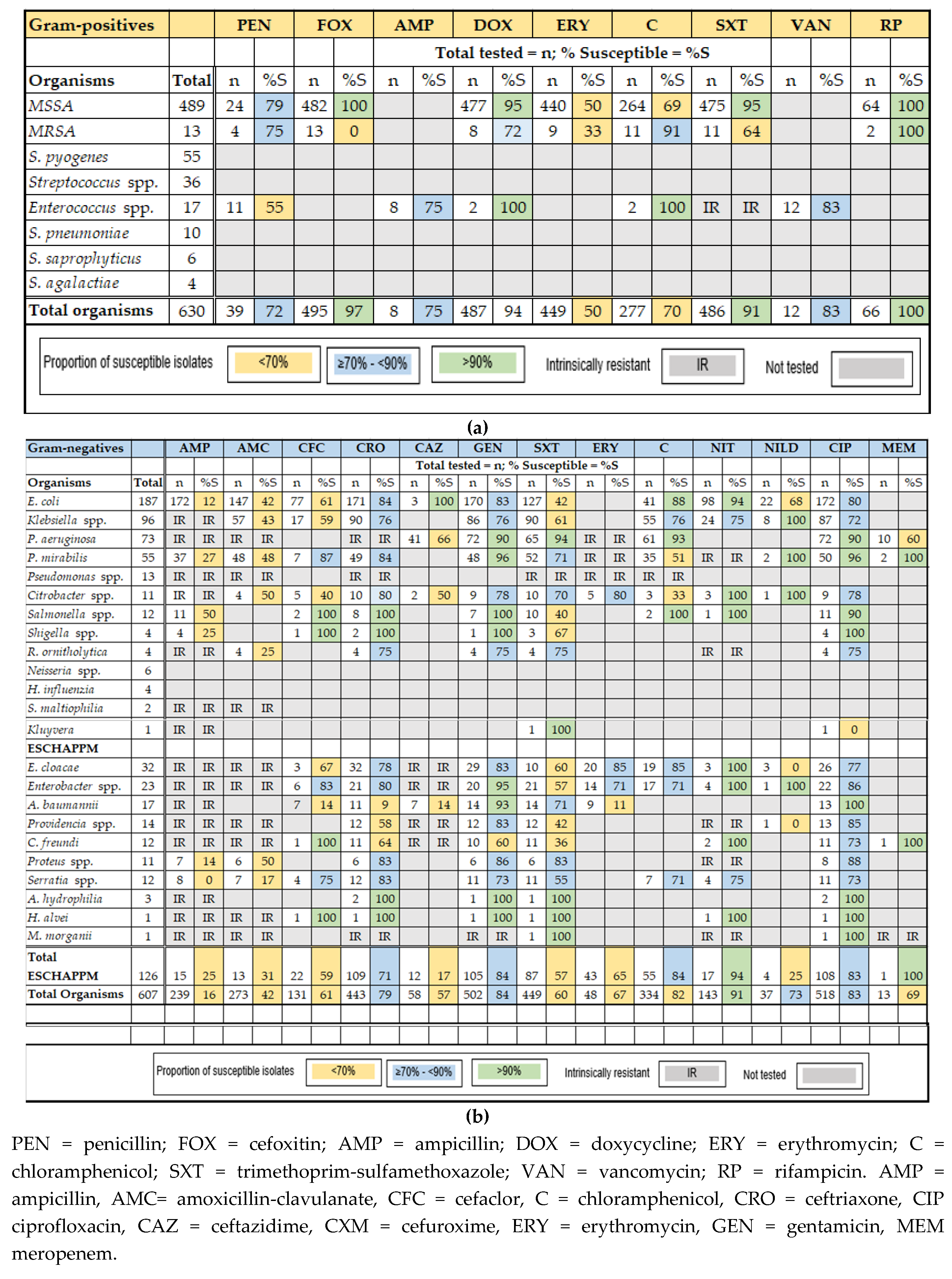
2.3. Antimicrobial Susceptibility Results
2.3.1. Gram-Positive Bacteria
2.3.2. Gram-Negative Bacteria
2.3.3. ESBL-Producing Pathogens, Carbapenem-Resistant P. aeruginosa (CRPA)
3. Discussion
Limitations of the Data
4. Materials and Methods
4.1. Location
4.2. Quality Assurance
4.3. Study Design
4.4. Microbiological Analysis
4.5. Data Analysis
4.6. Ethical Approval
5. Conclusions
- Advance laboratory capacity, including the training of staff, the creation of infrastructure to improve access to microbiology services, adopting international standards, and establishing a continuing laboratory education program;
- Implement antimicrobial stewardship programmes in healthcare settings, including AMR awareness raising, improving prescribing practices, and education for infection prevention and control; and
- Develop a regional AMR surveillance network to support national containment efforts and link surveillance data to enable collaborative efforts against AMR.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Available online: www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (accessed on 20 August 2019).
- World Health Organization Regional Office for the Western Pacific. A Primer for Media: Antimicrobial Resistance in the Western Pacific Region. Available online: iris.wpro.who.int/bitstream/handle/10665.1/13087/WPR_2016_DHS_002-007_eng.pdf (accessed on 12 December 2019).
- Averett, N. Pacific island countries and climate change: Examining associated human health vulnerabilities. Environ. Health Perspect. 2016, 124, A208. [Google Scholar] [CrossRef]
- United Nations Conference on Trade and Development. UN Recognition of Least Developed Countries (LDC). Available online: https://unctad.org/en/Pages/ALDC/Least%20Developed%20Countries/UN-recognition-of-LDCs.aspx (accessed on 25 October 2019).
- World Health Organization Regional Office for the Western Pacific Antimicrobial Resistance in the Asia Pacific Region: A Development Plan. Available online: iris.wpro.who.int/bitstream/handle/10665.1/13570/9789290618126-eng.pdf (accessed on 25 October 2019).
- Critchley, I.; Karlowsky, J. Optimal use of antibiotic resistance surveillance systems. Clin. Microbiol. Infect. 2004, 3, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Nkengasong, J.; Nsubuga, P.; Nwanyanwu, O.; Gershy-Damet, G.; Roscigno, G.; Bulterys, M.; Schoub, B.; DeCock, K.; Birx, D. Laboratory systems and services are critical in Global Health: Time to end the neglect? Am. J. Clin. Pathol. 2010, 134, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Ombelet, S.; Ronat, J.B.; Walsh, T.; Yansouni, C.P.; Cox, J.; Vlieghe, E.; Martiny, D.; Semret, M.; Vandenberg, O.; Jacobs, J. Clinical bacteriology in low-resource settings: Today’s solutions. Lancet Infect. Dis. 2018, 18, e248–e258. [Google Scholar] [CrossRef]
- Foxlee, N.D.; Townell, N.; McIver, L.; Lau, C.L. Antibiotic Resistance in Pacific Island Countries and Territories: A Systematic Scoping Review. Antibiotics (Basel) 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Health Care. Third Report on Antimicrobial Use and Resistance in Human Health; ACSQHC: Sydney, Australia, 2019; Available online: https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system-aura/aura-2019 (accessed on 28 February 2020).
- Coombs, G.; Daley, D.; Pearson, J.; Nimmo, G.; Collignon, P.; McLaws, M.L.; O Robinson, J.; Turnidge, D. Community-onset of Staphylococcus aureus Surveillance Programme annual report, 2012. Commun. Dis. Intell. 2014, 38, E59–E69. [Google Scholar]
- Davis, J.; Jones, C.; Cheng, A.; Howden, B. Australia’s response to the global threat of antimicrobial resistance: Past, present and future. Med. J. Aust. 2019, 211, 106–108e101. [Google Scholar] [CrossRef]
- Wozniak, T.; Paterson, D.; Halton, K. Review of the epidemiological data regarding antimicrobial resistance in Gram-negative bacteria in Australia. Infect. Dis. Health 2017, 22, 210–218. [Google Scholar] [CrossRef]
- Blakiston, M.; Heffernan, H.; Roberts, S.; Freeman, J. The clear and present danger of carbapenemase-producing Enterobacteriaceae (CPE) in New Zealand: Time for a national response plan. N. Z. Med. J. 2017, 130, 72–79. [Google Scholar]
- Heffernan, H.; Woodhouse, R.; Draper, J.; Ren, X. 2016 Survey of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae. Available online: https://surv.esr.cri.nz/PDF_surveillance/Antimicrobial/ESBL/ESBL_2016.pdf (accessed on 11 February 2020).
- Heffernan, H.; Bakker, S. 2017 Survey of Methicillin-Resistant Staphylococcus Aureus (MRSA). Available online: https://surv.esr.cri.nz/PDF_surveillance/Antimicrobial/MRSA/MRSA_2017.pdf (accessed on 11 February 2020).
- Williamson, D.; Baker, M.; French, N.; Thomas, M. Missing in action: An antimicrobial resistance strategy for New Zealand. N. Z. Med. J. 2015, 128, 65–67. [Google Scholar]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; DXF, F.; Milne, S.; Qiu, H.; Sun, M. Chinese venturers to Pacific Small Island Developing States: Travel and lifestyle. Int. J. Tour. Res. 2019, 21, 665–674. [Google Scholar] [CrossRef]
- Curtain, R.; Dornan, M.; Howes, S.; Sherrell, H. Pacific seasonal workers: Learning from the contrasting temporary migration outcomes in Australian and New Zealand horticulture. Asia Pac. Policy Stud. 2018, 5, 462–480. [Google Scholar] [CrossRef]
- Clarke, M.; Feney, S. The dragon versus the kangaroo: Perceptions of Chinese and Australian influence and development assistance in Vanuatu. Aust. J. Polit. Sci. 2019, 54, 334–354. [Google Scholar] [CrossRef]
- Frost, I.; van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic Resistant Bacteria to Guide Research Discovery and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 25 October 2019).
- Torok, E.; Moran, E.; Cooke, F. Oxford Handbook of Infectious Diseases and Microbiology, 2nd ed.; OUP: Oxford, UK, 2017. [Google Scholar]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance: 2014. Available online: https://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 24 October 2019).
- Izzard, A.; Jaworski, J.; Drekore, J.; Urakoko, B.; Poka, H.; Michael, A.; Greenhill, A. Methicillin-resistant staphylococcus aureus in Melanesian children with haematogenous osteomyelitis from the Central Highlands of Papua New Guinea. Int. J. Pediatr. 2018, 6, 8361–8370. [Google Scholar] [CrossRef]
- Alesana-Slater, J.; Ritchie, S.; Heffernan, H.; Camp, T.; Richardson, A.; Herbison, P.; Norris, P. Methicillin-resistant staphylococcus aureus: Samoa, 2007–2008. Emerg. Infect. Dis 2011, 17, 1023–1029. [Google Scholar] [CrossRef]
- Cook Islands Ministry of Health; Evert, R. Antibiotic Guidelines Cook Islands: 2018: Guidelines for Empiric and Targeted Antibiotic Treatment, Prophylaxis, Dosing and Allergies; Ministry of Health: Rarotonga, Cook Islands, 2018; Available online: https://www.health.gov.ck/wp-content/uploads/2018/03/Cook-Islands_Handbook_1_Mar_18-DIGITAL.pdf (accessed on 28 February 2020).
- Tonga Ministry of Health; Evert, R. Antibiotic Guidelines: Tonga 2018: Guidelines for Empiric and Targeted Antibiotic Treatment, Prophylaxis, Dosing and Allergies; Ministry of Health: Nkualofa, Tonga, 2018. [Google Scholar]
- Ferguson, K.; Joseph, J.; Kangapu, S.; Townell, N.; Duke, T.; Manning, L.; Lavu, E. Quality microbiological diagnostics and antimicrobial susceptibility testing, an essential component of antimicrobial resistance surveillance and control efforts in Pacific island nations. WPSAR 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Naidu, K.; Nabose, I.; Ram, S.; Viney, K.; Graham, S.M.; Bissell, K.A. A descriptive study of nosocomial infections in an adult intensive care unit in Fiji: 2011–12. J. Trop. Med. 2014, 1, 545160. [Google Scholar] [CrossRef]
- Melot, B.; Colot, J.; Guerrier, G. Bacteremic community-acquired infections due to Klebsiella pneumoniae: Clinical and microbiological presentation in New Caledonia, 2008-2013. Int. J. Infect. Dis. 2015, 41, 29–31. [Google Scholar] [CrossRef]
- Le Hello, S.; Falcot, V.; Lacassin, F.; Baumann, F.; Nordmann, P.; Naas, T. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in New Caledonia. Clin. Microbiol. Infect. 2008, 14, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Levy, M.; Hirschauer, C.; Marchandin, H.; Nordmann, P. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the carbapenemase OXA-23 in a tertiary care hospital of Papeete, French Polynesia. J. Clin. Microbiol. 2005, 43, 4826–4829. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Bagheri Nejad, S.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Bardossy, A.C.; Bardossy, A.C.; Zervos, J.; Zervos, M. Preventing Hospital-acquired Infections in Low-income and Middle-income Countries: Impact, Gaps, and Opportunities. Infect. Dis. Clin. North Am. 2016, 30, 805. [Google Scholar] [CrossRef]
- Van der Bij, A.; Pitout, J. The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J. Antimicrob. Chemother. 2012, 67, 2090–2100. [Google Scholar] [CrossRef]
- Mento, S.; Vanuatu National Statistics Office. Impact Analysis of International Visitor Arrivals to Vanuatu: Pre and Post Cycline Pam 2015. Available online: https://vnso.gov.vu/index.php/component/advlisting/?view=download&fileId=4959 (accessed on 13 January 2020).
- Roth, A.; Wiklund, A.; Palsson, A.; Melander, E.; Wullt, M.; Cronqvist, J.; Walder, M.; Sturegard, E. Reducing blood culture contamination by a simple informational intervention. J. Clin. Microbiol. 2010, 48, 4552–4558. [Google Scholar] [CrossRef]
- Kredo, T.; Bernhardsson, S.; Machingaidze, S.; Young, T.; Louw, Q.; Ochodo, E.; Grimmer, K. Guide to clinical practice guidelines: The current state of play. Int. J. Q. Health Care 2016, 28, 122–128. [Google Scholar] [CrossRef]
- Vanauatu National Statistcs Office. Mini Census Report: 2016 Post TC Pam. Available online: https://vnso.gov.vu/index.php/document-library (accessed on 13 January 2020).
- Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Kohlmann, R.; Gatermann, S. Analysis and presentation of cumulative antimicrobial susceptibility test data – the influence of different parameters in a routine clinical microbiology laboratory. PLoS ONE 2016, 11, e0147965. [Google Scholar] [CrossRef]
- Pacific Pathology Training Centre. Pacific Pathology Training Centre Overview. Available online: pptc.org.nz/regional-external-quality-assurance-programme/ (accessed on 12 January 2020).
| Ward or Department | Blood | Urine | Woun π/Pus | RespiratoryTract * | CSF ¥ | Stool | Body Fluids ‡ | Ear, Eye ±, Oral | Urogenital Tract £ | Totals |
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||||||
| Medicine | 57 (29) | 36 (17) | 16 (2) | 17 (49) | 0 | 0 | 5 (29) | 0 | 0 | 131 (11) |
| Paediatrics | 47 (24) | 18 (9) | 129 (17) | 7 (20) | 5 (83) | 1 (33) | 5 (29) | 1 (14) | 0 | 213 (17) |
| Nursery | 47 (24) | 3 (1) | 15 (2) | 0 | 0 | 0 | 0 | 2 (29) | 0 | 67 (5) |
| Surgery | 14 (7) | 39 (19) | 389 (51) | 2 (6) | 1 (17) | 0 | 4 (24) | 1 (14) | 1 (20) | 451 (36) |
| Obstetrics & Gynaecology | 0 | 8 (4) | 1 (0.1) | 0 | 0 | 0 | 2 (6) | 0 | 0 | 11 (1) |
| Maternity | 3 (2) | 17 (9) | 31 (4) | 0 | 0 | 0 | 0 | 0 | 0 | 51 (4) |
| Emergency | 14 (7) | 18 (8) | 24 (3) | 1 (3) | 0 | 1 (33) | 1 (3) | 0 | 0 | 59 (4) |
| Outpatient: adult/child | 11 (6) | 54(25) | 143 (19) | 6 (17) | 0 | 1 (33) | 0 | 0 | 2 (60) | 217 (18) |
| Specialist Outpatient Clinic | 0 | 9 (5) | 7 (1) | 2 (6) | 0 | 0 | 0 | 3 (43) | 2 (40) | 23 (2) |
| Hospital referred | 1 (1) | 8 (4) | 6 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 15 (1) |
| Total positive cultures | 194 (100) | 210 (100) | 761 (100) | 35 (100) | 6 (100) | 3 (100) | 17 (100) | 7 (100) | 5 (100) | 1238 (100) |
| Total lab contaminants or no growth | 2890 | 645 | 455 | 143 | 99 | 166 | 121 | 11 | 48 | 4578 |
| Total specimens cultured | 3084 | 855 | 1216 | 178 | 105 | 169 | 138 | 18 | 53 | 5816 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foxlee, N.D.; Townell, N.; Tosul, M.A.L.; McIver, L.; Lau, C.L. Bacteriology and Antimicrobial Resistance in Vanuatu: January 2017 to December 2019. Antibiotics 2020, 9, 151. https://doi.org/10.3390/antibiotics9040151
Foxlee ND, Townell N, Tosul MAL, McIver L, Lau CL. Bacteriology and Antimicrobial Resistance in Vanuatu: January 2017 to December 2019. Antibiotics. 2020; 9(4):151. https://doi.org/10.3390/antibiotics9040151
Chicago/Turabian StyleFoxlee, Nicola D., Nicola Townell, Mary Ann L. Tosul, Lachlan McIver, and Colleen L. Lau. 2020. "Bacteriology and Antimicrobial Resistance in Vanuatu: January 2017 to December 2019" Antibiotics 9, no. 4: 151. https://doi.org/10.3390/antibiotics9040151
APA StyleFoxlee, N. D., Townell, N., Tosul, M. A. L., McIver, L., & Lau, C. L. (2020). Bacteriology and Antimicrobial Resistance in Vanuatu: January 2017 to December 2019. Antibiotics, 9(4), 151. https://doi.org/10.3390/antibiotics9040151






