Rosmarinus officinalis L. (Rosemary) Extracts Containing Carnosic Acid and Carnosol are Potent Quorum Sensing Inhibitors of Staphylococcus aureus Virulence
Abstract
1. Introduction
2. Results
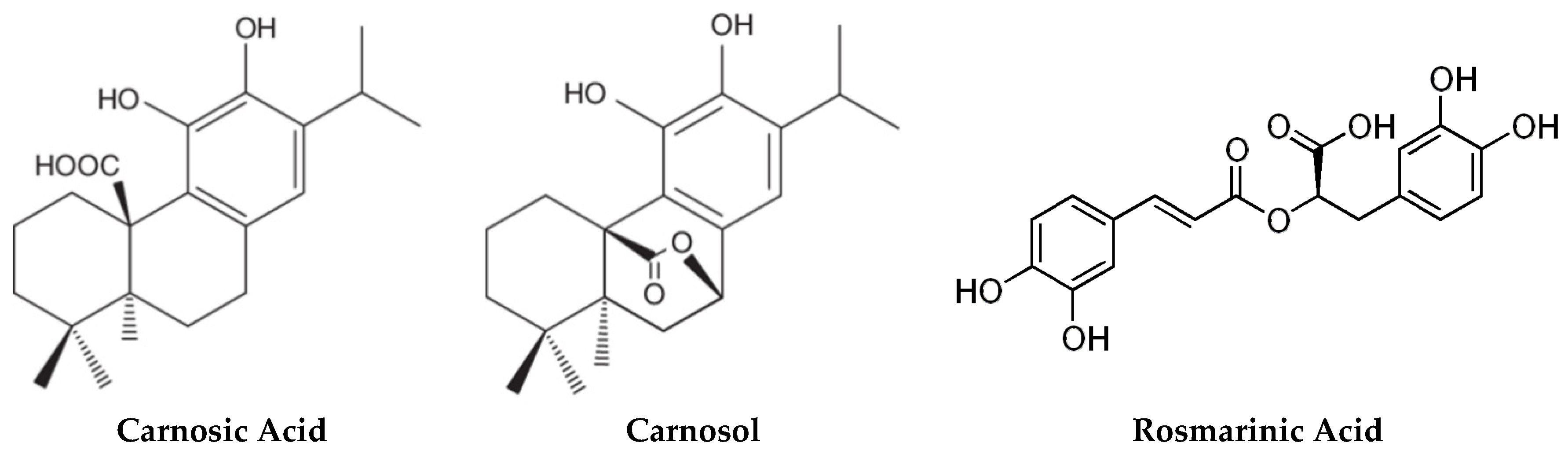
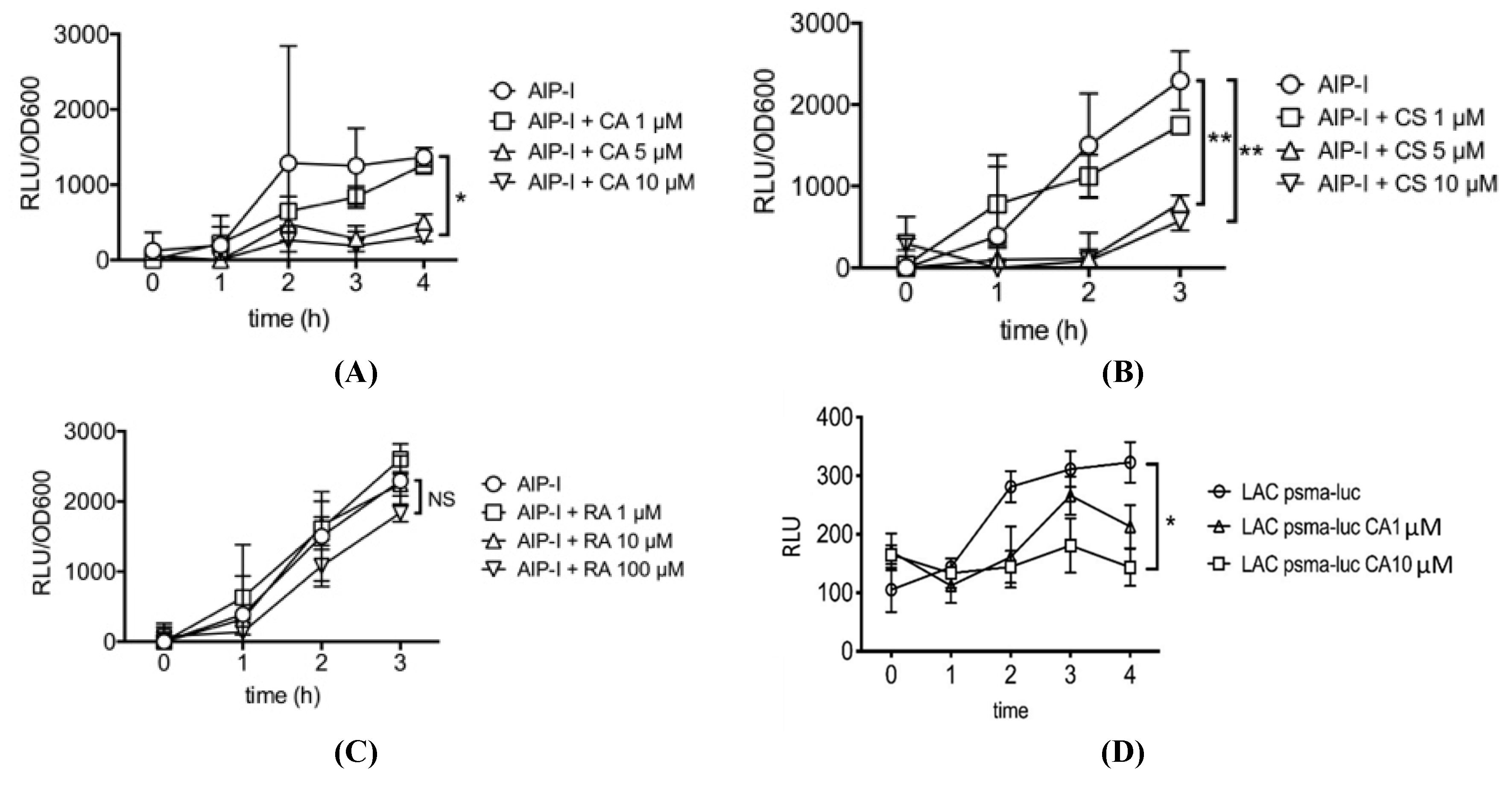
2.1. Carnosic Acid and Carnosol, but not Rosmarinic Acid, Specifically Inhibit agr Virulence Expression
2.2. Rosemary Extracts Containing Carnosic Acid Specifially Inhibit RNAIII agr Virulence Expression
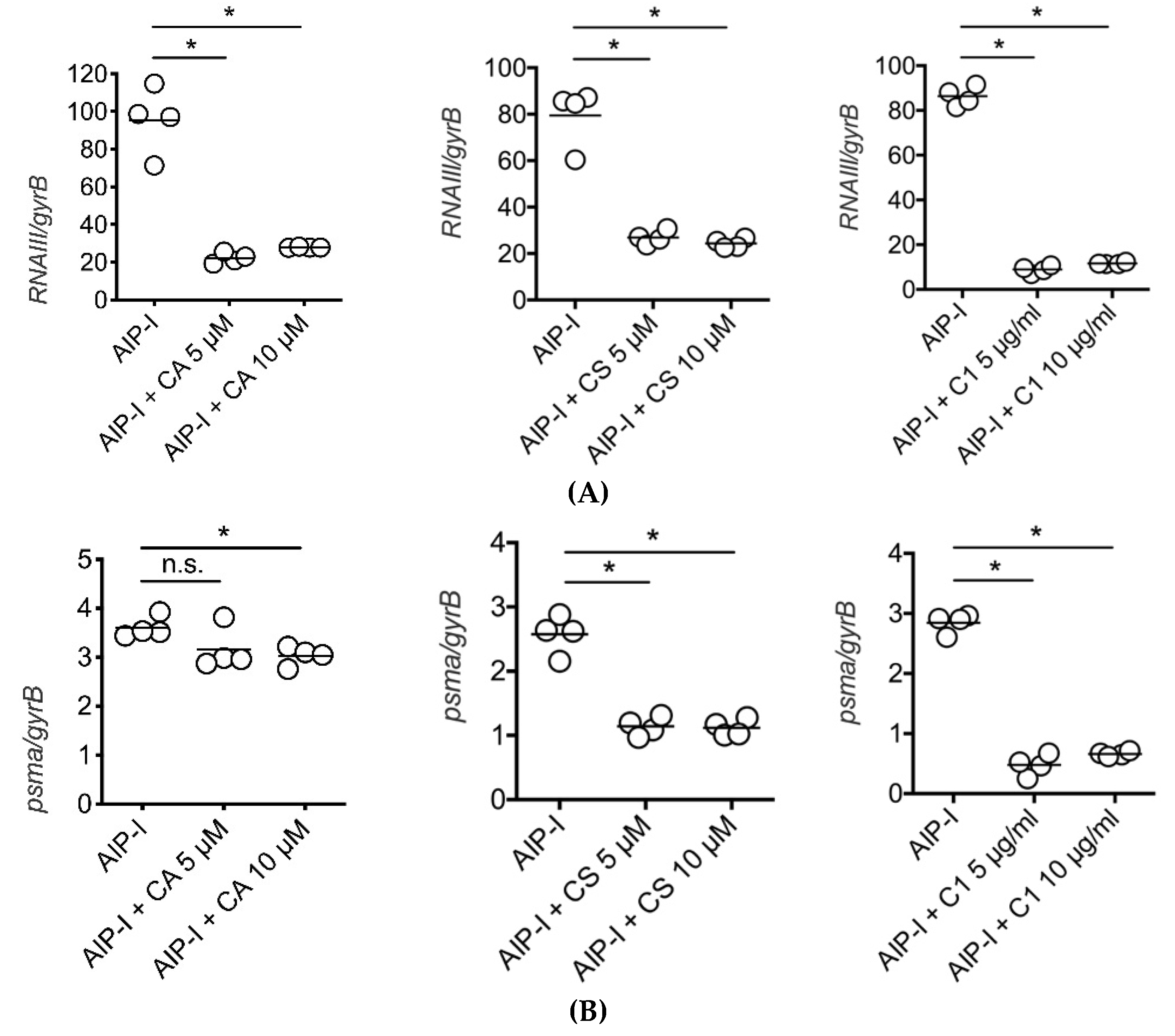
2.3. Carnosic Acid, Carnosol, and Rosemary Extracts Inhibit RNAIII and psmα Gene Expression in Clinical Strains of S. aureus from Atopic Dermatitis Patients
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Strains Utilized
5.2. Test Materials
5.3. Ultraperformance Liquid Chromatography
5.4. Reporter Assay
5.5. Quantitative Real Time PCR
5.6. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aryee, A.; Edgeworth, J.D. Carriage, Clinical Microbiology and Transmission of Staphylococcus aureus. Curr. Top. Microbiol. Immunol. 2017, 409, 1–19. [Google Scholar] [PubMed]
- Lowy, F.D. Medical progress: Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, D.; Harper, L.; Shopsin, B.; Torres, V.J. Staphylococcus aureus pathogenesis in diverse host environments. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Flohr, C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J. Allergy Clin. Immun. 2006, 118, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Rudikoff, D.; Lebwohl, M. Atopic dermatitis. Lancet 1998, 35, 1715–1721. [Google Scholar] [CrossRef]
- Leyden, J.J.; Marples, R.R.; Kligman, A.M. Staphylococcus aureus in the lesions of atopic dermatitis. Br. J. Dermatol. 1974, 90, 525. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Program, N.C.S.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; Prignano, G.; Sperduti, I.; Gurtner, A.; Trento, E.; Toma, L.; Pimpinelli, F.; Capitanio, B.; et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: a pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci. Rep. 2018, 8, 9573. [Google Scholar] [CrossRef]
- Paller, A.S.; Kabashima, K.; Bieber, T. Therapeutic pipeline for atopic dermatitis: End of the drought? J. Allergy Clin. Immun. 2017, 140, 633–643. [Google Scholar] [CrossRef]
- Catherine MacK Correa, M.; Nebus, J. Management of patients with atopic dermatitis: The role of emollient therapy. Dermatol. Res. Practice 2012, 2012. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Hirsch, R.L.; Schneider, L.; Moody, C.; Takaoka, R.; Li, S.H.; Meyerson, L.A.; Mariam, S.G.; Goldstein, G.; Hanifin, J.M. Thymopentin therapy reduces the clinical severity of atopic dermatitis. J. Allergy Clin. Immun. 1990, 85, 927–933. [Google Scholar] [CrossRef]
- Eriksson, S.; van der Plas, M.J.A.; Morgelin, M.; Sonesson, A. Antibacterial and antibiofilm effects of sodium hypochlorite against Staphylococcus aureus isolates derived from patients with atopic dermatitis. Br. J. Dermatol. 2017, 177, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal Infections: Mechanisms of Biofilm Maturation and Detachment as Critical Determinants of Pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef]
- Novick, R.P.; Geisinger, E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013, 11, 667–673. [Google Scholar] [CrossRef]
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci-an overview. Front Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-Independent Target. Gene Control. by the agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef]
- Cheung, G.Y.; Joo, H.S.; Chatterjee, S.S.; Otto, M. Phenol-soluble modulins--critical determinants of staphylococcal virulence. FEMS Microbiol. Rev. 2014, 38, 698–719. [Google Scholar] [CrossRef]
- Nakamura, Y.; Oscherwitz, J.; Cease, K.B.; Chan, S.M.; Munoz-Planillo, R.; Hasegawa, M.; Villaruz, A.E.; Cheung, G.Y.; McGavin, M.J.; Travers, J.B.; et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature 2013, 503, 397–401. [Google Scholar] [CrossRef]
- Nakagawa, S.; Matsumoto, M.; Katayama, Y.; Oguma, R.; Wakabayashi, S.; Nygaard, T.; Saijo, S.; Inohara, N.; Otto, M.; Matsue, H.; et al. Staphylococcus aureus Virulent PSMα Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe 2017, 22, 667–677.e5. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.K.; Vinothkumar, K.; Rajpara, N. Bacterial Quorum Sensing Inhibitors: Attractive Alternatives for Control. of Infectious Pathogens Showing Multiple Drug Resistance. Recent Patents Anti-Infective Drug Dis. 2013, 8, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Heras, B.; Scanlon, M.J.; Martin, J.L. Targeting virulence not viability in the search for future antibacterials. Br. J. Clin. Pharmacol. 2015, 79, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Baldry, M.; Nakamura, Y.; Nakagawa, S.; Frees, D.; Matsue, H.; Nunez, G.; Ingmer, H. Application of an agr-Specific Antivirulence Compound as Therapy for Staphylococcus aureus-Induced Inflammatory Skin Disease. J. Infect. Dis. 2018, 218, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Costa, S.K.; Zaramela, L.S.; Khalil, S.; Todd, D.A.; Winter, H.L.; Sanford, J.A.; O’Neill, A.M.; Liggins, M.C.; Nakatsuji, T.; et al. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med. 2019, 11, eaat8329. [Google Scholar] [CrossRef]
- Rahme, L.G.; Stevens, E.J.; Wolfort, S.F.; Shao, J.; Tompkins, R.G.; Ausubel, F.M. Common virulence factors for bacterial pathogenicity in plants and animals. Science 1995, 268, 1899. [Google Scholar] [CrossRef]
- Rahme, L.G.; Ausubel, F.M.; Cao, H.; Drenkard, E.; Goumnerov, B.C.; Lau, G.W.; Mahajan-Miklos, S.; Plotnikova, J.; Tan, M.-W.; Tsongalis, J.; et al. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. USA 2000, 97, 8815. [Google Scholar] [CrossRef]
- Mukherji, R.; Prabhune, A. Novel glycolipids synthesized using plant essential oils and their application in quorum sensing inhibition and as Antibiofilm agents. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Deryabin, D.; Galadzhieva, A.; Kosyan, D.; Duskaev, G. Plant-derived inhibitors of AHL-mediated quorum sensing in bacteria: Modes of action. Inter. J. Mol. Sci. 2019, 20, 5588. [Google Scholar] [CrossRef]
- Birtic, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant Physiol. 2001, 125, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtic, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act. through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Farahpour, M.R.; Mounesi Rad, S. Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif. Cells Nanomed. Biotechnol. 2019, 47, 980–988. [Google Scholar] [CrossRef]
- Dhariwala, M.Y.; Ravikumar, P. An overview of herbal alternatives in androgenetic alopecia. J. Cosmetic Dermatol. 2019, 18, 966–975. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Nunez, G.; Oscherwitz, J.; Cease, K.; Nakamura, Y.; Nygaard, T. Treatment of Staphylococcal Disorders. US Patent Application US 2016/0031973 Al, 14 February 2016. [Google Scholar]
- Ribet, D.; Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015, 17, 173–183. [Google Scholar] [CrossRef]
- Finlay, B.B.; Falkow, S. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 1997, 61, 136–169. [Google Scholar] [CrossRef]
- Adiliaghdam, F.; Almpani, M.; Gharedaghi, M.H.; Najibi, M.; Hodin, R.A.; Rahme, L.G. Targeting bacterial quorum sensing shows promise in improving intestinal barrier function following burnsite infection. Mol. Med. Rep. 2019, 19, 4057–4066. [Google Scholar]
- Baldry, M.; Kitir, B.; Frokiaer, H.; Christensen, S.B.; Taverne, N.; Meijerink, M.; Franzyk, H.; Olsen, C.A.; Wells, J.M.; Ingmer, H. The agr Inhibitors Solonamide B and Analogues Alter Immune Responses to Staphylococccus aureus but Do Not. Exhibit Adverse Effects on Immune Cell Functions. PLoS ONE 2016, 11, e0145618. [Google Scholar] [CrossRef]
- Wu, S.C.; Liu, F.; Zhu, K.; Shen, J.Z. Natural Products That Target Virulence Factors in Antibiotic-Resistant Staphylococcus aureus. J. Agric. Food Chem. 2019, 67, 13195–13211. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, Y.; Xu, N.; Yang, Q.; Ai, X. Morin Protects Channel Catfish From Aeromonas hydrophila Infection by Blocking Aerolysin Activity. Front Microbiol. 2018, 9, 2828. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, I.; Baig, M.H. Eugenol inhibits quorum sensing and biofilm of toxigenic MRSA strains isolated from food handlers employed in Saudi Arabia. Biotechnol. Biotechnol. Equipment 2017, 31, 387–396. [Google Scholar] [CrossRef]
- Noumi, E.; Merghni, A.; M, M.A.; Haddad, O.; Akmadar, G.; De Martino, L.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-Sieni, A.; et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules 2018, 23, 2672. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Mohammadzadeh, A.; Zahraei Salehi, T.; Mahmoodi, P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J. Appl. Microbiol. 2018, 124, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Li, H.E.; Qiu, J.Z.; Yang, Z.Q.; Dong, J.; Wang, J.F.; Luo, M.J.; Pan, J.; Dai, X.H.; Zhang, Y.; Song, B.L.; et al. Glycyrrhetinic acid protects mice from Staphylococcus aureus pneumonia. Fitoterapia 2012, 83, 241–248. [Google Scholar] [CrossRef]
- Endo, E.H.; Costa, G.M.; Makimori, R.Y.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Anti-biofilm activity of Rosmarinus officinalis, Punica granatum and Tetradenia riparia against methicillin-resistant Staphylococcus aureus (MRSA) and synergic interaction with penicillin. J. Herbal Med. 2018, 14, 48–54. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; de Jesus, D.; Figueira, L.W.; de Oliveira, F.E.; Pacheco Soares, C.; Camargo, S.E.A.; Jorge, A.O.C.; de Oliveira, L.D. Biological activities of Rosmarinus officinalis L. (rosemary) extract as analyzed in microorganisms and cells. Exp. Biol. Med. 2017, 242, 625–634. [Google Scholar] [CrossRef]
- Dastgheyb, S.S.; Villaruz, A.E.; Le, K.Y.; Tan, V.Y.; Duong, A.C.; Chatterjee, S.S.; Cheung, G.Y.; Joo, H.S.; Hickok, N.J.; Otto, M. Role of Phenol-Soluble Modulins in Formation of Staphylococcus aureus Biofilms in Synovial Fluid. Infect. Immun. 2015, 83, 2966–2975. [Google Scholar] [CrossRef]
- Li, M.; Cha, D.J.; Lai, Y.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 2007, 66, 1136–1147. [Google Scholar] [CrossRef]
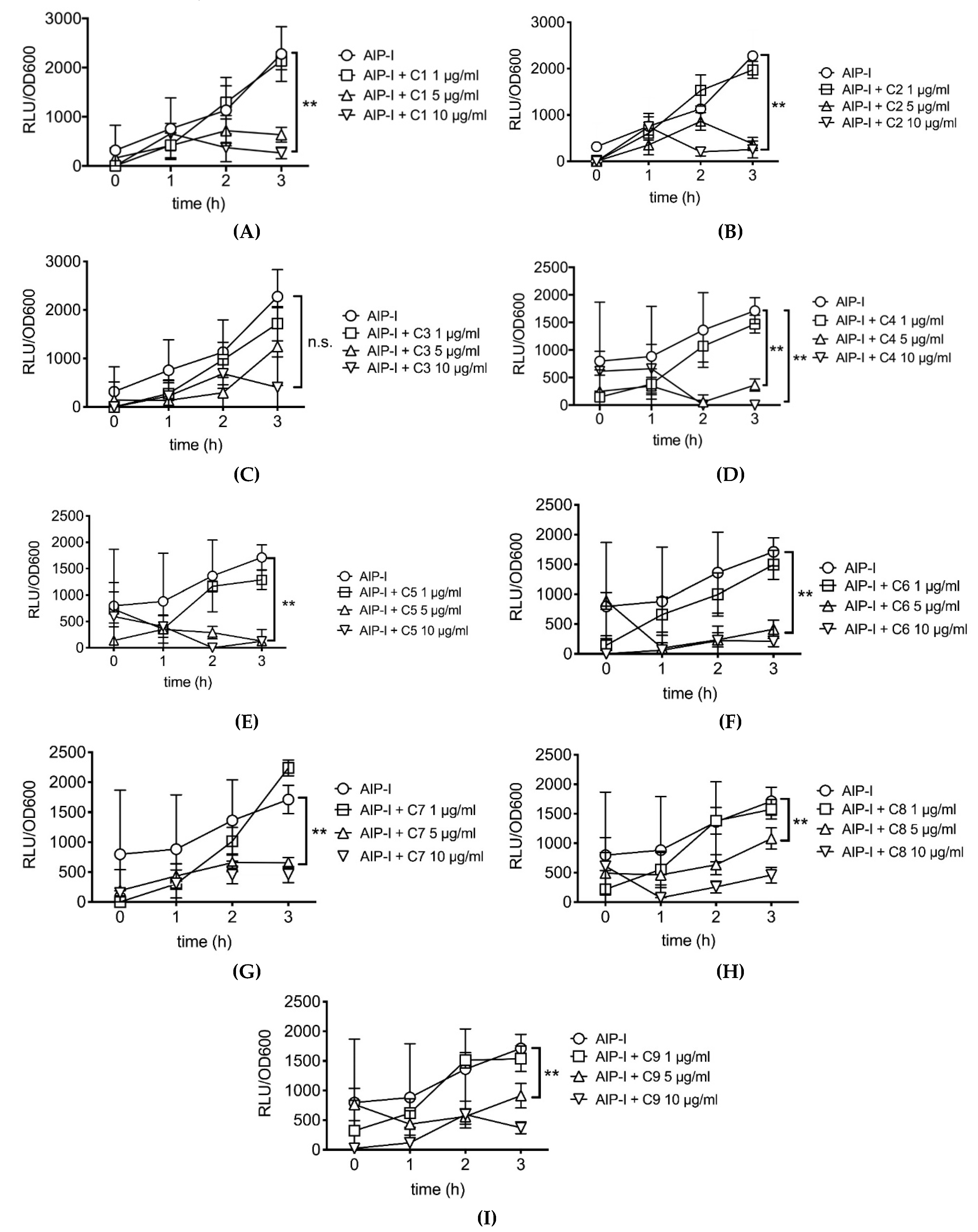
| Code | Carnosic Acid (%) | Carnosol (%) | Rosmarinic Acid (%) |
|---|---|---|---|
| C1 | 6.2 | NA | NA |
| C2 | 11.5 | 2.8 | NA |
| C3 | NA | NA | NA |
| C4 | 20.9 | 2.0 | NA |
| C5 | 21.29 | 24.19 | 1.18 |
| C6 | 15.29 | 4.74 | 0.63 |
| C7 | 16.39 | 3.70 | 6.53 |
| C8 | 13.42 | 6.77 | 6.48 |
| C9 | 13.83 | 2.50 | 6.48 |
| Primer/Probe | Sequence |
|---|---|
| RNAIII forward primer | AATTAGCAAGTGAGTAACATTTGCTAGT |
| RNAIII reverse primer | GATGTTGTTTACGATAGCTTACATGC |
| RNAIII probe | FAM-AGTTAGTTTCCTTGGACTCAGTGCTATGTATTTTTCTT-BHQ |
| psmα forward primer | TAAGCTTAATCGAACAATTC |
| psmα reverse primer | CCCCTTCAAATAAGATGTTCATATC |
| psmα probe | FAM-AAAGAVVTCCTTTGTTTGTTATGAAATCTTATTTACCAG-BHQ |
| gyrB forward primer | CAAATGATCACAGCATTTGGTACAG |
| gyrB reverse primer | CGGCATCAGTCATAATGACGAT |
| gyrB probe | FAM-AATCGGTGGCGACTTTGATCTAGCGAAAG-BHQ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, S.; Hillebrand, G.G.; Nunez, G. Rosmarinus officinalis L. (Rosemary) Extracts Containing Carnosic Acid and Carnosol are Potent Quorum Sensing Inhibitors of Staphylococcus aureus Virulence. Antibiotics 2020, 9, 149. https://doi.org/10.3390/antibiotics9040149
Nakagawa S, Hillebrand GG, Nunez G. Rosmarinus officinalis L. (Rosemary) Extracts Containing Carnosic Acid and Carnosol are Potent Quorum Sensing Inhibitors of Staphylococcus aureus Virulence. Antibiotics. 2020; 9(4):149. https://doi.org/10.3390/antibiotics9040149
Chicago/Turabian StyleNakagawa, Seitaro, Greg G. Hillebrand, and Gabriel Nunez. 2020. "Rosmarinus officinalis L. (Rosemary) Extracts Containing Carnosic Acid and Carnosol are Potent Quorum Sensing Inhibitors of Staphylococcus aureus Virulence" Antibiotics 9, no. 4: 149. https://doi.org/10.3390/antibiotics9040149
APA StyleNakagawa, S., Hillebrand, G. G., & Nunez, G. (2020). Rosmarinus officinalis L. (Rosemary) Extracts Containing Carnosic Acid and Carnosol are Potent Quorum Sensing Inhibitors of Staphylococcus aureus Virulence. Antibiotics, 9(4), 149. https://doi.org/10.3390/antibiotics9040149





