Antimicrobial Activity of Host-Derived Lipids
Abstract
1. Introduction
2. AML Sources
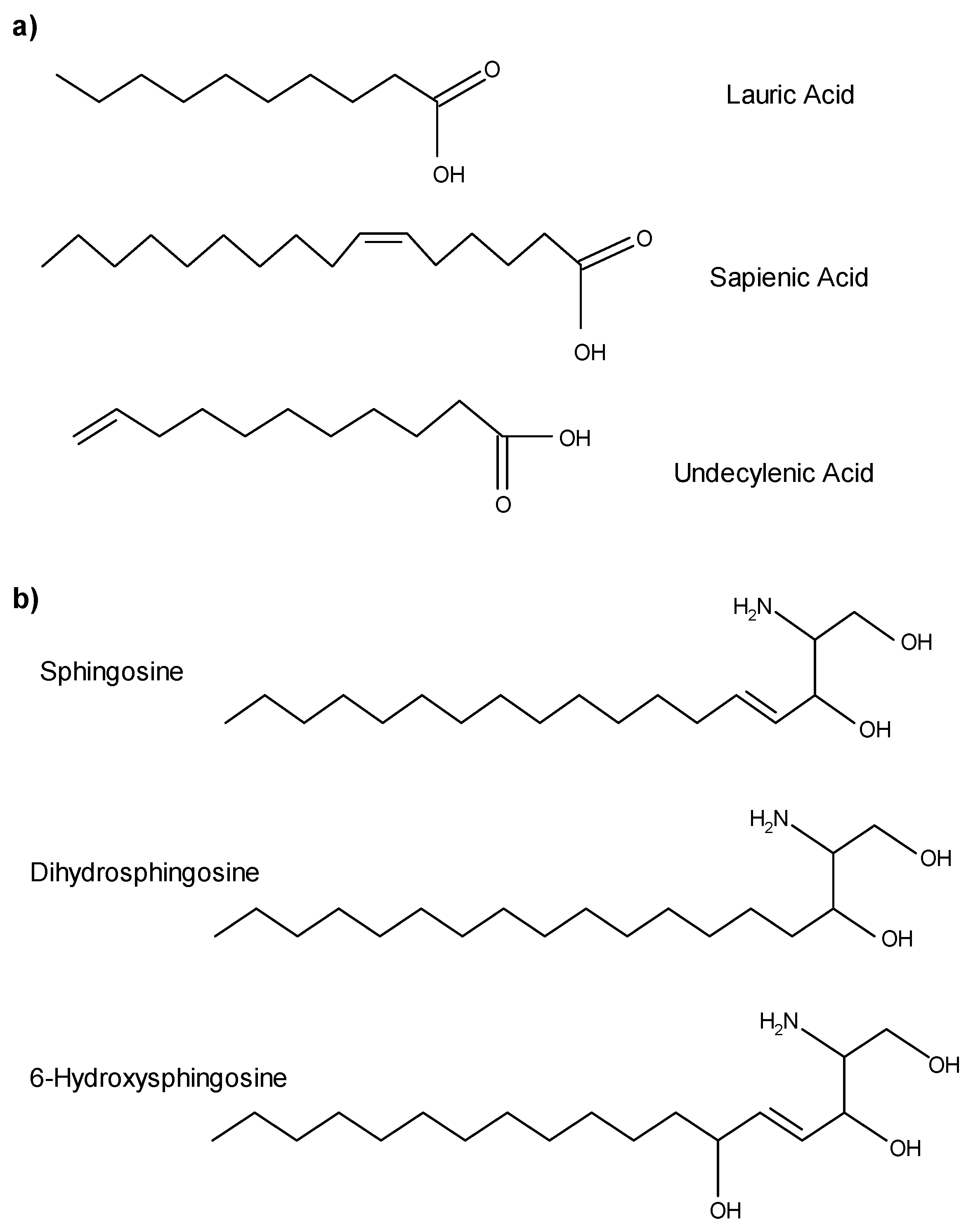
3. Fatty Acids as AMLs
4. Sphingoid Bases as AMLs
5. AML Lipid Mechanisms
6. Consequences of Endogenous AML Disruptions
7. Summary and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sierra, J.M.; Fusté, E.; Rabanal, F.; Vinuesa, T.; Viñas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert. Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Drake, D.R.; Brogden, K.A.; Dawson, D.V.; Wertz, P.W. Thematic review series: Skin lipids. Antimicrobial lipids at the skin surface. J. Lipid Res. 2008, 49, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Hilmarsson, H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem. Phys. Lipids. 2007, 150, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Ma, D.C.; Alvarez, S.; Faull, K.F. Antimicrobial lipids: Emerging effector molecules of innate host defense. World J. Immunol. 2015, 5, 51–61. [Google Scholar] [CrossRef]
- Brogden, K.A.; Drake, D.R.; Dawson, D.V.; Hill, J.R.; Bratt, C.L.; Wertz, P.W. Antimicrobial lipids of the skin and oral mucosa. In Innate Immune Systems of Skin and Oral Mucosa; Dayan, N., Wertz, P.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 75–81. [Google Scholar]
- Dawson, D.V.; Drake, D.R.; Hill, J.R.; Brogden, K.A.; Fischer, C.L.; Wertz, P.W. Organization, barrier function and antimicrobial lipids of the oral mucosa. Int. J. Cosmet Sci. 2013, 35, 220–223. [Google Scholar] [CrossRef]
- Downing, D.T.; Stewart, M.E.; Wertz, P.W.; Colton, S.W.; Strauss, J.S. Skin lipids. Comp. Biochem. Physiol. B. 1983, 76, 673–678. [Google Scholar] [CrossRef]
- Downing, D.T.; Stewart, M.E.; Wertz, P.W.; Colton, S.W.; Abraham, W.; Strauss, J.S. Skin lipids: An update. J. Investig. Dermatol. 1987, 88 (Suppl. 3), 2s–6s. [Google Scholar] [CrossRef]
- Agassandian, M.; Mallampalli, R.K. Surfactant phospholipid metabolism. Biochim. Biophys. Acta. 2013, 1831, 612–625. [Google Scholar] [CrossRef]
- Lee, J.T.; Jansen, M.; Yilma, A.N.; Nguyen, A.; Desharnais, R.; Porter, E. Antimicrobial lipids: Novel innate defense molecules are elevated in sinus secretions of patients with chronic rhinosinusitis. Am. J. Rhinol. Allergy 2010, 24, 99–104. [Google Scholar] [CrossRef]
- Do, T.Q.; Moshkani, S.; Castillo, P.; Anunta, S.; Pogosyan, A.; Cheung, A.; Marbois, B.; Faull, K.F.; Ernst, W.; et al. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J. Immunol. 2008, 181, 4177–4187. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Kim, K.S.; Thormar, H. Inactivation of enveloped viruses in human bodily fluids by purified lipids. Ann. NY Acad. Sci. 1994, 724, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Bortz, J.T.; Wertz, P.W.; Downing, D.T. Composition of cerumen lipids. J. Am. Acad Dermatol. 1990, 23, 845–849. [Google Scholar] [CrossRef]
- Brasser, A.J.; Barwacz, C.A.; Dawson, D.V.; Brogden, K.A.; Drake, D.R.; Wertz, P.W. Presence of wax esters and squalene in human saliva. Arch. Oral. Biol. 2011, 56, 588–591. [Google Scholar] [CrossRef]
- Rohrer, L.; Winterhalter, K.H.; Eckert, J.; Köhler, P. Killing of Giardia lamblia by human milk is mediated by unsaturated fatty acids. Antimicrob. Agents Chemother. 1986, 30, 254–257. [Google Scholar] [CrossRef]
- Field, C.J. The immunological components of human milk and their effect on immune development in infants. J. Nutr. 2005, 135, 1–4. [Google Scholar] [CrossRef]
- Isaacs, C.E.; Litov, R.E.; Thormar, H. Antimicrobial activity of lipids added to human milk, infant formula, and bovine milk. J. Nutr. Biochem. 1995, 6, 362–366. [Google Scholar] [CrossRef]
- Isaacs, C.E.; Kashyap, S.; Heird, W.C.; Thormar, H. Antiviral and antibacterial lipids in human milk and infant formula feeds. Arch. Dis Child. 1990, 65, 861–864. [Google Scholar] [CrossRef]
- Isaacs, C.E.; Thormar, H. The role of milk-derived antimicrobial lipids as antiviral and antibacterial agents. Adv. Exp. Med. Biol. 1991, 310, 159–165. [Google Scholar] [CrossRef]
- Chen, C.H.; Wang, Y.; Nakatsuji, T.; Liu, Y.T.; Zouboulis, C.; Gallo, R.; Zhang, L.; Hseih, M.H.; Huang, C.M. An innate bactericidal oleic acid effective against skin infection of methicillin-resistant Staphylococcus aureus: A therapy concordant with evolutionary medicine. J. Microbiol. Biotechnol. 2011, 21, 391–399. [Google Scholar]
- Hernell, O.; Ward, H.; Bläckberg, L.; Pereira, M.E. Killing of Giardia lamblia by human milk lipases: An effect mediated by lipolysis of milk lipids. J. Infect. Dis. 1986, 153, 715–720. [Google Scholar] [CrossRef]
- Downing, D.T.; Stewart, M.E.; Wertz, P.W.; Strauss, J.S. Essential fatty acids and acne. J. Am. Acad. Dermatol. 1986, 14, 221–225. [Google Scholar] [CrossRef]
- Downing, D.; Strauss, J. Synthesis and composition of surface lipids of human skin. J. Investig. Dermatol. 1974, 62, 228–244. [Google Scholar] [CrossRef]
- Nicolaides, N.; Wells, G.C. On the biogenesis of the free fatty acids in human skin surface fat. J. Investig. Dermatol. 1957, 29, 423–433. [Google Scholar] [CrossRef][Green Version]
- Downing, D.T.; Strauss, J.S.; Pochi, P.E. Variability in the chemical composition of human skin surface lipids. J. Investig. Dermatol. 1969, 53, 322–327. [Google Scholar] [CrossRef]
- Defagó, M.D.; Valentich, M.A.; Actis, A.B. Lipid characterization of human saliva. J. Calif. Dent. Assoc. 2011, 39, 874–880. [Google Scholar]
- Slomiany, B.L.; Murty, V.L.; Slomiany, A. Salivary lipids in health and disease. Prog Lipid Res. 1985, 24, 311–324. [Google Scholar] [CrossRef]
- Larsson, B.; Olivecrona, G.; Ericson, T. Lipids in human saliva. Arch. Oral Biol. 1996, 41, 105–110. [Google Scholar] [CrossRef]
- Undecylenic acid. Monograph. Altern Med. Rev. 2002, 7, 68–70.
- Wertz, P.W. (University of Iowa, Iowa City, Iowa, USA); Fischer, C.L. (Waldorf University, Forest City, Iowa, USA). Personal Communication, 2019.
- Wertz, P. Epidermal Lamellar Granules. Skin Pharmacol. Physiol. 2018, 31, 262–268. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Ceramidase activity in porcine epidermis. FEBS Lett. 1990, 268, 110–112. [Google Scholar] [CrossRef]
- Wertz, P.W. Integral lipids of hair and stratum corneum. EXS 1997, 78, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Law, S.L.; Squier, C.A.; Wertz, P.W. Free sphingosines in oral epithelium. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 110, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.L.; Walters, K.S.; Drake, D.R.; Blanchette, D.R.; Dawson, D.V.; Brogden, K.A.; Wertz, P.W. Sphingoid bases are taken up by Escherichia coli and Staphylococcus aureus and induce ultrastructural damage. Skin Pharmacol. Physiol. 2013, 26, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.L.; Walters, K.S.; Drake, D.R.; Dawson, D.V.; Blanchette, D.R.; Brogden, K.A.; Wertz, P.W. Oral mucosal lipids are antibacterial against Porphyromonas gingivalis, induce ultrastructural damage, and alter bacterial lipid and protein compositions. Int. J. Oral Sci. 2013, 5, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.L.; Drake, D.R.; Dawson, D.V.; Blanchette, D.R.; Brogden, K.A.; Wertz, P.W. Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrob. Agents Chemother. 2012, 56, 1157–1161. [Google Scholar] [CrossRef]
- Garaicoa, J.L.; Fischer, C.L.; Bates, A.M.; Holloway, J.; Avila-Ortiz, G.; Guthmiller, J.M.; Johnson, G.K.; Stanford, C.; Brogden, K.A. Promise of Combining Antifungal Agents in Denture Adhesives to Fight Candida Species Infections. J. Prosthodont. 2018, 27, 755–762. [Google Scholar] [CrossRef]
- Pavicic, T.; Wollenweber, U.; Farwick, M.; Korting, H.C. Anti-microbial and -inflammatory activity and efficacy of phytosphingosine: An in vitro and in vivo study addressing acne vulgaris. Int. J. Cosmet Sci. 2007, 29, 181–190. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Free sphingosine in human epidermis. J. Investig. Dermatol. 1990, 94, 159–161. [Google Scholar] [CrossRef]
- Burtenshaw, J.M. The mechanism of self-disinfection of the human skin and its appendages. J. Hyg. (Lond). 1942, 42, 184–210. [Google Scholar] [CrossRef]
- Burtenshaw, J.M. Self-disinfection of the skin; a short review and some original observations. Br. Med. Bull. 1945, 3, 161–164. [Google Scholar] [CrossRef]
- Rothman, S.; Lorincz, A.L. Defense mechanisms of the skin. Annu. Rev. Med. 1963, 14, 215–242. [Google Scholar] [CrossRef]
- Weitkamp, A.W.; Smiljanic, A.M.; Rothman, S. The free fatty acids of human hair fat. J. Am. Chem. Soc. 1947, 69, 1936–1939. [Google Scholar] [CrossRef]
- Rothman, S.; Smiljanic, A.M.; Weitkamp, A.W. Mechanism of spontaneous cure in puberty of ringworm of the scalp. Science 1946, 104, 201–203. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Kabara, J.J.; Conley, A.J.; Truant, J.P. Relationship of chemical structure and antimicrobial activity of alkyl amides and amines. Antimicrob. Agents Chemother. 1972, 2, 492–498. [Google Scholar] [CrossRef]
- Kabara, J.J.; Conley, A.J.; Swieczkowski, D.M. Antimicrobial action of isomeric fatty acids on group A Streptococcus. J. Med. Chem. 1973, 16, 1060–1063. [Google Scholar] [CrossRef]
- Kabara, J.J.; Haitsma, G.V. Aminimides: II. Antimicrobial effect of short chain fatty acid derivatives. J. Am. Oil Chem. Soc. 1975, 52, 444–447. [Google Scholar] [CrossRef]
- Kabara, J.J.; McKillip, W.J.; Sedor, E.A. Aminimidesi I. Antimicrobial effect of some long chain fatty acid derivatives. J. Am. Oil Chem. Soc. 1975, 52, 316–317. [Google Scholar] [CrossRef]
- Kabara, J.J.; Vrable, R.; Ikeda, I.; Okahara, M. Aminimides IV: Antimicrobial activity of 1, 1, 1-tris(2-hydroxyethyl)amine-2-acylimides. J. Am. Oil Chem. Soc. 1977, 54, 316–318. [Google Scholar] [CrossRef]
- Kabara, J.J. Aminimides: III antimicrobial effect of various hexadecyl and quaternary derivatives. J. Am. Oil Chem. Soc. 1977, 54, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J.; Vrable, R. Antimicrobial lipids: Natural and synthetic fatty acids and monoglycerides. Lipids 1977, 12, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Kao, M.C.; Fang, J.Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.M. Antimicrobial property of lauric acid against Propionibacterium acnes: Its therapeutic potential for inflammatory acne vulgaris. J. Investig. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.; Thormar, H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS 2001, 109, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Fischer, C.L.; Dawson, D.V.; Blanchette, D.R.; Drake, D.R.; Wertz, P.W.; Brogden, K.A. Protein Analysis of Sapienic Acid-Treated Porphyromonas gingivalis Suggests Differential Regulation of Multiple Metabolic Pathways. J. Bacteriol. 2016, 198, 157–167. [Google Scholar] [CrossRef]
- Wille, J.J.; Kydonieus, A. Palmitoleic acid isomer (C16:1delta6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol. Appl. Skin Physiol. 2003, 16, 176–187. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, T.H.; Chuang, L.T.; Li, Y.Y.; Zouboulis, C.C.; Tsai, P.J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Meana, C.; Guijas, C.; Pereira, L.; Lebrero, P.; Balboa, M.A.; Balsinde, J. Occurrence and biological activity of palmitoleic acid isomers in phagocytic cells. J. Lipid Res. 2018, 59, 237–249. [Google Scholar] [CrossRef]
- Bobiński, R.; Wyszomirski, M.; Machnickam, A.; Pielesz, A.; Kawecki, M.; Waksmańska, W.; Staniszewski, L. The Effect of Lauric Acid on Pathogens Colonizing the Burn Wound: A Pilot Study. Altern. Ther. Health Med. 2019. [Google Scholar]
- Isaacs, C.E.; Thormar, H.; Pessolano, T. Membrane-disruptive effect of human milk: Inactivation of enveloped viruses. J. Infect. Dis. 1986, 154, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, CE. The antimicrobial function of milk lipids. Adv. Nutr Res. 2001, 10, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. The antimicrobial properties of milkfat after partial hydrolysis by calf pregastric lipase. Chem. Biol. Interact. 2002, 140, 185–198. [Google Scholar] [CrossRef]
- Sprong, R.C.; Hulstein, M.F.; Van der Meer, R. Bactericidal activities of milk lipids. Antimicrob. Agents Chemother. 2001, 45, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Chen, J.W.; Rathod, J.; Jiang, Y.Z.; Tsai, P.J.; Hung, Y.P.; Ko, W.C.; Paredes-Sabia, D.; Huang, I.H. Lauric Acid Is an Inhibitor of Clostridium difficile Growth in Vitro and Reduces Inflammation in a Mouse Infection Model. Front. Microbiol. 2017, 8, 2635. [Google Scholar] [CrossRef]
- Numata, M.; Kandasamy, P.; Nagashima, Y.; Posey, J.; Hartshorn, K.; Woodland, D.; Voelker, D.R. Phosphatidylglycerol suppresses influenza A virus infection. Am. J. Respir. Cell Mol. Biol. 2012, 46, 479–487. [Google Scholar] [CrossRef]
- Numata, M.; Kandasamy, P.; Nagashima, Y.; Fickes, R.; Murphy, R.C.; Voelker, D.R. Phosphatidylinositol inhibits respiratory syncytial virus infection. J. Lipid Res. 2015, 56, 578–587. [Google Scholar] [CrossRef]
- Hilmarsson, H.; Kristmundsdóttir, T.; Thormar, H. Virucidal activities of medium- and long-chain fatty alcohols, fatty acids and monoglycerides against herpes simplex virus types 1 and 2: Comparison at different pH levels. APMIS 2005, 113, 58–65. [Google Scholar] [CrossRef]
- McLain, N.; Ascanio, R.; Baker, C.; Strohaver, R.A.; Dolan, J.W. Undecylenic acid inhibits morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2873–2875. [Google Scholar] [CrossRef]
- Bergsson, G.; Steingrímsson, O.; Thormar, H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents. 2002, 20, 258–262. [Google Scholar] [CrossRef]
- Petschow, B.W.; Batema, R.P.; Ford, L.L. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob. Agents Chemother. 1996, 40, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Petschow, B.W.; Batema, R.P.; Talbott, R.D.; Ford, L.L. Impact of medium-chain monoglycerides on intestinal colonisation by Vibrio cholerae or enterotoxigenic Escherichia coli. J. Med. Microbiol. 1998, 47, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yamamoto, Y.; Miura, M.; Konno, H.; Yano, S.; Nonomura, Y. Systematic Analysis of Selective Bactericidal Activity of Fatty Acids against Staphylococcus aureus with Minimum Inhibitory Concentration and Minimum Bactericidal Concentration. J. Oleo Sci. 2019, 68, 291–296. [Google Scholar] [CrossRef]
- Willett, N.P.; Morse, G.E. Long-chain fatty acid inhibition of growth of Streptococcus agalactiae in a chemically defined medium. J. Bacteriol. 1966, 91, 2245–2250. [Google Scholar] [CrossRef]
- Miller, S.J.; Aly, R.; Shinefeld, H.R.; Elias, P.M. In vitro and in vivo antistaphylococcal activity of human stratum corneum lipids. Arch. Dermatol. 1988, 124, 209–215. [Google Scholar] [CrossRef]
- Bibel, D.J.; Miller, S.J.; Brown, B.E.; Pandey, B.B.; Elias, P.M.; Shinefield, H.R.; Aly, R. Antimicrobial activity of stratum corneum lipids from normal and essential fatty acid-deficient mice. J. Investig. Dermatol. 1989, 92, 632–638. [Google Scholar] [CrossRef]
- Bibel, D.J.; Aly, R.; Shinefield, H.R. Antimicrobial activity of sphingosines. J. Investig. Dermatol. 1992, 98, 269–273. [Google Scholar] [CrossRef]
- Bibel, D.J.; Aly, R.; Shinefield, H.R. Inhibition of microbial adherence by sphinganine. Can. J. Microbiol. 1992, 38, 983–985. [Google Scholar] [CrossRef]
- Bibel, D.J.; Aly, R.; Shah, S.; Shinefield, H.R. Sphingosines: Antimicrobial barriers of the skin. Acta Derm. Venereol. 1993, 73, 407–411. [Google Scholar] [CrossRef]
- Bibel, D.J.; Aly, R.; Shinefield, H.R. Topical sphingolipids in antisepsis and antifungal therapy. Clin. Exp. Dermatol. 1995, 20, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Sun, L.; Smith, D.L.; Laxminarayan, R. The changing epidemiology of methicillin-resistant Staphylococcus aureus in the United States: A national observational study. Am. J. Epidemiol. 2013, 177, 666–674. [Google Scholar] [CrossRef]
- Klein, E.; Smith, D.L.; Laxminarayan, R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 2007, 13, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.P.; Schumacher, F.; Baker, J.; Soddemann, M.; Wilker, B.; Caldwell, C.C.; Gobble, R.M.; Kalmer, M.; Becker, K.A.; Beck, S.; et al. Sphingosine-coating of plastic surfaces prevents ventilator-associated pneumonia. J. Mol. Med. (Berl.) 2019, 97, 1195–1211. [Google Scholar] [CrossRef]
- Beck, S.; Sehl, C.; Voortmann, S.; Verhasselt, H.L.; Edwards, M.J.; Buer, J.; Hasenberg, M.; Gulbins, E.; Becher, K.A. Sphingosine is able to prevent and eliminate Staphylococcus epidermidis biofilm formation on different orthopedic implant materials in vitro. J. Mol. Med. (Berl.) 2019. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kobayashi, M.; Matsuzaki, E.; Higashi, K.; Takahashi-Yanaga, F.; Takano, A.; Hirata, M.; Nishimura, F. Sphingosine-1-phosphate-enhanced Wnt5a promotes osteogenic differentiation in C3H10T1/2 cells. Cell Biol. Int. 2016, 40, 1129–1136. [Google Scholar] [CrossRef]
- Carstens, H.; Schumacher, F.; Keitsch, S.; Kramer, M.; Kühn, C.; Sehl, C.; Woddemann, M.; Hermann, D.; Swaidan, A. Clinical Development of Sphingosine as Anti-Bacterial Drug: Inhalation of Sphingosine in Mini Pigs has no Adverse Side Effects. Cell Physiol. Biochem. 2019, 53, 1015–1028. [Google Scholar] [CrossRef]
- Galbraith, H.; Miller, T.B.; Paton, A.M.; Thompson, J.K. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J. Appl. Bacteriol. 1971, 34, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef] [PubMed]
- Khulusi, S.; Ahmed, H.A.; Patel, P.; Mendall, M.A.; Northfield, T.C. The effects of unsaturated fatty acids on Helicobacter pylori in vitro. J. Med. Microbiol. 1995, 42, 276–282. [Google Scholar] [CrossRef]
- Nichols, F.C. Novel ceramides recovered from Porphyromonas gingivalis: Relationship to adult periodontitis. J. Lipid Res. 1998, 39, 2360–2372. [Google Scholar]
- Nichols, F.C.; Riep, B.; Mun, J.; Morton, M.D.; Kawai, T.; Dewhirst, F.E.; Smith, M.B. Structures and biological activities of novel phosphatidylethanolamine lipids of Porphyromonas gingivalis. J. Lipid Res. 2006, 47, 844–853. [Google Scholar] [CrossRef]
- Nichols, F.C.; Riep, B.; Mun, J.; Morton, M.D.; Bojarski, M.T.; Dewhirst, F.E.; Smith, M.B. Structures and biological activity of phosphorylated dihydroceramides of Porphyromonas gingivalis. J. Lipid Res. 2004, 45, 2317–2330. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.S.; Lee, T.G.; Cho, H.Y.; Kim, Y.H.; Kim, W.G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef]
- Sado-Kamdem, S.L.; Vannini, L.; Guerzoni, M.E. Effect of alpha-linolenic, capric and lauric acid on the fatty acid biosynthesis in Staphylococcus aureus. Int. J. Food Microbiol. 2009, 129, 288–294. [Google Scholar] [CrossRef]
- Malina, A.; Shai, Y. Conjugation of fatty acids with different lengths modulates the antibacterial and antifungal activity of a cationic biologically inactive peptide. Biochem. J. 2005, 390, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A.; De Lucca, A.J.; Bland, J.; Elliott, S. Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc. Natl. Acad. Sci. USA 1996, 93, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Kalfa, V.C.; Jia, H.P.; Kunkle, R.A.; McCray, P.B.; Tack, B.F.; Brogden, K.A. Congeners of SMAP29 kill ovine pathogens and induce ultrastructural damage in bacterial cells. Antimicrob. Agents Chemother. 2001, 45, 3256–3261. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J. Biol Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Ohki, K.; Shimamoto, Y.; Kohashi, O. Morphology of defensin-treated Staphylococcus aureus. Infect. Immun. 1995, 63, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.; Burnell, K.; Qian, F.; Brogden, K.; Wertz, P.; Drake, D. Synergistic activity of human skin lipids and LL37. J. Dent. Res. 2006, 85A, 2113. [Google Scholar]
- Gell, G.; Drake, D.R.; Wertz, P.W. Peridex and Lauric Acid Exhibit a Synergistic Effect on Streptococcus mutans. J. Dent. Res. 1995, 74, 76. [Google Scholar]
- Bratt, C.L.; Dayan, N. Coryneform species and their role in the generation of human malodor. In Innate Immune System of Skin and Oral Mucosa; Wertz, P.W., Dayan, N., Eds.; Wiley: Hobokern, NJ, USA, 2011; pp. 333–349. [Google Scholar]
- Arikawa, J.; Ishibashi, M.; Kawashima, M.; Takagi, Y.; Ichikawa, Y.; Imokawa, G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J. Investig. Dermatol. 2002, 119, 433–439. [Google Scholar] [CrossRef]
- Takigawa, H.; Nakagawa, H.; Kuzukawa, M.; Mori, H.; Imokawa, G. Deficient production of hexadecenoic acid in the skin is associated in part with the vulnerability of atopic dermatitis patients to colonization by Staphylococcus aureus. Dermatology 2005, 211, 240–248. [Google Scholar] [CrossRef]
- Higuchi, K.; Hara, J.; Okamoto, R.; Kawashima, M.; Imokawa, G. The skin of atopic dermatitis patients contains a novel enzyme, glucosylceramide sphingomyelin deacylase, which cleaves the N-acyl linkage of sphingomyelin and glucosylceramide. Biochem. J. 2000, 350, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Ogata, J.; Higaki, Y.; Kawashima, M.; Yada, Y.; Higuchi, K.; Tsuchiva, T.; Kawainami, S.; Imokawa, G. Abnormal expression of sphingomyelin acylase in atopic dermatitis: An etiologic factor for ceramide deficiency? J. Investig. Dermatol. 1996, 106, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Hara, J.; Higuchi, K.; Okamoto, R.; Kawashima, M.; Imokawa, G. High-expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. J. Investig. Dermatol. 2000, 115, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Pewzner-Jung, Y.; Tavakoli Tabazavareh, S.; Grassmé, H.; Becker, K.A.; Japtok, L.; Steinmann, J.; Joseph, T.; Lang, S.; Tuemmier, B.; Schuchman, E.H. Sphingoid long chain bases prevent lung infection by Pseudomonas aeruginosa. EMBO Mol. Med. 2014, 6, 1205–1214. [Google Scholar] [CrossRef]
- Grassmé, H.; Henry, B.; Ziobro, R.; Becker, K.A.; Riethmüller, J.; Gardner, A.; Seitz, A.P.; Steinmann, J.; Lang, S.; Ward, C. β1-Integrin Accumulates in Cystic Fibrosis Luminal Airway Epithelial Membranes and Decreases Sphingosine, Promoting Bacterial Infections. Cell Host Microbe. 2017, 21, 707–718. [Google Scholar] [CrossRef]
- Freedman, S.D.; Blanco, P.G.; Zaman, M.M.; Shea, J.C.; Ollero, M.; Hopper, I.K.; Weed, D.A.; Gelrud, A.; Regan, M.M.; Laposata, M.; et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 2004, 350, 560–569. [Google Scholar] [CrossRef]
- Strandvik, B.; Gronowitz, E.; Enlund, F.; Martinsson, T.; Wahlström, J. Essential fatty acid deficiency in relation to genotype in patients with cystic fibrosis. J. Pediatr. 2001, 139, 650–655. [Google Scholar] [CrossRef]
- Strandvik, B. Fatty acid metabolism in cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids. 2010, 83, 121–129. [Google Scholar] [CrossRef]
- Georgel, P.; Crozat, K.; Lauth, X.; Makrantonaki, E.; Seltmann, H.; Sovath, S.; Hoebe, K.; Du, X.; Rutschmann, S.; Jiang, Z.; et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect. Immun. 2005, 73, 4512–4521. [Google Scholar] [CrossRef]
- Kluytmans, J.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Dewan, P.; Ganz, T. Innate antimicrobial activity of nasal secretions. Infect. Immun. 1999, 67, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Nouwen, J.L.; van Belkum, A.; Verbrugh, H.A. Determinants of Staphylococcus aureus nasal carriage. Neth J. Med. 2001, 59, 126–133. [Google Scholar] [CrossRef][Green Version]
- Bourne, N.; Ireland, J.; Stanberry, L.R.; Bernstein, D.I. Effect of undecylenic acid as a topical microbicide against genital herpes infection in mice and guinea pigs. Antiviral Res. 1999, 40, 139–144. [Google Scholar] [CrossRef]
- Shafran, S.D.; Sacks, S.L.; Aoki, F.Y.; Tyrrell, D.L.; Schlech, W.F.; Mendelson, J.; Rosenthal, D.; Gill, M.J.; Bader, R.L.; Chang, L. Topical undecylenic acid for herpes simplex labialis: A multicenter, placebo-controlled trial. J. Infect. Dis. 1997, 176, 78–83. [Google Scholar] [CrossRef]
- Klee, S.K.; Farwick, M.; Lersch, P. The effect of sphingolipids as a new therapeutic option for acne treatment. Basic Clin. Derm. 2007, 40, 155–166. [Google Scholar]
| Lipids | µg/mL Saliva | µg/cm2 Surface |
|---|---|---|
| Squalene | 2.0 | 13.1 |
| Cholesterol esters | 0.2 | 1.1 |
| Wax esters | 1.7 | 9.4 |
| Triglycerides | 26.4 | 21.8 |
| Fatty acids | 2.0 | 7.8 |
| Cholesterol | 10.0 | 15.0 |
| Total | 42.3 | 68.2 |
| Microbe | Sapienic Acid | Lauric Acid | Undecylenic Acid |
|---|---|---|---|
| Aggregatibacter actinomycetemcomitans | [38] | [38] | |
| Candida albicans | [47,56] | [30,72] | |
| Clostridium difficile | [68] | ||
| Epidermophyton inguinale | [30] | ||
| Escherichia coli | [4,63] | ||
| Fusobacterium nucleatum | [38] | [38] | |
| Helicobacter pylori | [73,74] | ||
| Herpes simplex virus 1 and 2 | [4,71] | [30] | |
| Micrococci | [47] | ||
| Microsporum audouinii | [54] | [30] | |
| Nocardia asteroides | [47] | ||
| Pneumococci | [47] | ||
| Porphyromonas gingivalis | [37] | [37] | |
| Propionibacterium acnes | [55,61,74] | ||
| Respiratory syncytial virus | [4] | ||
| Shigella sonnei | [75] | ||
| Staphylococcus aureus | [38,47,55,63,76] | ||
| Staphylococcus epidermidis | [4,47,55,76] | ||
| Streptococci | [4,42,47,57,77] | ||
| Streptococcus mitis | [38] | [38] | |
| Streptococcus mutans | [58] | ||
| Streptococcus sanguinis | [38] | [38] | |
| Trichophyton rubrum | [30] | ||
| Trichophyton mentagrophytes | [30] | ||
| Vaccinia virus | [4] |
| Microbe | Sphingosine | Dihydrosphingosine (Sphinganine) | 6-hydroxy- Sphingosine |
|---|---|---|---|
| Acinetobacter baumannii | [89] | [89] | |
| Acinetobacter lwoffii | [82] | ||
| Bacillus subtilis | [82] | ||
| Candida albicans | [82] | [80,82,83] | |
| Corynebacteria | [38] | [38] | |
| Epidermophyton floccosum | [82] | ||
| Escherichia coli | [38] | [38,80] | |
| Fusobacterium nucleatum | [38] | [38] | |
| Micrococcus luteus | [80] | ||
| Neisseria meningitides | [82] | ||
| Porphyromonas gingivalis | [37] | [37] | [37] |
| Propionibacterium acnes | [40] | [40,80] | |
| Pseudomonas aeruginosa | [89] | [80,89] | |
| Serratia marcescens | [80] | ||
| Staphylococcus aureus | [80,89,90] | [80,81,82,83,89,90] | [80] |
| Staphylococcus epidermidis | [38,90] | [38,80] | |
| Streptococcus mitis | [38] | [38,81] | |
| Streptococcus sanguinis | [38] | [38] | |
| Streptococcus pyogenes | [80] | ||
| Trichophyton mentagrophytes | [82] | ||
| Trichophyton tonsurans | [82] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, C.L. Antimicrobial Activity of Host-Derived Lipids. Antibiotics 2020, 9, 75. https://doi.org/10.3390/antibiotics9020075
Fischer CL. Antimicrobial Activity of Host-Derived Lipids. Antibiotics. 2020; 9(2):75. https://doi.org/10.3390/antibiotics9020075
Chicago/Turabian StyleFischer, Carol L. 2020. "Antimicrobial Activity of Host-Derived Lipids" Antibiotics 9, no. 2: 75. https://doi.org/10.3390/antibiotics9020075
APA StyleFischer, C. L. (2020). Antimicrobial Activity of Host-Derived Lipids. Antibiotics, 9(2), 75. https://doi.org/10.3390/antibiotics9020075





