Simple and Accurate HPTLC-Densitometric Method for Quantification of Delafloxacin (A Novel Fluoroquinolone Antibiotic) in Plasma Samples: Application to Pharmacokinetic Study in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Analytical Conditions
2.3. Sample Preparation
2.4. Sample Extraction
2.5. Method Validation
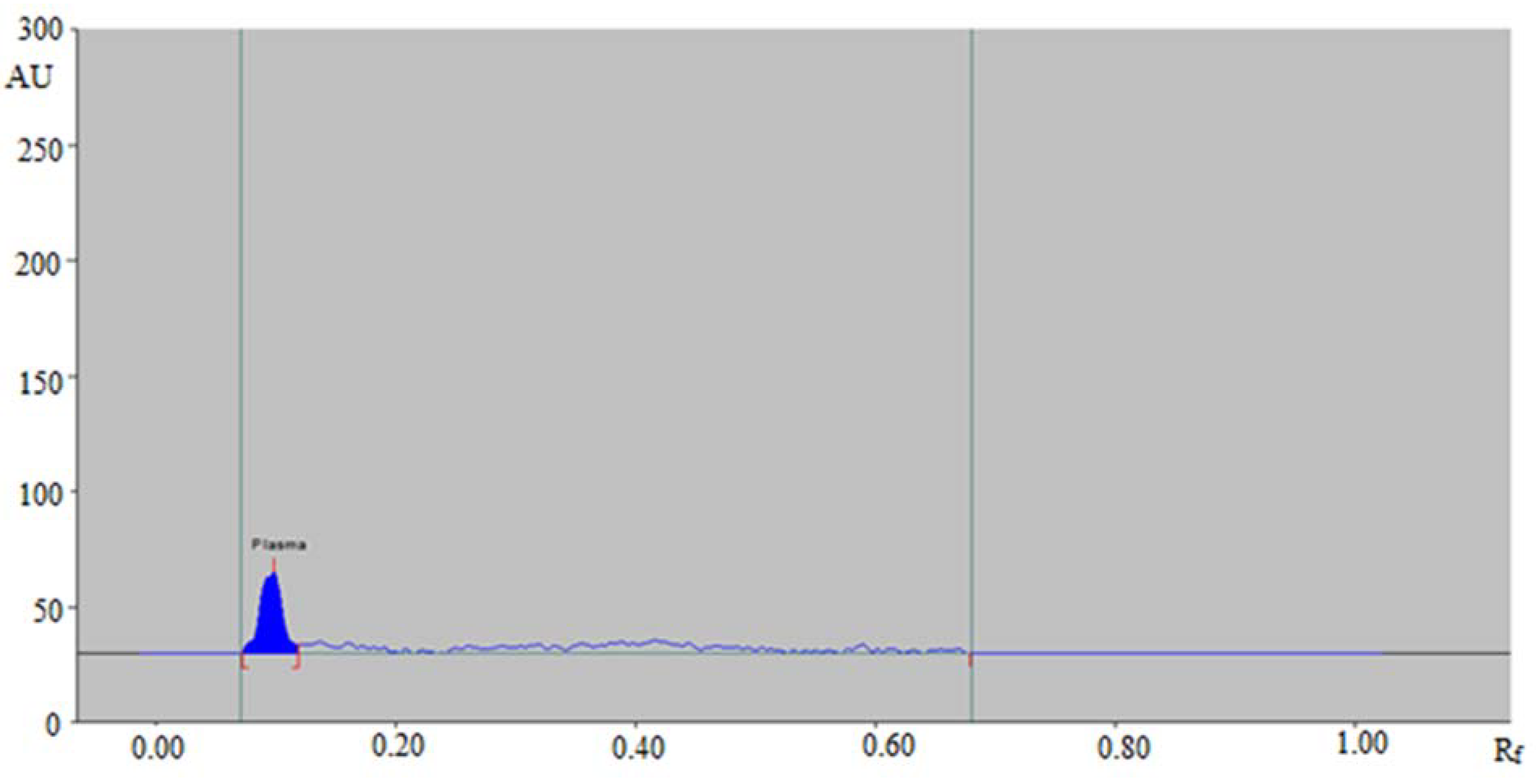
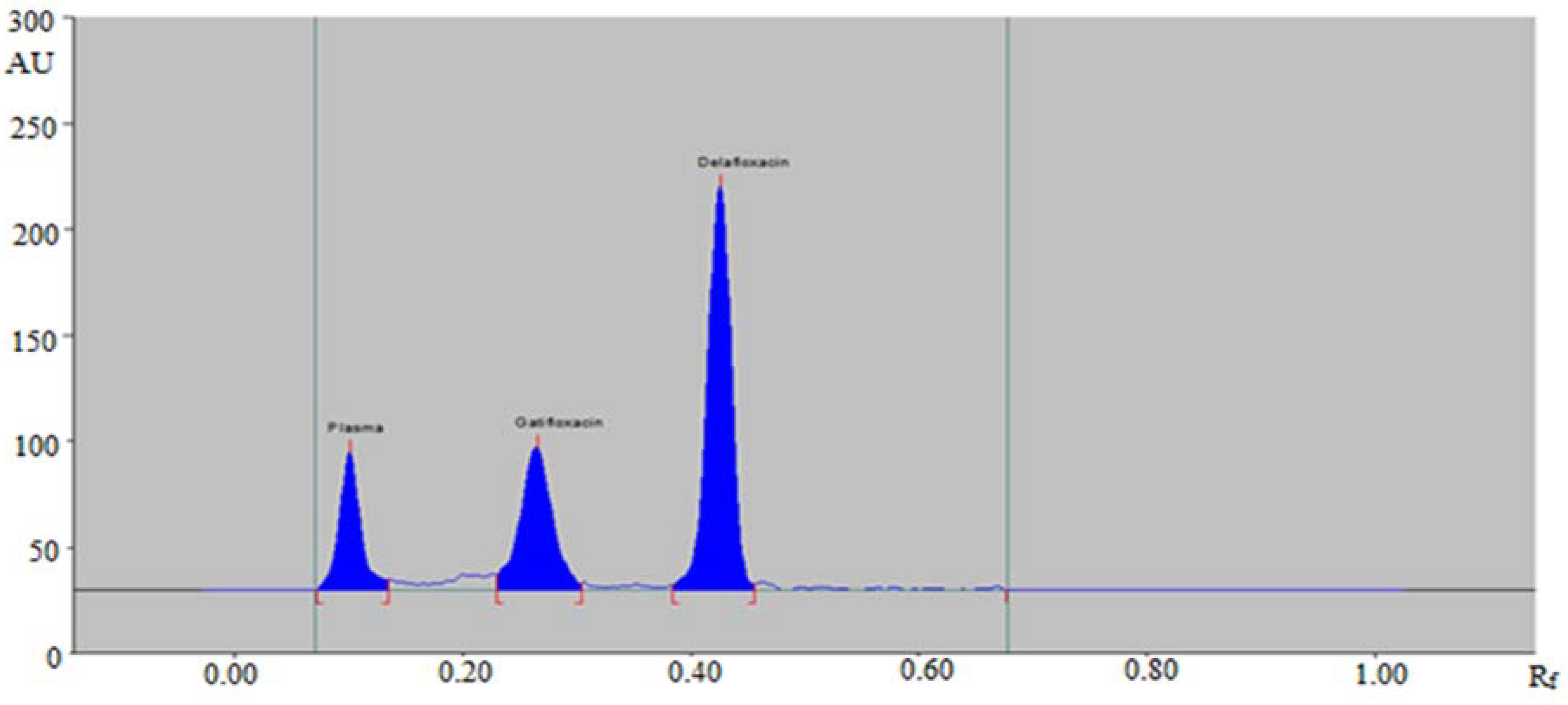
2.5.1. Selectivity and Specificity
2.5.2. CCs and Linearity
2.5.3. Sensitivity
2.5.4. Precision and Accuracy
2.5.5. Robustness
2.5.6. Recovery Studies
2.5.7. Stability Study
2.6. Application of HPTLC Assay to Pharmacokinetic Study in Rats
3. Results and Discussion
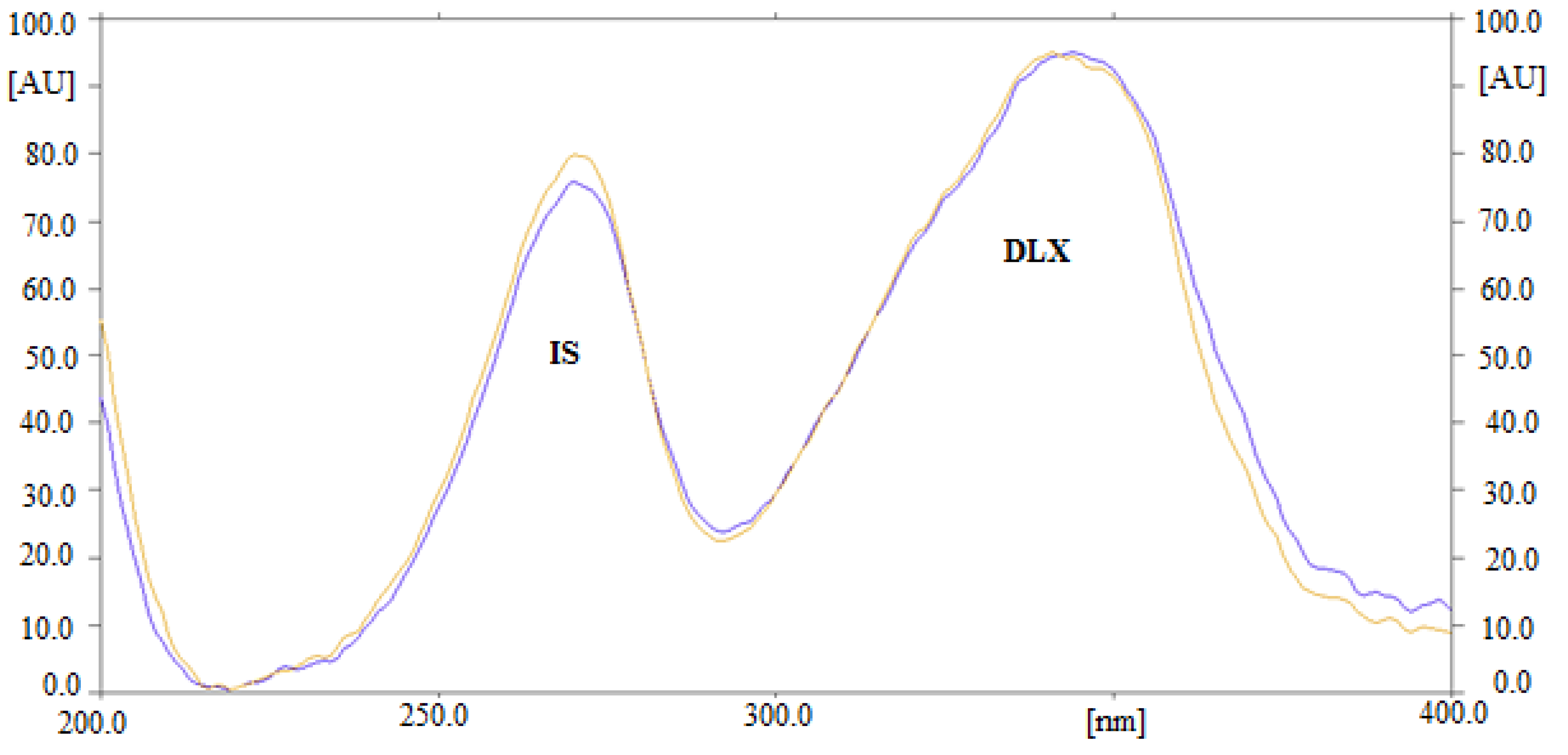
3.1. Optimization of Analytical Procedures
3.2. Method Validation
3.2.1. Selectivity and Specificity
3.2.2. CCs and Linearity
3.2.3. Sensitivity
3.2.4. Accuracy and Precision
3.2.5. Robustness
3.2.6. Recovery Studies
3.2.7. Stability Studies
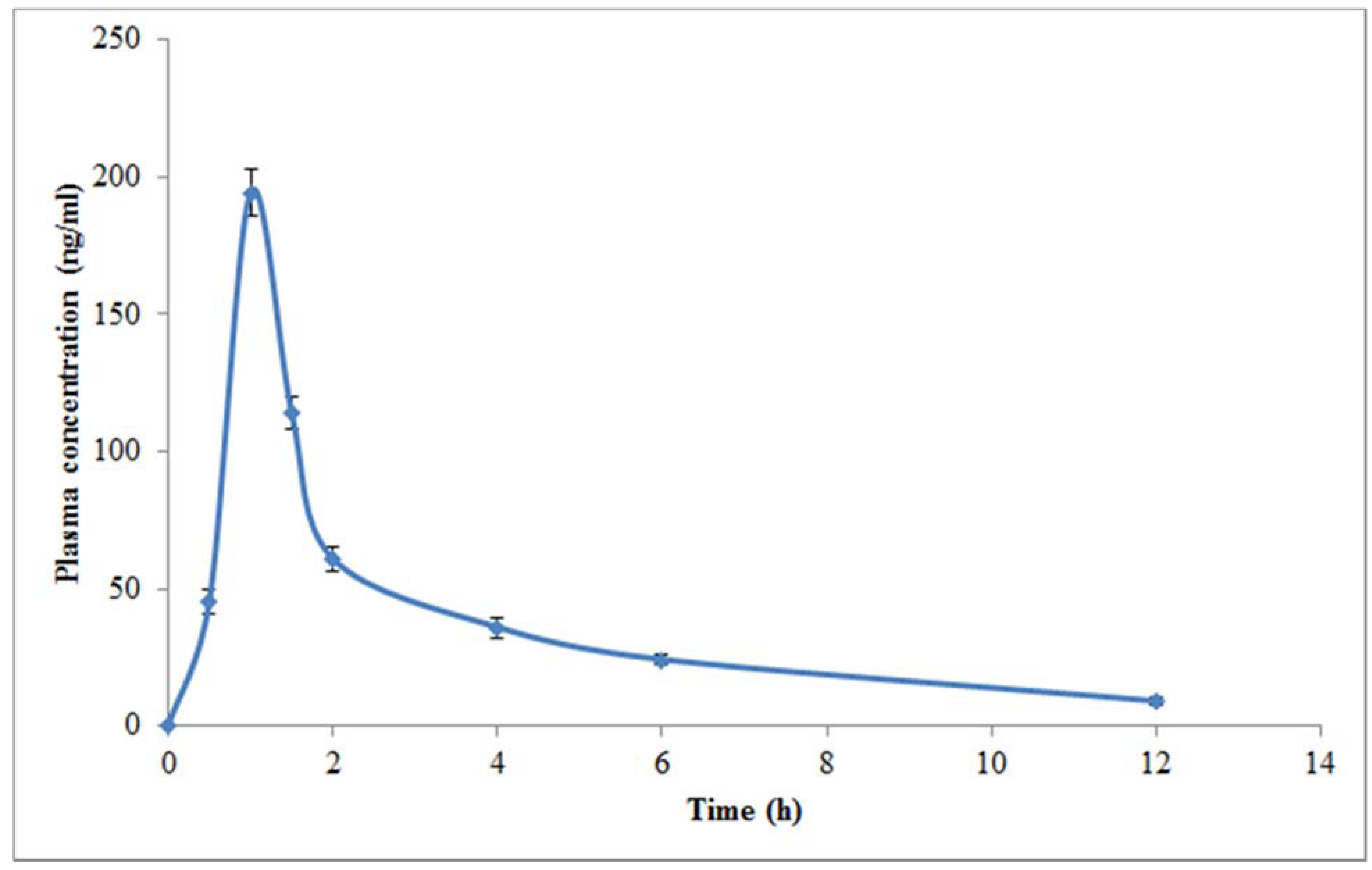
3.3. Pharmacokinetic Study in Rats
3.4. Literature Comparison
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falcone, M.; Concia, E.; Giusti, M.; Mazzone, A.; Santini, C.; Stefani, S.; Violi, F. Acute bacterial skin and skin structure infections in internal medicine wards: Old and new drugs. Intern. Emerg. Med. 2016, 11, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Ray, G.T.; Suaya, J.A.; Baxter, R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: A retrospective population based study. BMC Infect. Dis. 2013, 13, E252. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Siega, P.D.; Pecori, D.; Scarparo, C.; Righi, E. Delafloxacin for the treatment of respiratory and skin infections. Expert Opin. Investig. Drugs 2015, 24, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.C.; Crotty, M.P.; White, B.P.; Worley, M.V. What is old is new again: Delafloxacin, a modern fluoroquinolone. Pharmacotherapy 2018, 38, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Delafloxacin: First global approval. Drugs 2017, 77, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.T.; Akhras, K.S.; Lin, S.J. The attributable clinical and economic burden of skin and skin structure infections in hospitalized patients: A matched cohort study. Diagn. Microbiol. Infect. Dis. 2009, 64, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Thabit, A.K.; Crandon, J.L.; Nicolau, D.P. Pharmacodynamic and pharmacokinetic profiling of delafloxacin in a murine lung model against community-acquired respiratory tract pathogens. Int. J. Antimicrob. Agents 2016, 48, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Pecori, D.; Cojutti, P.; Righi, E.; Pea, F. Clinical and pharmacokinetic drug evaluation of delafloxacin for the treatment of acute bacterial skin and skin structure infections. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1193–1200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiu, J.; Ting, G.; Kiang, T.K. Clinical pharmacokinetics and pharmacodynamics of delafloxacin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ezzeldin, E.; Harqash, R.N.; Anwer, M.K.; Azam, F. Development and validation of a novel UPLC-MS/MS method for quantification of delafloxacin in plasma and aqueous humour for pharmacokinetic analyses. J. Chromatogr. B 2020, 1138, E121961. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Hunt, T.; Benedict, M.; Paulson, S.K.; Lawrence, L.; Cammarata, S.; Sun, E. Single and multiple ascending-dose studies of oral delafloxacin: Effects of food, sex, and age. Clin. Ther. 2016, 38, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Kardas, P. Patient compliance with antibiotic treatment for respiratory tract infections. J. Antimicrob. Chemother. 2002, 49, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Alcorn, H., Jr.; Lawrence, L.; Paulson, S.K.; Quintas, M.; Cammarata, S.K. Pharmacokinetics of intravenous delafloxacin in patients with end-stage renal disease. J. Clin. Pharmacol. 2018, 58, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.K.; Alcorn, H., Jr.; Lawrence, L.; Paulson, S.K.; Quintas, M.; Cammarata, S.K. Delafloxacin pharmacokinetics in subjects with varying degrees of renal function. J. Clin. Pharmacol. 2018, 58, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ezzeldin, E.; Al-Rashood, K.A.; Asiri, Y.A.; Rezk, N.L. Rapid determination of canagliflozin in rat plasma by UHPLC-MS/MS using negative ionization mode to avoid adduct-ions formation. Talanta 2015, 132, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Ezzeldin, E.; Iqbal, M.; Anwer, M.K.; Mostafa, G.A.E.; Alqarni, M.H.; Foudah, A.I.; Shakeel, F. Ecofriendly densitometric RP-HPTLC method for determination of rivaroxaban in nanoparticle formulations using green solvents. RSC Adv. 2020, 10, 2133–2140. [Google Scholar] [CrossRef]
- El-Gizawy, S.M.; El-Shaboury, S.R.; Atia, N.N.; Abo-Zeid, M.N. New, simple and sensitive HPTLC method for simultaneous determination of anti-hepatitis C sofosbuvir and ledipasvir in rabbit plasma. J. Chromatogr. B 2018, 1092, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Abo-Zeid, M.N.; El-Gizawy, S.M.; Atia, N.N.; El-Shaboury, S.R. Efficient HPTLC-dual wavelength spectrodensitometric method for simultaneous determination of sofosbuvir and daclatasvir: Biological and pharmaceutical analysis. J. Pharm. Biomed. Anal. 2018, 156, 358–365. [Google Scholar] [CrossRef] [PubMed]
- El-Yazbi, F.A.; Amin, O.A.; El-Kimary, E.I.; Khamis, E.F.; Younis, S.E. High-performance thin-layer chromatographic methods for the determination of febuxostat and febuxostat/diclofenac combination in human plasma. J. Chromatogr. B 2018, 1086, 89–96. [Google Scholar] [CrossRef] [PubMed]
- El-Koussi, W.M.; Atia, N.N.; Mahmoud, A.M.; El-Shabouri, S.R. HPTLC method for direct determination of gemifloxacin mesylate in human plasma. J. Chromatogr. B 2014, 967, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research (CDER) (Ed.) Guidance for Industry on Bioanalytical Method Validation; US Food and Drug Administration: Rockville, MD, USA, 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 5 February 2020).

| Linearity Range (ng/Spot) | 16–400 |
|---|---|
| Regression Equation | Y = 0.0091x + 0.7493 |
| Correlation Coefficient (r) | 0.9996 |
| Determination Coefficient (r2) | 0.9992 |
| Slope ± SD | 0.0091 ± 0.0003 |
| Intercept ± SD | 0.7493 ± 0.0008 |
| λmax (nm) | 344 |
| Rf | 0.43 ± 0.01 |
| LOD (ng/band) | 5.80 |
| LOQ (ng/band) | 16.0 |
| Conc. Added (ng/Band) | Intra-Day Assay (n = 6) | Inter-Day Assay (n = 18) | ||||
|---|---|---|---|---|---|---|
| Conc. Found (ng/Band) a | Precision (% RSD) | Accuracy (% Recovery) | Conc. Found (ng/Band) a | Precision (% RSD) | Accuracy (% Recovery) | |
| 16 | 16.28 ± 0.46 | 2.82 | 101.75 | 14.94 ± 0.44 | 2.94 | 93.37 |
| 200 | 192.52 ± 5.94 | 3.08 | 96.26 | 192.97 ± 6.10 | 3.16 | 96.48 |
| 400 | 410.12 ± 13.24 | 3.22 | 102.53 | 414.28 ± 15.42 | 3.72 | 103.57 |
| Conc. (ng/Bnd) | Mobile Phase Composition (EA:Methanol:Ammonia) | Recovery (%) ± SD | RSD (%) | Rf | ||
|---|---|---|---|---|---|---|
| Original | Used | Level | ||||
| 200 | 5:4:2 | 4.9:4.1:2 | +0.1 | 95.38 ± 2.69 | 2.82 | 0.41 |
| 5:4:2 | 0.0 | 104.10 ± 3.11 | 2.98 | 0.42 | ||
| 5.1:3.9:2 | −0.1 | 103.61 ± 3.74 | 3.60 | 0.43 | ||
| Spiked Conc. (ng/Band) | Conc. Found (ng/Band) | Recovery (%) ± SD | RSD (%) |
|---|---|---|---|
| 16 | 14.93 | 93.31 ± 3.13 | 3.35 |
| 200 | 197.41 | 98.70 ± 3.97 | 4.02 |
| 400 | 403.75 | 100.93 ± 5.22 | 5.17 |
| Stability | Nominal Conc. (ng/Band) | Conc. Found (ng/Band) ± SD | Precision (% RSD) | Accuracy (% Recovery) |
|---|---|---|---|---|
| Bench Top (12 h) | 16 | 15.08 ± 0.85 | 5.63 | 94.25 |
| 400 | 396.45 ± 4.68 | 1.18 | 99.11 | |
| Refrigeration (Overnight) | 16 | 15.32 ± 0.96 | 6.26 | 95.75 |
| 400 | 397.77 ± 5.25 | 1.31 | 99.44 | |
| Freeze Thaw (3 Cycles) | 16 | 17.05 ± 1.02 | 5.98 | 106.56 |
| 400 | 405.28 ± 6.91 | 1.70 | 101.32 | |
| Freezer at −80 °C (30 days) | 16 | 17.65 ± 1.12 | 6.34 | 110.31 |
| 400 | 401.13 ± 7.13 | 1.77 | 100.28 |
| Parameters | Values (Mean ± SD) |
|---|---|
| Cmax (ng/mL) | 194.19 ± 8.20 |
| Tmax (h) | 1.00 ± 0.01 |
| AUC0-t (ng.h/mL) | 396.49 ± 8.53 |
| Analytical Method | Linearity Range | Extraction Method | Detection Method | Cost | Accuracy (% Recovery) | Precision (% RSD) | Ref. |
|---|---|---|---|---|---|---|---|
| UPLC-MS/MS | 3.5–5000 ng/ml | LLE a | MS/MS | High | 86.80–111.20 | 4.08–11.30 | [10] |
| HPTLC | 16–400 ng/band | PP b | UV | Low | 93.37–103.57 | 2.82–3.72 | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, P.; Iqbal, M.; Ezzeldin, E.; Khalil, N.Y.; Foudah, A.I.; Alqarni, M.H.; Shakeel, F. Simple and Accurate HPTLC-Densitometric Method for Quantification of Delafloxacin (A Novel Fluoroquinolone Antibiotic) in Plasma Samples: Application to Pharmacokinetic Study in Rats. Antibiotics 2020, 9, 134. https://doi.org/10.3390/antibiotics9030134
Alam P, Iqbal M, Ezzeldin E, Khalil NY, Foudah AI, Alqarni MH, Shakeel F. Simple and Accurate HPTLC-Densitometric Method for Quantification of Delafloxacin (A Novel Fluoroquinolone Antibiotic) in Plasma Samples: Application to Pharmacokinetic Study in Rats. Antibiotics. 2020; 9(3):134. https://doi.org/10.3390/antibiotics9030134
Chicago/Turabian StyleAlam, Prawez, Muzaffar Iqbal, Essam Ezzeldin, Nasr Y. Khalil, Ahmed I. Foudah, Mohammed H. Alqarni, and Faiyaz Shakeel. 2020. "Simple and Accurate HPTLC-Densitometric Method for Quantification of Delafloxacin (A Novel Fluoroquinolone Antibiotic) in Plasma Samples: Application to Pharmacokinetic Study in Rats" Antibiotics 9, no. 3: 134. https://doi.org/10.3390/antibiotics9030134
APA StyleAlam, P., Iqbal, M., Ezzeldin, E., Khalil, N. Y., Foudah, A. I., Alqarni, M. H., & Shakeel, F. (2020). Simple and Accurate HPTLC-Densitometric Method for Quantification of Delafloxacin (A Novel Fluoroquinolone Antibiotic) in Plasma Samples: Application to Pharmacokinetic Study in Rats. Antibiotics, 9(3), 134. https://doi.org/10.3390/antibiotics9030134








