Whole Genome Sequencing for the Analysis of Drug Resistant Strains of Mycobacterium tuberculosis: A Systematic Review for Bedaquiline and Delamanid
Abstract
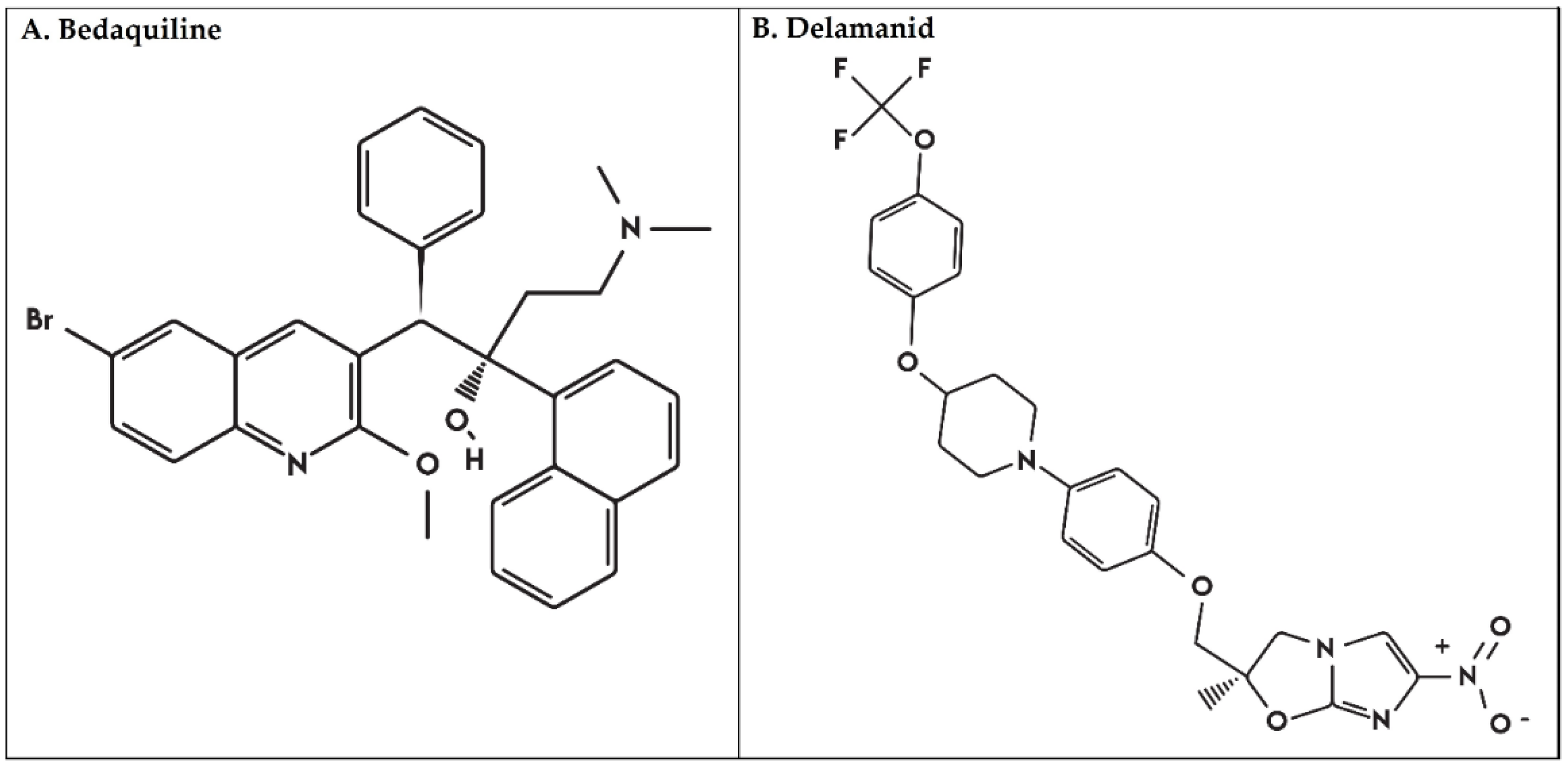
1. Introduction
2. Results and Discussion
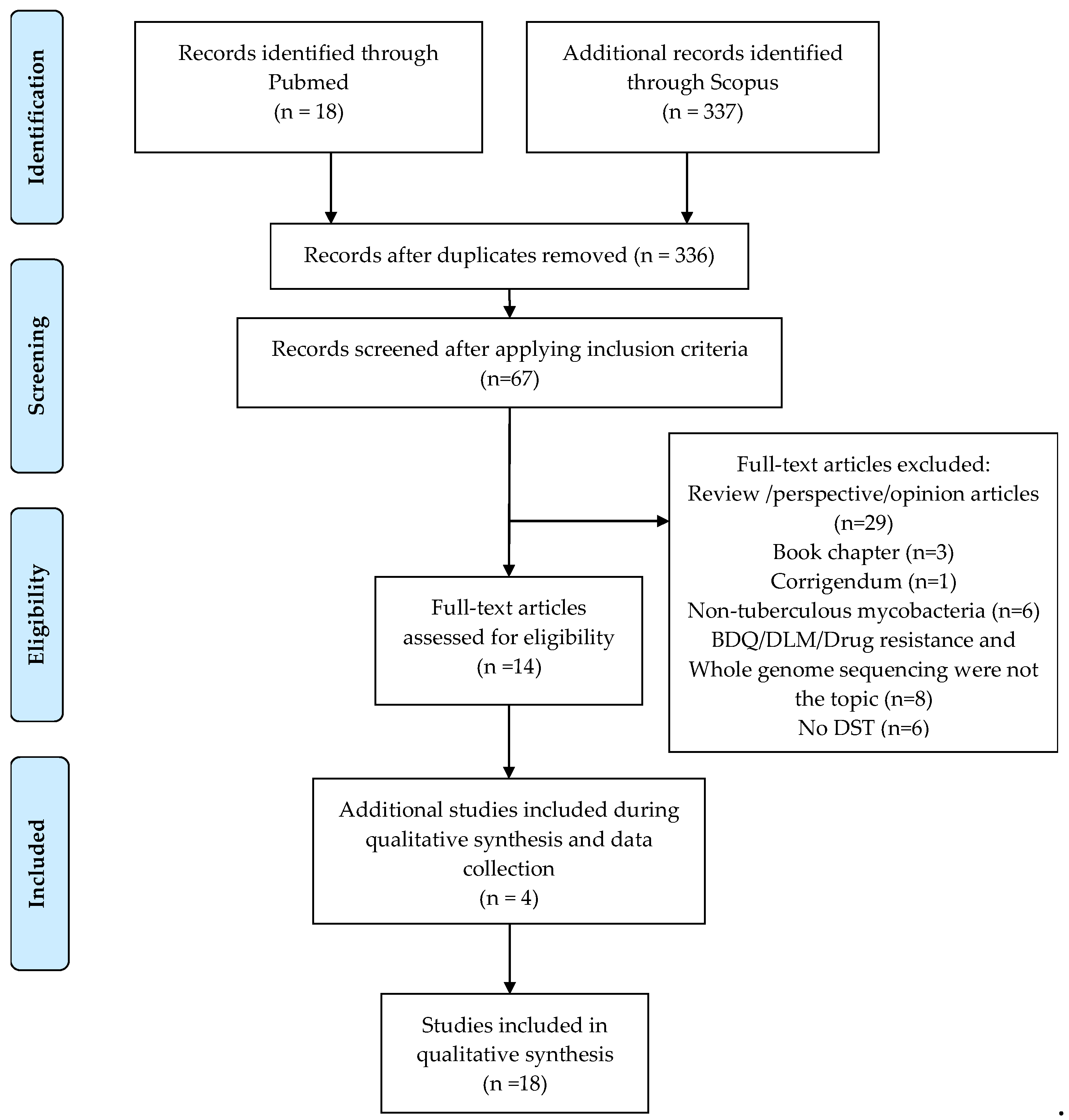
2.1. Description of Collected Articles
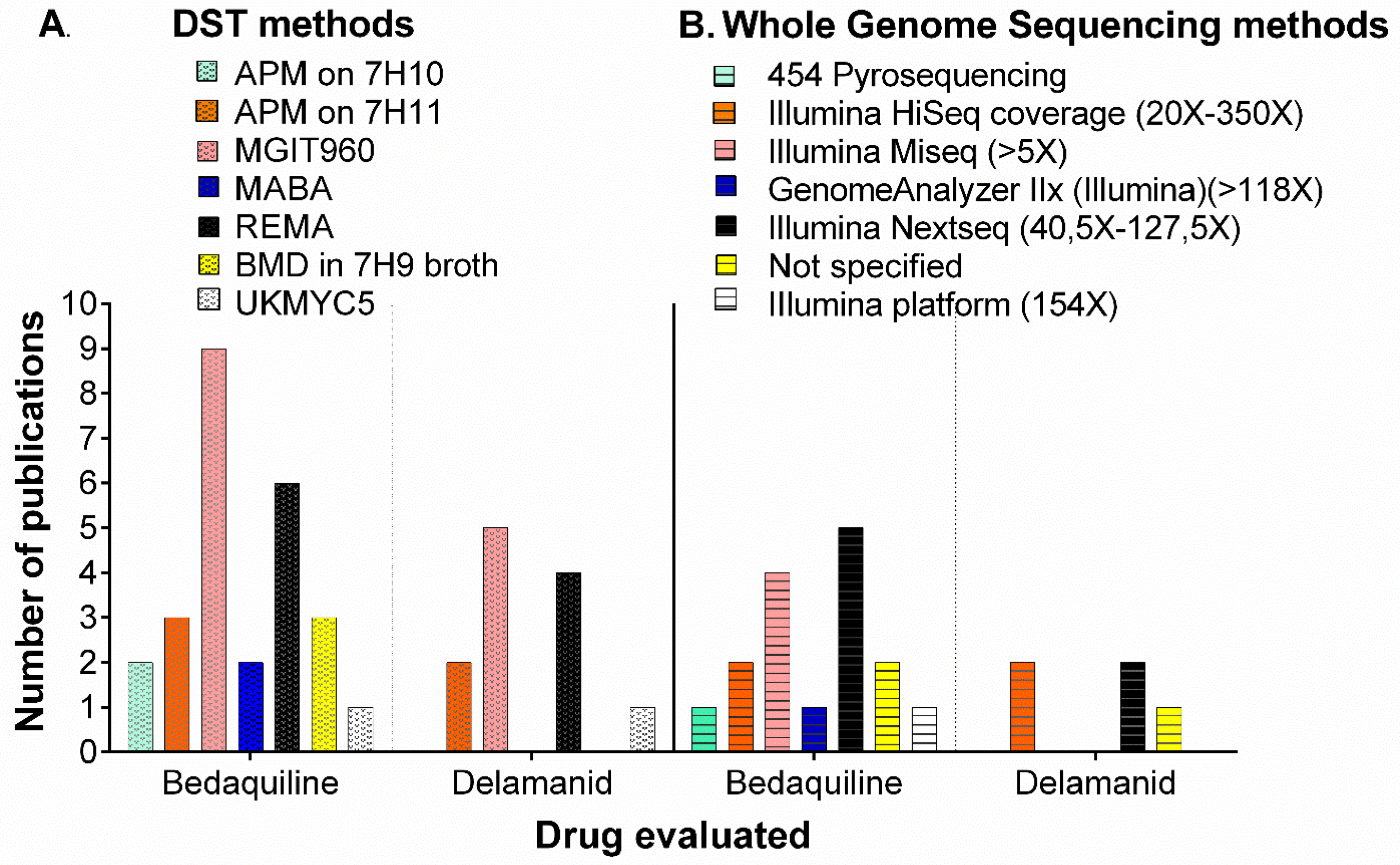
2.2. Phenotypic Methods for Drug Susceptibility Testing (DST) for BDQ and DLM
| DST Method | CC for Bedaquiline (BDQ) (mg/L) | CC for Delamanid (DLM) (mg/L) |
|---|---|---|
| (Bactec) MGIT960 | 0.8 [6] 1.0 [29,30,32,34] 2.0 [35] | 0.04 [5,6] 0.06 [30] 0.12-0.125 [10,32] |
| Resazurin microtiter assay (REMA) | 0.125 [27] 0.25 [21,26] | 0.03 [30] |
| Agar proportion method (APM on 7H10 or 7H11) | 0.12 [24] 0.25 [23,28,32,36] | 0.06 [32] 0.2 [10] |
| Broth Microdilution (BMD) | 0.25 [35,37] | Not defined |
| Microplate alamarBlue Assay (MABA) | 0.25 [37] | Not defined |
2.3. Mtb Genes Associated with BDQ and DLM Resistance
2.3.1. Mutations Associated with BDQ Susceptibility
2.3.2. Mutations Associated with DLM Susceptibility
2.4. Other Findings: Mutations in Drug-Susceptible Strains, Cross-Resistance, and Heteroresistance
3. Materials and Methods
3.1. Data Collection
3.2. Data Items and Quality Assessment
3.3. Inclusion and Exclusion Criteria
3.4. Data Extraction Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 (accessed on 15 January 2020).
- Zumla, A.I.; Gillespie, S.H.; Hoelscher, M.; Philips, P.P.; Cole, S.T.; Abubakar, I.; McHugh, T.D.; Schito, M.; Maeurer, M.; Nunn, A.J. New antituberculosis drugs, regimens, and adjunct therapies: Needs, advances, and future prospects. Lancet Infect. Dis. 2014, 14, 327–340. [Google Scholar] [CrossRef]
- Gler, M.T.; Skripconoka, V.; Sanchez-Garavito, E.; Xiao, H.; Cabrera-Rivero, J.L.; Vargas-Vasquez, D.E.; Gao, M.; Awad, M.; Park, S.K.; Shim, T.S.; et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N. Engl. J. Med. 2012, 366, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Use of Delamanid in the Treatment of Multidrug-Resistant Tuberculosis: Interim Policy Guidance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Hoffmann, H.; Kohl, T.A.; Hofmann-Thiel, S.; Merker, M.; Beckert, P.; Jaton, K.; Nedialkova, L.; Sahalchyk, E.; Rothe, T.; Keller, P.M.; et al. Delamanid and Bedaquiline Resistance in Mycobacterium tuberculosis Ancestral Beijing Genotype Causing Extensively Drug-Resistant Tuberculosis in a Tibetan Refugee. Am. J. Respir. Crit. Care Med. 2016, 193, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Bloemberg, G.V.; Keller, P.M.; Stucki, D.; Stuckia, D.; Trauner, A.; Borrell, S.; Latshang, T.; Coscolla, M.; Rothe, T.; Hömke, R.; et al. Acquired Resistance to Bedaquiline and Delamanid in Therapy for Tuberculosis. N. Engl. J. Med. 2015, 373, 1986–1988. [Google Scholar] [CrossRef] [PubMed]
- Technical Report on Critical Concentrations for Drug Susceptibility Testing of Medicines Used in the Treatment of Drug-Resistant Tuberculosis; World Health Organization: Geneva, Switzerland, 2018.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of Mics and Zone Diameters; Version 10.0; EUCAST: Basel, Switzerland, 2020; Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 13 January 2020).
- Villellas, C.; Coeck, N.; Meehan, C.J.; Lounis, N.; de Jong, B.; Rigouts, L.; Andries, K. Unexpected high prevalence of resistance-associated Rv0678 variants in MDR-TB patients without documented prior use of clofazimine or bedaquiline. J. Antimicrob. Chemother. 2017, 72, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Nedialkova, L.; Borroni, E.; Battaglia, S.; Cabibbe, A.M.; Niemann, S.; Utpatel, C.; Merker, M.; Trovato, A.; Hofmann-Thiel, S.; et al. Delamanid susceptibility testing of Mycobacterium tuberculosis using the resazurin microtitre assay and the BACTEC™ MGIT™ 960 system. J. Antimicrob. Chemother. 2016, 71, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Dookie, N.; Rambaran, S.; Padayatchi, N.; Mahomed, S.; Naidoo, K. Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J. Antimicr. Chemother. 2018, 12, 1098–1113. [Google Scholar] [CrossRef]
- Miotto, P.; Zhang, Y.; Cirillo, D.M.; Yam, W.C. Drug resistance mechanisms and drug susceptibility testing for tuberculosis. Respirology 2018, 23, 1098–1113. [Google Scholar] [CrossRef]
- Cohen, K.A.; Manson, A.L.; Desjardins, C.A.; Abeel, T.; Earl, A.M. Deciphering drug resistance in Mycobacterium tuberculosis using whole-genome sequencing: Progress, promise, and challenges. Genome Med. 2019, 11, 45. [Google Scholar] [CrossRef]
- Nathavitharana, R.R.; Hillemann, D.; Schumacher, S.G.; Schlueter, B.; Ismail, N.; Omar, S.V.; Sikhondze, W.; Havumaki, J.; Valli, E.; Boehme, C.; et al. Multicenter Noninferiority Evaluation of Hain GenoType MTBDRplus Version 2 and Nipro NTM+MDRTB Line Probe Assays for Detection of Rifampin and Isoniazid Resistance. J. Clin. Microbiol. 2016, 54, 1624–1630. [Google Scholar] [CrossRef]
- Marlowe, E.M.; Novak-Weekley, S.M.; Cumpio, J.; Sharp, S.E.; Momeny, M.A.; Babst, A.; Carlson, J.S.; Kawamura, M.; Pandori, M. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J. Clin. Microbiol. 2011, 49, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; McCallum, N.; Sintchenko, V.; Howden, B.P. Whole genome sequencing in clinical and public health microbiology. Pathology 2015, 47, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Senghore, M.; Otu, J.; Witney, A.; Gehre, F.; Doughty, E.L.; Kay, G.L.; Butcher, P.; Salako, K.; Kehinde, A.; Onyejepu, N.; et al. Whole-genome sequencing illuminates the evolution and spread of multidrug-resistant tuberculosis in Southwest Nigeria. PLoS ONE 2017, 12, e0184510. [Google Scholar] [CrossRef] [PubMed]
- Zakham, F.; Laurent, S.; Esteves Carreira, A.L.; Corbaz, A.; Bertelli, C.; Masserey, E.; Nicod, L.; Greub, G.; Jaton, K.; Mazza-Stalder, J.; et al. Whole-genome sequencing for rapid, reliable and routine investigation of Mycobacterium tuberculosis transmission in local communities. N. Microbes N. Infect. 2019, 31, 100582. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Hartkoorn, R.C.; Uplekar, S.; Cole, S.T. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 2979–2981. [Google Scholar] [CrossRef]
- World Health Organization. The Use of Bedaquiline in the Treatment of Multidrug-Resistant Tuberculosis: Interim Policy Guidance; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Ismail, N.; Omar, S.V.; Ismail, N.A.; Peters, R.P.H. In vitro approaches for generation of Mycobacterium tuberculosis mutants resistant to bedaquiline, clofazimine or linezolid and identification of associated genetic variants. J. Microbiol. Methods 2018, 153, 1–9. [Google Scholar] [CrossRef]
- Almeida, D.; Ioerger, T.; Tyagi, S.; Li, S.Y.; Mdluli, K.; Andries, K.; Grosset, J.; Sacchettini, J.; Nuermberger, E. Mutations in pepQ Confer Low-Level Resistance to Bedaquiline and Clofazimine in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2016, 60, 4590–4599. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.Y.; Almeida, D.V.; Tasneen, R.; Barnes-Boyle, K.; Converse, P.J.; Upton, A.M.; Mdluli, K.; Fotouhi, N.; Nuermberger, E.L. Contribution of Pretomanid to Novel Regimens Containing Bedaquiline with either Linezolid or Moxifloxacin and Pyrazinamide in Murine Models of Tuberculosis. Antimicrob. Agents Chemother. 2019, 63, e00021-19. [Google Scholar] [CrossRef]
- Andries, K.; Villellas, C.; Coeck, N.; Thys, K.; Gevers, T.; Vranckx, L.; Lounis, N.; de Jong, B.C.; Koul, A. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS ONE 2014, 9, e102135. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Hennessy, D.; Jelfs, P.; Crighton, T.; Chen, S.C.A.; Sintchenko, V. Mutations associated with in vitro resistance to bedaquiline in Mycobacterium tuberculosis isolates in Australia. Tuberculosis 2018, 111, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Ghodousi, A.; Rizvi, A.H.; Baloch, A.Q.; Ghafoor, A.; Khanzada, F.M.; Qadir, M.; Borroni, E.; Trovato, A.; Tahseen, S.; Cirillo, D.M. Acquisition of Cross-Resistance to Bedaquiline and Clofazimine following Treatment for Tuberculosis in Pakistan. Antimicrobial. Agents Chemother. 2019, 63, e00915-19. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Ley, S.D.; Wiggins, K.B.; Derendinger, B.; Dippenaar, A.; Grobbelaar, M.; Reuter, A.; Dolby, T.; Burns, S.; Schito, M.; et al. Bedaquiline microheteroresistance after cessation of tuberculosis treatment. N. Engl. J. Med. Lett. 2019, 380, 2178–2180. [Google Scholar] [CrossRef] [PubMed]
- Polsfuss, S.; Hofmann-Thiel, S.; Merker, M.; Krieger, D.; Niemann, S.; Rüssmann, H.; Schönfeld, N.; Hoffmann, H.; Kranzer, K. Emergence of Low-level Delamanid and Bedaquiline Resistance during Extremely Drug-resistant Tuberculosis Treatment. Clin. Infect. Dis. 2019, 69, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Cancino-Munoz, I.; Moreno-Molina, M.; Furio, V.; Goig, G.A.; Torres-Puente, M.; Chiner-Oms, A.; Villamayor, L.M.; Sanz, F.; Guna-Serrano, M.R.; Comas, I. Cryptic Resistance Mutations Associated with Misdiagnoses of Multidrug-Resistant Tuberculosis. J. Infect. Dis. 2019, 220, 316–320. [Google Scholar] [CrossRef]
- Rancoita, P.M.V.; Cugnata, F.; Gibertoni Cruz, A.L.; Borroni, E.; Hoosdally, S.J.; Walker, T.M.; Grazian, C.; Davies, T.J.; Peto, T.E.A.; Crook, D.W.; et al. Validating a 14-drug microtiter plate containing bedaquiline and delamanid for large-scale research susceptibility testing of mycobacterium tuberculosis. Antimicrobial. Agents Chemother. 2018, 62, e00344-18. [Google Scholar] [CrossRef]
- Tiberi, S.; Cabibbe, A.M.; Tomlins, J.; Cirillo, D.M.; Migliori, G.B. Bedaquiline Phenotypic and Genotypic Susceptibility Testing, Work in Progress! EBioMedicine 2018, 29, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Peters, R.P.H.; Ismail, N.A.; Omar, S.V. Clofazimine Exposure In Vitro Selects Efflux Pump Mutants and Bedaquiline Resistance. Antimicrob. Agents Chemother. 2019, 63, e02141-18. [Google Scholar] [CrossRef]
- Ismail, N.A.; Omar, S.V.; Joseph, L.; Govender, N.; Blows, L.; Ismail, F.; Koornhof, H.; Dreyer, A.W.; Kaniga, K.; Ndjeka, N. Defining Bedaquiline Susceptibility, Resistance, Cross-Resistance and Associated Genetic Determinants: A Retrospective Cohort Study. EBioMedicine 2018, 28, 136–142. [Google Scholar] [CrossRef]
- Ismail, N.; Ismail, N.A.; Omar, S.V.; Peters, R.P.H. Study of Stepwise Acquisition of of rv0678 and atpE Mutations Conferring Bedaquiline Resistance. Antimicrob. Agents Chemother. 2019, 63, e00292-19. [Google Scholar] [CrossRef]
- Ghajavand, H.; Kargarpour Kamakoli, M.; Khanipour, S.; Pourazar Dizaji, S.; Masoumi, M.; Rahimi Jamnani, F.; Fateh, A.; Siadat, S.D.; Vaziri, F. High Prevalence of Bedaquiline Resistance in Treatment-Naive Tuberculosis Patients and Verapamil Effectiveness. Antimicrob. Agents Chemother. 2019, 63, e02530-18. [Google Scholar] [CrossRef] [PubMed]
- Daum, L.T.; Konstantynovska, O.S.; Solodiankin, O.S.; Poteiko, P.I.; Bolotin, V.I.; Rodriguez, J.D.; Gerilovych, A.P.; Chambers, J.P.; Fischer, G.W. Characterization of novel Mycobacterium tuberculosis pncA gene mutations in clinical isolates from the Ukraine. Diagn. Microbiol. Infect. Dis. 2017, 93, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, B.; Hu, M.; Huo, F.; Guo, S.; Jing, W.; Nuermberger, E.; Lu, Y. Primary Clofazimine and Bedaquiline Resistance among Isolates from Patients with Multidrug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2017, 61, e00239-17. [Google Scholar] [CrossRef] [PubMed]
- Torrea, G.; Coeck, N.; Desmaretz, C.; Van De Parre, T.; Van Poucke, T.; Lounis, N.; de Jong, B.C.; Rigouts, L. Bedaquiline susceptibility testing of Mycobacterium tuberculosis in an automated liquid culture system. J. Antimicrob. Chemother. 2015, 70, 2300–2305. [Google Scholar] [CrossRef]
- Zimenkov, D.V.; Nosova, E.Y.; Kulagina, E.V.; Antonova, O.V.; Arslanbaeva, L.R.; Isakova, A.I.; Krylova, L.Y.; Peretokina, I.V.; Makarova, M.V.; Safonova, S.G.; et al. Examination of bedaquiline- and linezolid-resistant Mycobacterium tuberculosis isolates from the Moscow region. J. Antimicrob. Chemother. 2017, 72, 1901–1906. [Google Scholar] [CrossRef]
- Huitric, E.; Verhasselt, P.; Koul, A.; Andries, K.; Hoffner, S.; Andersson, D.I. Rates and mechanisms of resistance development in Mycobacterium tuberculosis to a novel diarylquinoline ATP synthase inhibitor. Antimicrob. Agents Chemother. 2010, 54, 1022–1028. [Google Scholar] [CrossRef]
- Haver, H.L.; Chua, A.; Ghode, P.; Lakshminarayana, S.B.; Singhal, A.; Mathema, B.; Wintjens, R.; Bifani, P. Mutations in genes for the F420 biosynthetic pathway and a nitroreductase enzyme are the primary resistance determinants in spontaneous in vitro-selected PA-824-resistant mutants of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 5316–5323. [Google Scholar] [CrossRef]
- Feuerriegel, S.; Köser, C.U.; Baù, D.; Rüsch-Gerdes, S.; Summers, D.K.; Archer, J.A.; Marti-Renom, M.A.; Niemann, S. Impact of Fgd1 and ddn diversity in Mycobacterium tuberculosis complex on in vitro susceptibility to PA-824. Antimicrob. Agents Chemother. 2011, 55, 5718–5722. [Google Scholar] [CrossRef]
- Keller, P.M.; Hömke, R.; Ritter, C.; Valsesia, G.; Bloemberg, G.V.; Böttger, E.C. Determination of MIC distribution and epidemiological cutoff values for bedaquiline and delamanid in Mycobacterium tuberculosis using the MGIT 960 system equipped with TB eXiST. Antimicrob. Agents Chemother. 2015, 59, 4352–4355. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Kumar, N.; Wright, C.C.; Chou, T.H.; Tringides, M.L.; Bolla, J.R.; Lei, H.T.; Rajashankar, K.R.; Su, C.C.; Purdy, G.E.; et al. Crystal structure of the transcriptional regulator Rv0678 of Mycobacterium tuberculosis. J. Biol. Chem. 2014, 289, 16526–16540. [Google Scholar] [CrossRef] [PubMed]
- Milano, A.; Pasca, M.R.; Provvedi, R.; Lucarelli, A.P.; Manina, G.; Ribeiro, A.L.; Manganelli, R.; Riccardi, G. Azole resistance in Mycobacterium tuberculosis is mediated by the MmpS5-MmpL5 efflux system. Tuberculosis 2009, 89, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Segala, E.; Sougakoff, W.; Nevejans-Chauffour, A.; Jarlier, V.; Petrella, S. New mutations in the mycobacterial ATP synthase: New insights into the binding of the diarylquinoline TMC207 to the ATP synthase C-ring structure. Antimicrob. Agents Chemother. 2012, 56, 2326–2334. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Dendouga, N.; Vergauwen, K.; Molenberghs, B.; Vranckx, L.; Willebrords, R.; Ristic, Z.; Lill, H.; Dorange, I.; Guillemont, J.; et al. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 2007, 3, 323–324. [Google Scholar] [CrossRef]
- De Jonge, M.R.; Koymans, L.H.; Guillemont, J.E.; Koul, A.; Andries, K. A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910. Proteins 2007, 67, 971–980. [Google Scholar] [CrossRef]
- Andersson, D.I.; Levin, B.R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 1999, 2, 489–493. [Google Scholar] [CrossRef]
- Rifat, D.; Campodónico, V.L.; Tao, J.; Miller, J.A.; Alp, A.; Yao, Y.; Karakousis, P.C. In vitro and in vivo fitness costs associated with Mycobacterium tuberculosis RpoB mutation H526D. Future Microbiol. 2014, 12, 753–765. [Google Scholar] [CrossRef]
- Koch, A.; Mizrahi, V.; Warner, D.F. The impact of drug resistance on Mycobacterium tuberculosis physiology: What can we learn from rifampicin? Emerg. Mic. Infect. 2014, 3, e17. [Google Scholar] [CrossRef]
- Kodio, O.; Georges Togo, A.; Sadio Sarro, Y.; Fane, B.; Diallo, F.; Somboro, A.; Degoga, B.; Kone, M.; Coulibaly, G.; Tolofoudje, M.; et al. Competitive fitness of Mycobacterium tuberculosis in vitro. Int. J. Mycobacteriol. 2019, 8, 287. [Google Scholar] [CrossRef]
- Lamichhane, G.; Tyagi, S.; Bishai, W.R. Designer arrays for defined mutant analysis to detect genes essential for survival of Mycobacterium tuberculosis in mouse lungs. Infect. Immun. 2005, 73, 2533–2540. [Google Scholar] [CrossRef]
- Yu, X.; Gao, X.; Li, C.; Luo, J.; Wen, S.; Zhang, T.; Ma, Y.; Dong, L.; Wang, F.; Huang, H. Activities of Bedaquiline and Delamanid against Nontuberculous Mycobacteria Isolated in Beijing, China. Antimicrob. Agents Chemother. 2019, 63, e00031-19. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, U.H.; Lahiri, R.; Randhawa, B.; Dowd, C.S.; Krahenbuhl, J.L.; Barry, C.E. Mycobacterium leprae is naturally resistant to PA-824. Antimicrob. Agents Chemother. 2006, 50, 3350–3354. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hashizume, H.; Tomishige, T.; Kawasaki, M.; Tsubouchi, H.; Sasaki, H.; Shimokawa, Y.; Komatsu, M. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006, 3, e466. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Kawasaki, M.; Hariguchi, N.; Liu, Y.; Matsumoto, M. Mechanisms of resistance to delamanid, a drug for Mycobacterium tuberculosis. Tuberculosis 2018, 108, 186–194. [Google Scholar] [CrossRef]
- Lee, B.M.; Harold, L.K.; Almeida, D.V.; Afriat-Jurnou, L.; Aung, H.L.; Forde, B.M.; Hards, K.; Pidot, S.J.; Ahmed, F.H.; Mohamed, A.E.; et al. Predicting nitroimidazole antibiotic resistance mutations in Mycobacterium tuberculosis with protein engineering. PLoS Pathog. 2020, 16, e1008287. [Google Scholar] [CrossRef]
- El-Halfawy, O.M.; Valvano, M.A. Antimicrobial heteroresistance: An emerging field in need of clarity. Clin. Microbiol. Rev. 2015, 28, 191–207. [Google Scholar] [CrossRef]
- Bedaquiline Country Regulatory Status Overview. Available online: https://www.jnj.com/_document/bedaquiline-country-regulatory-status-overview?id=0000016e-0467-db13-a9ef-566f1a520000 (accessed on 5 January 2020).
- Assessment Report for Paediatric Studies Submitted According to Article 46 of the Regulation (EC) No 1901/2006; Deltyba. European Medicines Agency: London, UK, 2018; Available online: https://www.ema.europa.eu/en/documents/variation-report/deltyba-h-c-2552-p46-007-epar-assessment-report_en.pdf (accessed on 3 January 2020).
- Chawla, K.; Martinez, E.; Kumar, A.; Shenoy, V.P.; Sintchenko, V. Whole-genome sequencing reveals genetic signature of bedaquiline resistance in a clinical isolate of Mycobacterium tuberculosis. J. Glob. Antimicrob. Res. 2018, 15, 103–104. [Google Scholar] [CrossRef]
- Dheda, K.; Limberis, J.D.; Pietersen, E.; Phelan, J.; Esmail, A.; Lesosky, M.; Fennelly, K.P.; te Riele, J.; Mastrapa, B.; Streicher, E.M.; et al. Outcomes, Infect.iousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: A prospective cohort study. Lancet Res. Med. 2019, 5, 269–281. [Google Scholar] [CrossRef]
- Karmakar, M.; Rodrigues, C.H.M.; Holt, K.E.; Dunstan, S.J.; Denholm, J.; Ascher, D.B. Empirical ways to identify novel Bedaquiline resistance mutations in AtpE. PLoS ONE 2019, 14, e0217169. [Google Scholar] [CrossRef]
- Makhado, N.A.; Matabane, E.; Faccin, M.; Pincon, C.; Jouet, A.; Boutachkourt, F.; Goeminne, L.; Gaudin, C.; Maphalala, G.; Beckert, P.; et al. Outbreak of multidrug-resistant tuberculosis in South Africa undetected by WHO-endorsed commercial tests: An observational study. Lancet Infect. Dis. 2018, 18, 1350–1359. [Google Scholar] [CrossRef]
- Feuerriegel, S.; Köser, C.U.; Niemann, S. Phylogenetic polymorphisms in antibiotic resistance genes of the Mycobacterium tuberculosis complex. J. Antimicrob. Chemother. 2014, 69, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Advani, J.; Verma, R.; Chatterjee, O.; Pachouri, P.K.; Upadhyay, P.; Singh, R.; Yadav, J.; Naaz, F.; Ravikumar, R.; Buggi, S.; et al. Whole Genome Sequencing of Mycobacterium tuberculosis Clinical Isolates from India Reveals Genetic Heterogeneity and Region-Specific Variations That Might Affect Drug Susceptibility. Front. Microbiol. 2019, 10, 309. [Google Scholar] [CrossRef] [PubMed]

| Genes Linked to BDQ Susceptibility | Total Mutations | R | I a | S | Genes Linked to DLM Susceptibility | Total Mutations | R | S |
|---|---|---|---|---|---|---|---|---|
| Rv0678 | 48 | 39 | 1 | 9 b | fbiA | 6 | 4 | 3 f |
| atpE | 10 | 8 | 0 | 2 | fgd1 | 4 | 2 | 2 |
| Rv1979c | 4 | 2 c | 1 | 3 d | ddn | 4 | 2 | 2 |
| pepQ | 101 | 3 | 0 | 98 | fbiC | 3 | 1 | 2 |
| mmpL5 | 2 | 1 | 0 | 1 | fbiB | 3 | 0 | 3 |
| atpB | 3 | 2 e | 0 | 1 | − | − | − | |
| ppsC | 1 | 1 c | 0 | 0 | − | − | − |
| Gene | Mutations Identified Through WGS by the Articles Reviewed Here | Studies That also Identified the Mutation by PCR-Sanger Sequencing * |
|---|---|---|
| Bedaquiline | ||
| Rv0678 (mmpR) | −11 C>A | [9] |
| T2C (Val1Ala) | Reported as fMet1Ala [45] | |
| T136C (Cys46Arg) | [41] | |
| 136_137 insG | [41] | |
| 138_139 insG | [41] | |
| 141_142 insC | [41] | |
| C189A (Ser63Arg) | [40] | |
| 192_193_InsG | [9] | |
| T350G (Leu117Arg) | [9] | |
| T407C (Leu136Pro) | [40] | |
| atpE | A83T (Asp28Val) | [42] |
| G183T (Glu61Asp) | [42] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto Ramirez, L.M.; Quintero Vargas, K.; Diaz, G. Whole Genome Sequencing for the Analysis of Drug Resistant Strains of Mycobacterium tuberculosis: A Systematic Review for Bedaquiline and Delamanid. Antibiotics 2020, 9, 133. https://doi.org/10.3390/antibiotics9030133
Nieto Ramirez LM, Quintero Vargas K, Diaz G. Whole Genome Sequencing for the Analysis of Drug Resistant Strains of Mycobacterium tuberculosis: A Systematic Review for Bedaquiline and Delamanid. Antibiotics. 2020; 9(3):133. https://doi.org/10.3390/antibiotics9030133
Chicago/Turabian StyleNieto Ramirez, Luisa Maria, Karina Quintero Vargas, and Gustavo Diaz. 2020. "Whole Genome Sequencing for the Analysis of Drug Resistant Strains of Mycobacterium tuberculosis: A Systematic Review for Bedaquiline and Delamanid" Antibiotics 9, no. 3: 133. https://doi.org/10.3390/antibiotics9030133
APA StyleNieto Ramirez, L. M., Quintero Vargas, K., & Diaz, G. (2020). Whole Genome Sequencing for the Analysis of Drug Resistant Strains of Mycobacterium tuberculosis: A Systematic Review for Bedaquiline and Delamanid. Antibiotics, 9(3), 133. https://doi.org/10.3390/antibiotics9030133





