A Rapid Fluorescence-Based Microplate Assay to Investigate the Interaction of Membrane Active Antimicrobial Peptides with Whole Gram-Positive Bacteria
Abstract
:1. Introduction
2. Results
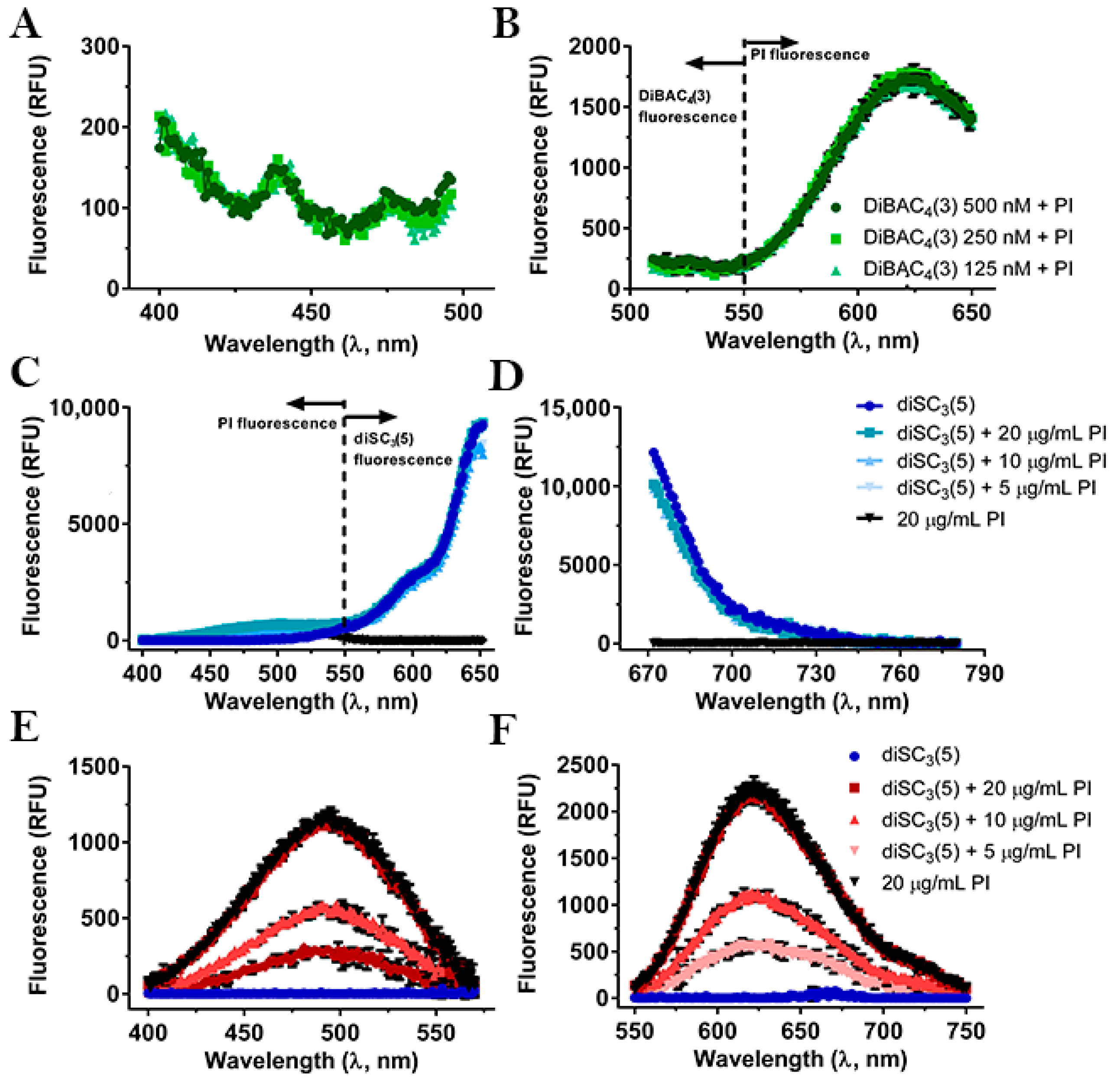
2.1. Compatibility between the Fluorescent Dyes
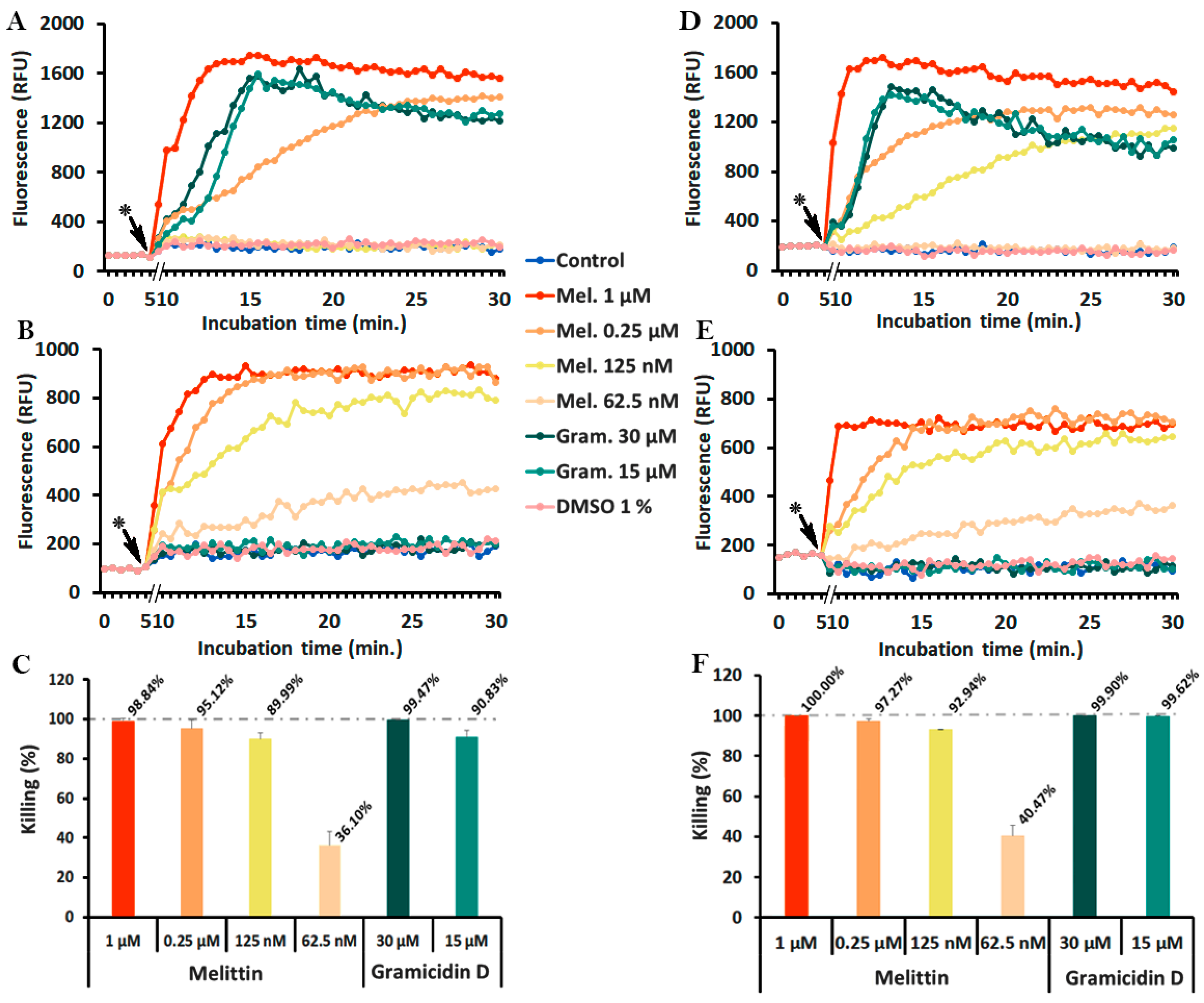
2.2. Selection of Membrane-Active Peptides
2.3. Interference of Peptides and Uncouplers with the Fluorescent Dyes
2.4. Monitoring of Peptide-Induced Membrane Alterations with the Combination of diSC3(5) and PI
2.5. Morphology of Peptide-Treated S. epidermidis
3. Discussion
4. Materials and Methods
4.1. Equipment
4.2. Peptides and Other Reagents
4.3. Bacteria and Bacterial Cultures
4.4. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.5. Excitation and Emission Spectra
4.6. Interference of Uncouplers and Peptides with the Fluorescent Dyes
4.7. Kinetic Fluorescence Measurements to Detect Membrane Depolarization and Permeabilization
4.8. Field Emission Scanning Electron Microscopy (FE-SEM) of S. epidermidis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Antimicrobial resistance. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 18 February 2020).
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E.W. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef]
- Rončević, T.; Puizina, J.; Tossi, A. Antimicrobial Peptides as Anti-Infective Agents in Pre-Post-Antibiotic Era? Int. J. Mol. Sci. 2019, 20, 5713. [Google Scholar] [CrossRef] [Green Version]
- Peschel, A.; Sahl, H.-G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.; O’Neill, A.; Lee, R. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2012, 9, 62–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetti, M.; Gennaro, R.; Skerlavaj, B.; Tomasinsig, L.; Circo, R. Cathelicidin Peptides as Candidates for a Novel Class of Antimicrobials. Curr. Pharm. Des. 2002, 8, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Strempel, N.; Strehmel, J.; Overhage, J. Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr. Pharm. Des. 2015, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Oren, Z.; Shai, Y. Mode of action of linear amphipathic α-helical antimicrobial peptides. Biopolymers 1998, 47, 451–463. [Google Scholar] [CrossRef]
- Matsuzaki, K. Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim. Biophys. Acta—Rev. Biomembr. 1998, 1376, 391–400. [Google Scholar] [CrossRef]
- Huang, H.W.; Charron, N.E. Understanding membrane-active antimicrobial peptides. Q. Rev. Biophys. 2017, 50, e10. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Strahl, H.; Errington, J. Bacterial Membranes: Structure, Domains, and Function. Annu. Rev. Microbiol. 2017, 71, 519–538. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Wimley, W.C.; Hristova, K. Antimicrobial Peptides: Successes, Challenges and Unanswered Questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sochacki, K.A.; Barns, K.J.; Bucki, R.; Weisshaar, J.C. Real-time attack on single Escherichia coli cells by the human antimicrobial peptide LL-37. Proc. Natl. Acad. Sci. USA 2011, 108, E77–E81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta-Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidenmaier, C.; Peschel, A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008, 6, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.W.; May, J.M.; Sherman, D.J.; Kahne, D.; Ruiz, N. Lipopolysaccharide transport to the cell surface: Biosynthesis and extraction from the inner membrane. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 1–7. [Google Scholar] [CrossRef]
- May, J.M.; Sherman, D.J.; Simpson, B.W.; Ruiz, N.; Kahne, D. Lipopolysaccharide transport to the cell surface: Periplasmic transport and assembly into the outer membrane. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 1–8. [Google Scholar] [CrossRef]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.-G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, E7077–E7086. [Google Scholar]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Sträuber, H.; Müller, S. Viability states of bacteria-Specific mechanisms of selected probes. Cytom. Part. A 2010, 77A, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Poot, M.; Yue, S.T.; Millard, P.J. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 1997, 63, 2421–2431. [Google Scholar] [CrossRef] [Green Version]
- Silverman, J.A.; Perlmutter, N.G.; Howard, M.; Shapiro, H.M. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef] [Green Version]
- Farha, M.A.; Verschoor, C.P.; Bowdish, D.; Brown, E.D. Collapsing the proton motive force to identify synergistic combinations against Staphylococcus aureus. Chem. Biol. 2013, 20, 1168–1178. [Google Scholar] [CrossRef] [Green Version]
- Epps, D.E.; Wolfe, M.L.; Groppi, V. Characterization of the steady-state and dynamic fluorescence properties of the potential-sensitive dye bis-(1,3-dibutylbarbituric acid)trimethine oxonol (Dibac4(3)) in model systems and cells. Chem. Phys. Lipids 1994, 69, 137–150. [Google Scholar] [CrossRef]
- Shapiro, H.M. Membrane Potential Estimation by Flow Cytometry. Methods 2000, 21, 271–279. [Google Scholar] [CrossRef]
- Wu, M.; Maier, E.; Benz, R.; Hancock, R.E.W. Mechanism of Interaction of Different Classes of Cationic Antimicrobial Peptides with Planar Bilayers and with the Cytoplasmic Membrane of Escherichia coli †. Biochemistry 1999, 38, 7235–7242. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, A. Optical probes of membrane potential. J. Membr. Biol. 1976, 27, 317–334. [Google Scholar] [CrossRef] [PubMed]
- te Winkel, J.D.; Gray, D.A.; Seistrup, K.H.; Hamoen, L.W.; Strahl, H. Analysis of Antimicrobial-Triggered Membrane Depolarization Using Voltage Sensitive Dyes. Front. Cell Dev. Biol. 2016, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, P.J.; Waggoner, A.S.; Wang, C.-H.; Hoffman, J.F. Studies on the Mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry 1974, 13, 3315–3330. [Google Scholar] [CrossRef]
- Cabrini, G.; Verkman, A.S. Potential-sensitive response mechanism of diS-C3-(5) in biological membranes. J. Membr. Biol. 1986, 92, 171–182. [Google Scholar] [CrossRef]
- Mason, D.J.; Lopéz-Amorós, R.; Allman, R.; Stark, J.M.; Lloyd, D. The ability of membrane potential dyes and calcafluor white to distinguish between viable and non-viable bacteria. J. Appl. Bacteriol. 1995, 78, 309–315. [Google Scholar] [CrossRef]
- López-Amorós, R.; Comas, J.; Vives-Rego, J. Flow cytometric assessment of Escherichia coli and Salmonella typhimurium starvation-survival in seawater using rhodamine 123, propidium iodide, and oxonol. Appl. Environ. Microbiol. 1995, 61, 2521–2526. [Google Scholar] [CrossRef] [Green Version]
- Ordóñez, J.V.; Wehman, N.M. Rapid flow cytometric antibiotic susceptibility assay for Staphylococcus aureus. Cytometry 1993, 14, 811–818. [Google Scholar] [CrossRef]
- Gauthier, C.; St-Pierre, Y.; Villemur, R. Rapid antimicrobial susceptibility testing of urinary tract isolates and samples by flow cytometry. J. Med. Microbiol. 2002, 51, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, C.J.; Nebe-Von-Caron, G. The Application of Multi-Parameter Flow Cytometry to Monitor Individual Microbial Cell Physiological State. Adv. Biochem. Eng. Biotechnol. 2004, 89, 197–223. [Google Scholar]
- Okuda, K.; Zendo, T.; Sugimoto, S.; Iwase, T.; Tajima, A.; Yamada, S.; Sonomoto, K.; Mizunoe, Y. Effects of Bacteriocins on Methicillin-Resistant Staphylococcus aureus Biofilm. Antimicrob. Agents Chemother. 2013, 57, 5572–5579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, C.L.; Moyles, D.; Beveridge, T.J.; Hancock, R.E.W. Antibacterial Action of Structurally Diverse Cationic Peptides on Gram-Positive Bacteria. Antimicrob. Agents Chemother. 2000, 44, 2086–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestro, L.; Weiser, J.N.; Axelsen, P.H. Antibacterial and Antimembrane Activities of Cecropin A in Escherichia coli. Antimicrob. Agents Chemother. 2000, 44, 602–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, N.; Lanneluc, I.; Connil, N.; Cottenceau, M.; Pons, A.M.; Sablé, S. Mechanism of Bactericidal Activity of Microcin L in Escherichia coli and Salmonella enterica. Antimicrob. Agents Chemother. 2011, 55, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Huang, J.X.; Ramu, S.; Butler, M.S.; Cooper, M.A. Ramoplanin at Bactericidal Concentrations Induces Bacterial Membrane Depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 6819–6827. [Google Scholar] [CrossRef] [Green Version]
- Clementi, E.A.; Marks, L.R.; Roche-Håkansson, H.; Håkansson, A.P. Monitoring changes in membrane polarity, membrane integrity, and intracellular ion concentrations in Streptococcus pneumoniae using fluorescent dyes. J. Vis. Exp. 2014, e51008. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug Resistant Bacterial Infections, Including Tuberculosis. 2017. Available online: https://www.who.int/medicines/areas/rational_use/PPLreport_2017_09_19.pdf?ua=1 (accessed on 18 February 2020).
- Sabaté Brescó, M.; Harris, L.G.; Thompson, K.; Stanic, B.; Morgenstern, M.; O’Mahony, L.; Richards, R.G.; Moriarty, T.F. Pathogenic Mechanisms and Host Interactions in Staphylococcus epidermidis Device-Related Infection. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Kelkar, D.A.; Chattopadhyay, A. The gramicidin ion channel: A model membrane protein. Biochim. Biophys. Acta - Biomembr. 2007, 1768, 2011–2025. [Google Scholar] [CrossRef] [Green Version]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A Membrane-active Peptide with Diverse Functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef]
- Arndt-Jovin, D.J.; Jovin, T.M. Chapter 16 Fluorescence Labeling and Microscopy of DNA. Methods Cell Biol. 1989, 30, 417–448. [Google Scholar]
- Johnson, I.; Spence, M.T.Z. (Eds.) Chapter 22–Probes for Membrane Potential. In The Molecular Probes Handbook. A Guide to Fluorescent Probes and Labeling Technologies; Thermo Fisher Scientific: Waltham, MA, USA, 2010; Volume 76, pp. 923–936. ISBN 978-0-9829279-1-5. [Google Scholar]
- Chapter 8—Nucleic Acid Detection and Analysis. In The Molecular Probes Handbook. A guide to fluorescent probes and labeling technologies; Johnson, I.; Spence, M.T.Z. (Eds.) ThermoFisher Scientific: Waltham, MA, USA, 2010; pp. 302–360. ISBN 0-9710636-4-8. [Google Scholar]
- Boman, H.G.; Steiner, H. Humoral Immunity in Cecropia Pupae. Curr. Top. Microbiol. Immunol. 1981, 94–95, 75–91. [Google Scholar]
- Berkowitz, B.A.; Bevins, C.L.; Zasloff, M.A. Magainins: A new family of membrane-active host defense peptides. Biochem. Pharmacol. 1990, 39, 625–629. [Google Scholar] [CrossRef]
- Bechinger, B. Structure and Functions of Channel-Forming Peptides: Magainins, Cecropins, Melittin and Alamethicin. J. Membr. Biol. 1997, 156, 197–211. [Google Scholar] [CrossRef]
- Dubos, R.J.; Hotchkiss, R.D. The Production of Bactericidal Substances by Aerobic Sporulating Bacilli. J. Exp. Med. 1941, 73, 629–640. [Google Scholar] [CrossRef]
- Yang, X.; Yousef, A.E. Antimicrobial peptides produced by Brevibacillus spp.: Structure, classification and bioactivity: A mini review. Worldj. Microbiol. Biotechnol. 2018, 34, 57. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Berditsch, M.; Hawecker, J.; Ardakani, M.F.; Gerthsen, D.; Ulrich, A.S. Damage of the Bacterial Cell Envelope by Antimicrobial Peptides Gramicidin S and PGLa as Revealed by Transmission and Scanning Electron Microscopy. Antimicrob. Agents Chemother. 2010, 54, 3132–3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rončević, T.; Krce, L.; Gerdol, M.; Pacor, S.; Benincasa, M.; Guida, F.; Aviani, I.; Čikeš-Čulić, V.; Pallavicini, A.; Maravić, A.; et al. Membrane-active antimicrobial peptide identified in Rana arvalis by targeted DNA sequencing. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 651–659. [Google Scholar]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Comparative mode of action of the antimicrobial peptide melimine and its derivative Mel4 against Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Virta, M.; Lineri, S.; Kankaanpää, P.; Karp, M.; Peltonen, K.; Nuutila, J.; Lilius, E.M. Determination of complement-mediated killing of bacteria by viability staining and bioluminescence. Appl. Environ. Microbiol. 1998, 64, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Boulos, L.; Prévost, M.; Barbeau, B.; Coallier, J.; Desjardins, R. LIVE/DEAD® BacLight™: Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 1999, 37, 77–86. [Google Scholar] [CrossRef]
- Herrera, G.; Martinez, A.; Blanco, M.; O’Connor, J.-E. Assessment of Escherichia coli B with enhanced permeability to fluorochromes for flow cytometric assays of bacterial cell function. Cytometry 2002, 49, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, H.; Li, L.; Fan, X.; Ding, J.; Li, X.; Xi, T.; Shen, Z. Membrane aggregation and perturbation induced by antimicrobial peptide of S-thanatin. Biochem. Biophys. Res. Commun. 2010, 395, 31–35. [Google Scholar] [CrossRef] [PubMed]
- D’Este, F.; Benincasa, M.; Cannone, G.; Furlan, M.; Scarsini, M.; Volpatti, D.; Gennaro, R.; Tossi, A.; Skerlavaj, B.; Scocchi, M. Antimicrobial and host cell-directed activities of Gly/Ser-rich peptides from salmonid cathelicidins. Fish. Shellfish Immunol. 2016, 59, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hao, D.; Chen, Y.; Xu, Y.; Tan, J.; Huang, Y.; Li, F.; Chen, Y. Inhibitory effects and mechanisms of physiological conditions on the activity of enantiomeric forms of an α-helical antibacterial peptide against bacteria. Peptides 2011, 32, 1488–1495. [Google Scholar] [CrossRef]
- D’Este, F.; Oro, D.; Boix-Lemonche, G.; Tossi, A.; Skerlavaj, B. Evaluation of free or anchored antimicrobial peptides as candidates for the prevention of orthopaedic device-related infections. J. Pept. Sci. 2017, 23, 777–789. [Google Scholar] [CrossRef]
- Smart, O.S.; Goodfellow, J.M.; Wallace, B.A. The pore dimensions of gramicidin A. Biophys. J. 1993, 65, 2455–2460. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.K.; Kim, Y.-C.; Nan, Y.H.; Shin, S.Y. Cell selectivity, mechanism of action and LPS-neutralizing activity of bovine myeloid antimicrobial peptide-18 (BMAP-18) and its analogs. Peptides 2011, 32, 1123–1130. [Google Scholar] [CrossRef]
- Shang, D.; Sun, Y.; Wang, C.; Wei, S.; Ma, L.; Sun, L. Membrane interaction and antibacterial properties of chensinin-1, an antimicrobial peptide with atypical structural features from the skin of Rana chensinensis. Appl. Microbiol. Biotechnol. 2012, 96, 1551–1560. [Google Scholar] [CrossRef]
- Madhuri; Shireen, T.; Venugopal, S.K.; Ghosh, D.; Gadepalli, R.; Dhawan, B.; Mukhopadhyay, K. In vitro antimicrobial activity of alpha-melanocyte stimulating hormone against major human pathogen Staphylococcus aureus. Peptides 2009, 30, 1627–1635. [Google Scholar] [CrossRef]
- Gee, M.L.; Burton, M.; Grevis-James, A.; Hossain, M.A.; McArthur, S.; Palombo, E.A.; Wade, J.D.; Clayton, A.H.A. Imaging the action of antimicrobial peptides on living bacterial cells. Sci. Rep. 2013, 3, 1557. [Google Scholar] [CrossRef]
- Faust, J.E.; Yang, P.-Y.; Huang, H.W. Action of Antimicrobial Peptides on Bacterial and Lipid Membranes: A Direct Comparison. Biophys. J. 2017, 112, 1663–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, R.; Hakemi Vala, M.; Hashemi, A.; Aghazadeh, H.; Sabatier, J.-M.; Pooshang Bagheri, K. Action mechanism of melittin-derived antimicrobial peptides, MDP1 and MDP2, de novo designed against multidrug resistant bacteria. Amino Acids 2018, 50, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Ladokhin, A.S.; Selsted, M.E.; White, S.H. Sizing membrane pores in lipid vesicles by leakage of co-encapsulated markers: Pore formation by melittin. Biophys. J. 1997, 72, 1762–1766. [Google Scholar] [CrossRef] [Green Version]


 ), blebs (
), blebs ( ), indents (
), indents ( ), bacteria with altered morphology (
), bacteria with altered morphology ( ), and deflated bags (
), and deflated bags ( ). Representative images from two experiments performed in duplicate are shown.
). Representative images from two experiments performed in duplicate are shown.
 ), blebs (
), blebs ( ), indents (
), indents ( ), bacteria with altered morphology (
), bacteria with altered morphology ( ), and deflated bags (
), and deflated bags ( ). Representative images from two experiments performed in duplicate are shown.
). Representative images from two experiments performed in duplicate are shown.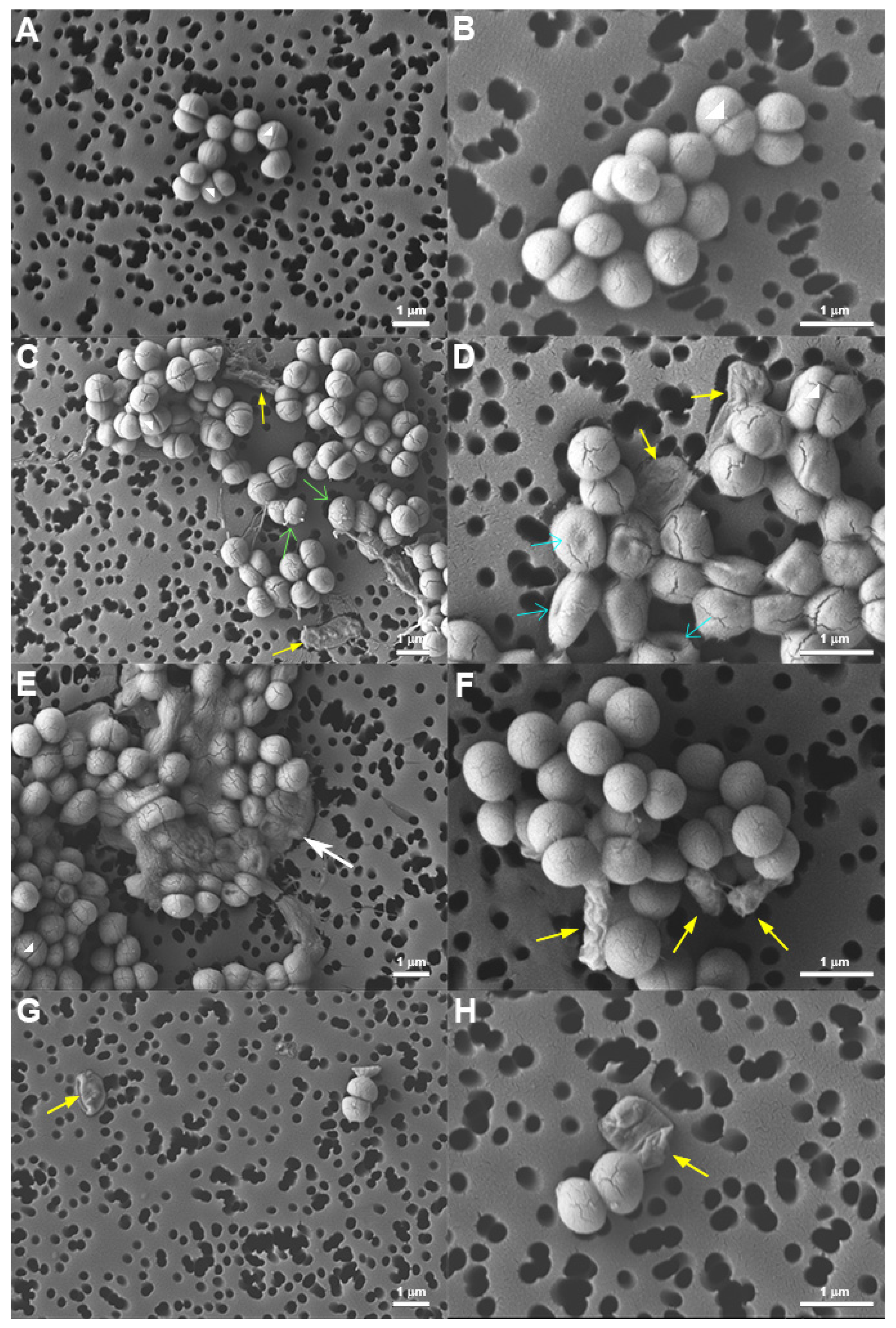
| Cecropin A | Melittin | Magainin 2 | Gramicidin D | |
|---|---|---|---|---|
| MIC (MBC) (µM) a,b | ||||
| S. epidermidis ATCC 35984 | >128 | 0.5 (0.5) | >128 | 2 (4) |
| S. aureus ATCC 25923 | >128 | 0.5 (1) | 64 (128) | 4 (8) |
| E. coli ATCC 25922 | 0.75 (0.75) | 1 (1) | 8 (16) | >32 |
| P. aeruginosa ATCC 27853 | 1 (2) | 1 (2) | 64 (64) | >32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boix-Lemonche, G.; Lekka, M.; Skerlavaj, B. A Rapid Fluorescence-Based Microplate Assay to Investigate the Interaction of Membrane Active Antimicrobial Peptides with Whole Gram-Positive Bacteria. Antibiotics 2020, 9, 92. https://doi.org/10.3390/antibiotics9020092
Boix-Lemonche G, Lekka M, Skerlavaj B. A Rapid Fluorescence-Based Microplate Assay to Investigate the Interaction of Membrane Active Antimicrobial Peptides with Whole Gram-Positive Bacteria. Antibiotics. 2020; 9(2):92. https://doi.org/10.3390/antibiotics9020092
Chicago/Turabian StyleBoix-Lemonche, Gerard, Maria Lekka, and Barbara Skerlavaj. 2020. "A Rapid Fluorescence-Based Microplate Assay to Investigate the Interaction of Membrane Active Antimicrobial Peptides with Whole Gram-Positive Bacteria" Antibiotics 9, no. 2: 92. https://doi.org/10.3390/antibiotics9020092
APA StyleBoix-Lemonche, G., Lekka, M., & Skerlavaj, B. (2020). A Rapid Fluorescence-Based Microplate Assay to Investigate the Interaction of Membrane Active Antimicrobial Peptides with Whole Gram-Positive Bacteria. Antibiotics, 9(2), 92. https://doi.org/10.3390/antibiotics9020092





