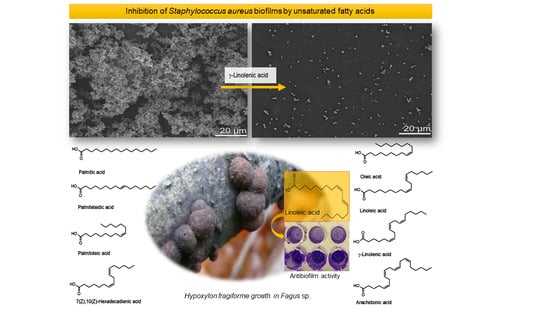
Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus aureus and Other Gram-Positive Bacteria
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Microorganisms
4.3. Isolation, Fungal Identification and Fermentation
4.4. Minimum Inhibitory Concentration (MIC) and Biofilm Assays
4.5. Purification and Identification of the Compound
4.6. Light and Field Emission Scanning Electron Microscopy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flemming, H.C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Burmølle, M.; Thomsen, T.R.; Fazli, M.; Dige, I.; Christensen, L.; Homøe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity—Monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef]
- Potera, C. Forging a link between biofilms and disease. Science 1999, 283, 1837–1839. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. Apmis Suppl. 2013, 136, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Bayles, K.W. The biological role of death and lysis in biofilm development. Nat. Rev. Microbiol. 2007, 5, 721–726. [Google Scholar] [CrossRef]
- Estrela, A.B.; Abraham, W.-R. Combining biofilm controlling compounds and antibiotics as a promising new way to control biofilm infections. Pharmaceuticals 2010, 3, 1374–1393. [Google Scholar] [CrossRef]
- Houbraken, J.; Frisvad, J.C.; Seifert, K.A.; Overy, D.P.; Tuthill, D.M.; Valdez, J.G.; Samson, R.A. New penicillin-producing Penicillium species and an overview of section Chrysogena. Persoonia 2012, 29, 78–100. [Google Scholar] [CrossRef]
- Brian, P.W.; Curtis, P.J.; Howland, S.R.; Jefferys, E.G.; Raudnitz, H. Three new antibiotics from a species of Gliocladium. Exp. 1951, 7, 266–267. [Google Scholar]
- Jiang, Y.; Wong, J.H.; Fu, M.; Ng, T.B.; Liu, Z.K.; Wang, C.R.; Li, N.; Qiao, W.T.; Wen, T.Y.; Liu, F. Isolation of adenosine, iso-sinensetin and dimethylguanosine with antioxidant and HIV-1 protease inhibiting activities from fruiting bodies of Cordyceps militaris. Phytomedicine 2011, 18, 189–193. [Google Scholar] [CrossRef]
- Chen, N.H.; Liu, J.W.; Zhong, J.J. Ganoderic acid me inhibits tumor invasion through down-regulating matrix metalloproteinases 2/9 gene expression. J. Pharmacol. Sci. 2008, 108, 212–216. [Google Scholar] [CrossRef]
- Manzoni, M.; Rollini, M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564. [Google Scholar] [PubMed]
- Yuyama, K.T.; Neves, T.S.P.C.; Memória, M.T.; Tartuci, I.T.; Abraham, W.-R. Aurantiogliocladin inhibits biofilm formation at subtoxic concentrations. Aims Microbiol. 2017, 3, 50–60. [Google Scholar] [CrossRef]
- Stadler, M.; Quang, D.N.; Tomita, A.; Hashimoto, T.; Asakawa, Y. Changes in secondary metabolism during stromatal ontogeny of Hypoxylon fragiforme. Mycol. Res. 2006, 110, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Persoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Chepkirui, C.; Wendt, L.; Fortkamp, D.; Stadler, M.; Abraham, W.-R. Bioactive compounds produced by Hypoxylon fragiforme against Staphylococcus aureus biofilm. Microorganisms 2017, 5, 80. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Wendt, L.; Surup, F.; Kretz, R.; Chepkirui, C.; Wittstein, K.; Boonlarppradab, C.; Wongkanoun, S.; Luangsa-Ard, J.; Stadler, M.; et al. Cytochalasans act as inhibitors of biofilm formation of Staphylococcus aureus. Biomolecules 2018, 8, 129. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Abraham, W.-R. cis-2-Alkenoic acids as promising drugs for the control of biofilm infections. Med. Chem. 2017, 13, 3–12. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L.; Thibane, V.S. Antifungal free fatty acids: A review. Sci. Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 3, 61–71. [Google Scholar]
- de Toledo-Piza, A.R.; de Oliveira, M.I.; Negri, G.; Mendonça, R.Z.; Figueiredo, C.A. Polyunsaturated fatty acids from Phyllocaulis boraceiensis mucus block the replication of influenza virus. Arch. Microbiol. 2018, 200, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Mayer, A.; Anke, H.; Sterner, O. Fatty acids and other compounds with nematicidal activity from cultures of Basidiomycetes. Planta Med. 1994, 60, 128–132. [Google Scholar] [CrossRef]
- Wendel, M.; Heller, A.R. Anticancer actions of omega-3 fatty acids—Current state and future perspectives. Anticancer Agents Med. Chem. 2009, 9, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Martorelli Di Genova, B.; Wilson, S.K.; Dubey, J.P.; Knoll, L.J. Intestinal delta-6-desaturase activity determines host range for Toxoplasma sexual reproduction. PLoS Biol. 2019, 17, e3000364. [Google Scholar] [CrossRef]
- Kordes, A.; Grahl, N.; Koska, M.; Preuße, M.; Rodriguez, A.A.; Abraham, W.-R.; Kaever, V.; Häussler, S. Establishment of an induced memory response in Pseudomonas aeruginosa during infection of a eukaryotic host. ISME J. 2019, 13, 2018–2030. [Google Scholar] [CrossRef]
- Marounek, M.; Skřivanová, E.; Rada, V. Susceptibility of Escherichia coli to C2-C18 fatty acids. Folia Microbiol. 2003, 48, 731–735. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus Aureus. Food Control 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Lee, J.-H.; Raorane, C.J.; Oh, S.T.; Park, J.G.; Lee, J. Herring oil and omega fatty acids inhibit Staphylococcus aureus biofilm formation and virulence. Front. Microbiol. 2018, 9, 1241. [Google Scholar] [CrossRef]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef]
- Kenny, J.G.; Ward, D.; Josefsson, E.; Jonsson, I.M.; Hinds, J.; Rees, H.H.; Lindsay, J.A.; Tarkowski, A.; Horsburgh, M.J. The Staphylococcus aureus response to unsaturated long chain free fatty acids: Survival mechanisms and virulence implications. PLoS ONE 2009, 4, e4344. [Google Scholar] [CrossRef]
- Arsic, B.; Zhu, Y.; Heinrichs, D.E.; McGavin, M.J. Induction of the staphylococcal proteolytic cascade by antimicrobial fatty acids in community acquired methicillin resistant Staphylococcus aureus. PLoS ONE 2012, 7, e45952. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.S.; Lee, T.G.; Cho, H.Y.; Kim, Y.H.; Kim, W.G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef]
- Chanda, W.; Joseph, T.P.; Padhiar, A.A.; Guo, X.; Min, L.; Wang, W.; Lolokote, S.; Ning, A.; Cao, J.; Huang, M.; et al. Combined effect of linolenic acid and tobramycin on Pseudomonas aeruginosa biofilm formation and quorum sensing. Exp. Ther. Med. 2017, 14, 4328–4338. [Google Scholar] [CrossRef]
- Soni, K.A.; Jesudhasan, P.; Cepeda, M.; Widmer, K.; Jayaprakasha, G.K.; Patil, B.S.; Hume, M.E.; Pillai, S.D. Identification of ground beef–derived fatty acid inhibitors of autoinducer-2-based cell signaling. J. Food Protect. 2008, 71, 134–138. [Google Scholar] [CrossRef]
- DSMZ—German Collection of Microorganisms and Cell Cultures, Catalogue. Available online: https://bacdive.dsmz.de/strain/14448 (accessed on 21 October 2020).
- Chepkirui, C.; Yuyama, K.T.; Wanga, L.; Decock, C.; Matasyoh, J.; Abraham, W.-R.; Stadler, M. Microporenic acids A-G biofilm inhibitors and antimicrobials agents from the basidiomycete Microporus sp. J. Nat. Prod. 2018, 81, 778–784. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef]
- Pažoutová, S.; Follert, S.; Bitzer, J.; Keck, M.; Surup, F.; Šrůtka, P.; Holuša, J.; Stadler, M. A new endophytic insect-associated Daldinia species, recognised from a comparison of secondary metabolite profiles and molecular phylogeny. Fungal Divers. 2013, 60, 107–123. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.I.; Tsiafoulis, C.G.; Gerothanassis, I.P. High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef]
- Vancanneyt, M.; Witt, S.; Abraham, W.-R.; Kersters, K.; Fredrickson, H.L. Fatty acid content in whole-cell hydrolysates and phospholipid fractions of pseudomonads: A taxonomic evaluation. Syst. Appl. Microbiol. 1996, 19, 528–540. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Jogler, M.; Wiegand, S.; Peeters, S.H.; Heuer, A.; Boedeker, C.; Jetten, M.S.M.; Rohde, M.; Jogler, C. Three novel Rubripirellula species isolated from plastic particles submerged in the Baltic Sea and the estuary of the river Warnow in northern Germany. Antonie Leeuwenhoek 2019, in press. [Google Scholar] [CrossRef]
- Desbois, A.P. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef]
- Becker, K.; Pfütze, S.; Kuhnert, E.; Cox, R.; Stadler, M.; Surup, F. Hybridorubrins A–D, novel azaphilone heterodimers from stromata of Hypoxylon fragiforme and insights into the biosynthetic machinery for azaphilone diversification. Chem. Eur. J. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.-R. Going beyond the control of quorum-sensing to combat biofilm infections. Antibiotics 2016, 5, 3. [Google Scholar] [CrossRef]
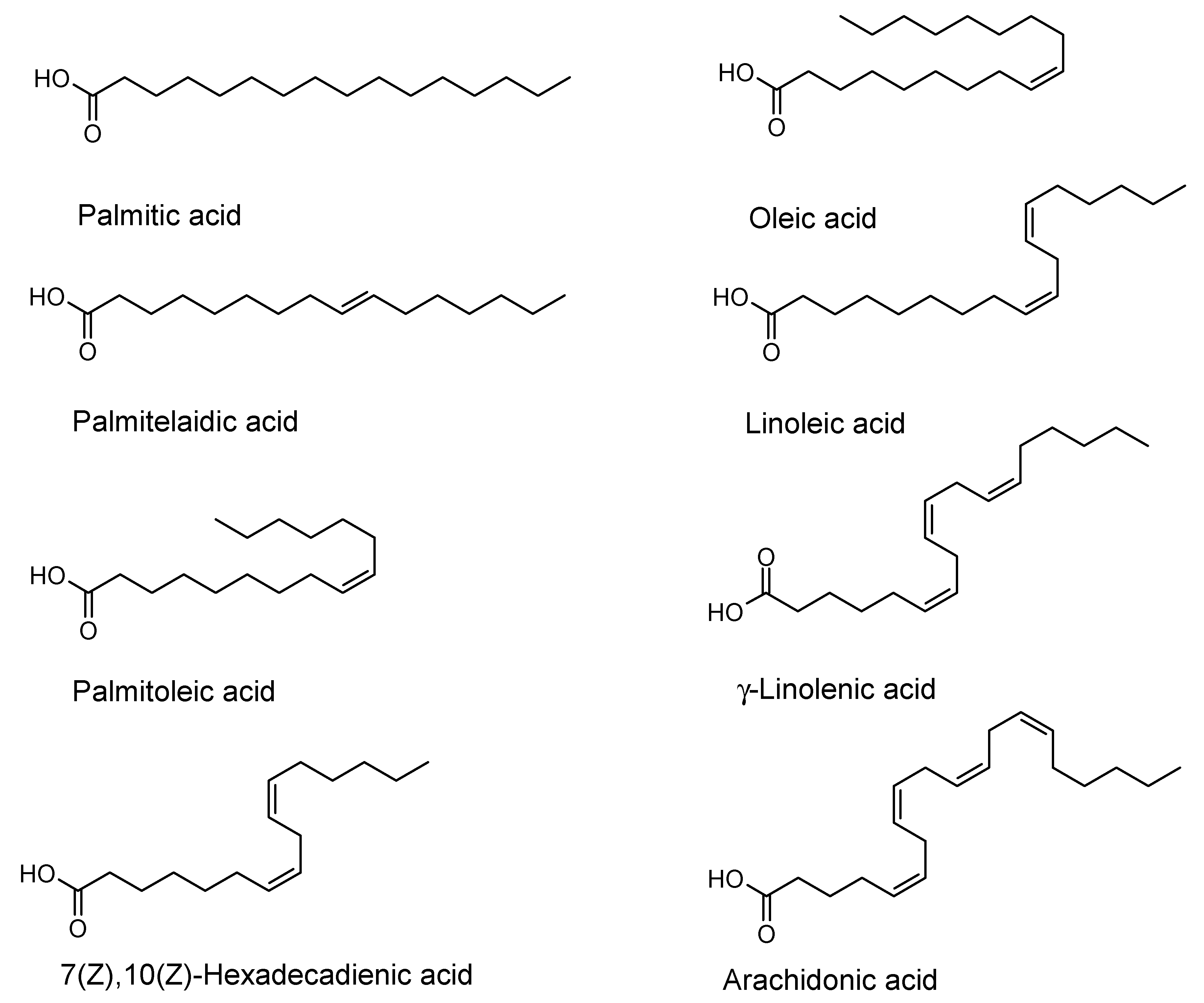
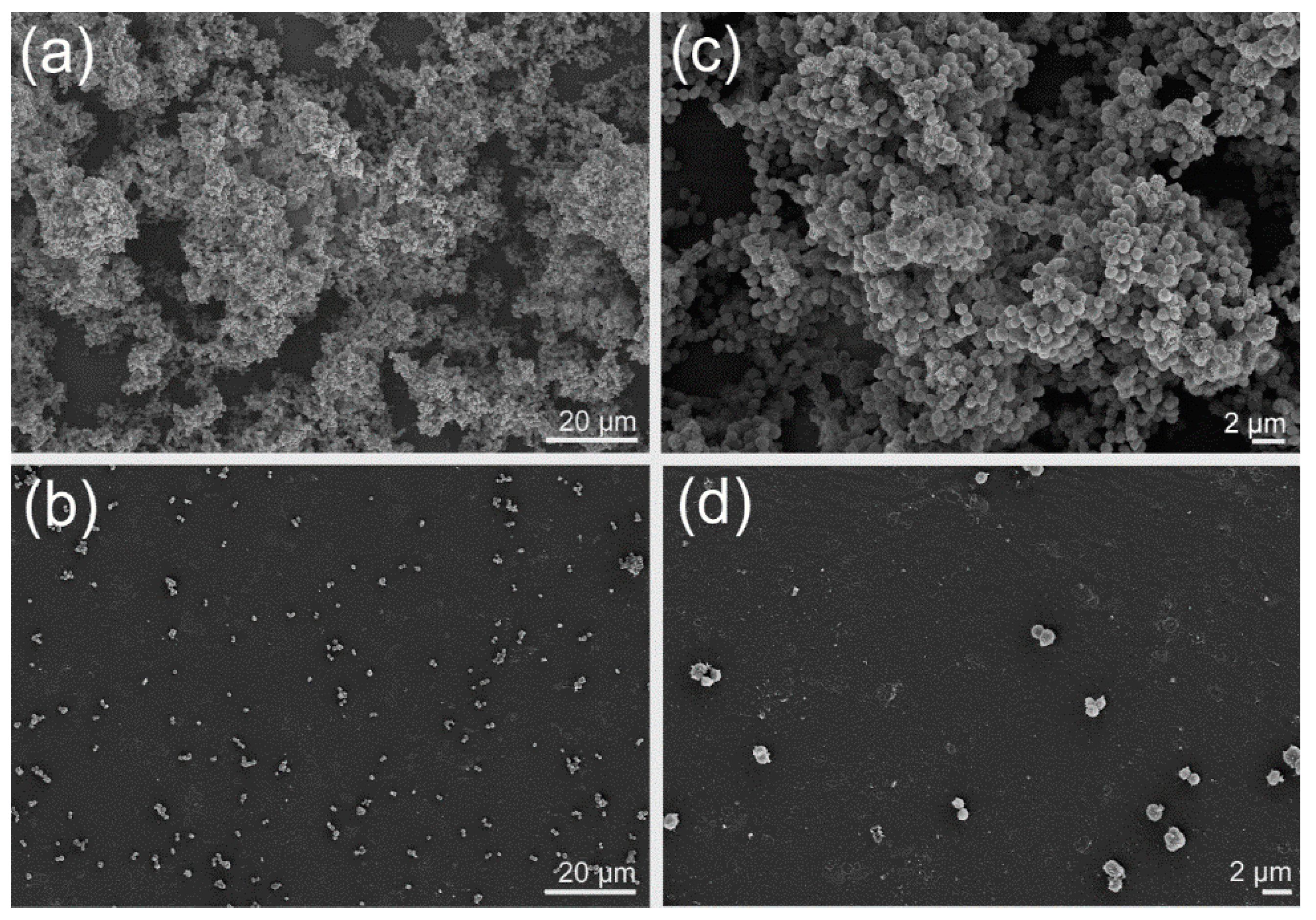
| Fatty Acid | Bacillus cereus | Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | Staphylococcus epidermidis | Streptococcus mutans |
|---|---|---|---|---|---|---|
| Palmitic acid | MIC (>256) - | MIC (>256) - | MIC (>256) - | MIC (>256) - | MIC (>256) - | MIC (>256) - |
| Palmitoleic acid | MIC (>256) - | MIC (>256) - | MIC (>256) - | MIC (>256) 49 ± 2 (128) 54 ± 2 (64) | MIC (>256) - | MIC (>256) - |
| Palmitelai- dic acid | MIC (>256) - | MIC (>256) 25 ± 4 (256) | MIC (>256) - | MIC (>256) 21 ± 4 (16) | MIC (>256) - | MIC (>256) - |
| 7(Z),10(Z)- Hexadeca- dienoic acid | MIC (16) | MIC (>256) 62 ± 8 (128) 47 ± 21 (32) | MIC (>256) - | MIC (32) 91 ± 2 (32) 70 ± 2 (16) 60 ± 9 (8) 52 ± 10 (4) | MIC (16) 22 ± 14 (8) | MIC (>256) 38 ± 12 (128) |
| Linoleic acid | MIC (32) 35 ± 15 (16) | MIC (>256) - | MIC (>256) - | MIC (64) 70 ± 9 (64) 71 ± 4 (32) 54 ± 4 (8) 44 ± 2 (4) | MIC (>256) 16 ± 7 (256) | MIC (>256) - |
| γ-Linolenic acid | MIC (16) | MIC (>256) - | MIC (>256) - | MIC (128) 81 ± 2 (128) 61 ± 6 (64) 58 ± 1 (4) | MIC (>256) 44 ± 19 (128) 27 ± 15 (64) | MIC (>256) - |
| Arachidonic acid | MIC (>256) 36 ± 0.2 (64) | MIC (>256) - | MIC (>256) - | MIC (>256) 31 ± 4 (256) 35 ± 11 (64) | MIC (>256) - | MIC (>256) - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuyama, K.T.; Rohde, M.; Molinari, G.; Stadler, M.; Abraham, W.-R. Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus aureus and Other Gram-Positive Bacteria. Antibiotics 2020, 9, 788. https://doi.org/10.3390/antibiotics9110788
Yuyama KT, Rohde M, Molinari G, Stadler M, Abraham W-R. Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus aureus and Other Gram-Positive Bacteria. Antibiotics. 2020; 9(11):788. https://doi.org/10.3390/antibiotics9110788
Chicago/Turabian StyleYuyama, Kamila Tomoko, Manfred Rohde, Gabriella Molinari, Marc Stadler, and Wolf-Rainer Abraham. 2020. "Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus aureus and Other Gram-Positive Bacteria" Antibiotics 9, no. 11: 788. https://doi.org/10.3390/antibiotics9110788
APA StyleYuyama, K. T., Rohde, M., Molinari, G., Stadler, M., & Abraham, W.-R. (2020). Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus aureus and Other Gram-Positive Bacteria. Antibiotics, 9(11), 788. https://doi.org/10.3390/antibiotics9110788