Abstract
We studied the presence of the mobile colistin resistance gene mcr-1 in Escherichia coli isolates recovered from fecal and urine samples of companion animals, that were collected from South Korea in 2018 and 2019. The mcr-1 gene was detected in one colistin-resistant E. coli isolated from a diarrheic dog. The isolate exhibited additional resistance to multiple antimicrobials, including fluoroquinolones and third-generation cephalosporins. The mcr-1 carrying isolate belonged to ST160. The pulsed-field gel electrophoresis pattern of our strain differed from those ST160 E. coli strains previously identified from chickens in Korea. The mcr-1 gene was identified in the IncI2 plasmid. It was also transferred to E. coli J53 recipient strain, with a conjugation efficiency of 2.8 × 10−4. Average nucleotide identity analysis demonstrated that the mcr-1-carrying plasmid in this study was closely related to those from patients in Korea. To the best of our knowledge, this is the first report of mcr-1 carrying E. coli from a companion animal in South Korea. Our findings support One Health approach is necessary to prevent the dissemination of this high-risk gene.
1. Introduction
Colistin is the last-resort antibiotic against multidrug-resistant Gram-negative bacteria. Since the first report of mcr-1 gene in China in 2016 [1], 10 mcr genes have been described so far i.e., mcr-1 to mcr-10 [2]. The mcr genes code for phosphoethanolamine transferases which catalyze the attachment of phosphoethanolamine to lipopolysaccharides-lipid A in the outer membrane of Gram-negative bacteria. This leads to a reduction of the negative charge of lipopolysaccharides upon structural alteration of lipid A and confers resistance to colistin [3]. Horizontal transfer of the mcr genes contributes to the rapid spread of colistin resistance among Enterobacteriaceae [4].
Escherichia coli plays an important role in colistin resistance because it is the main mcr gene carrier [4] and can be easily transferred among different animal hosts [5]. Plasmid-mediated mobile colistin resistance gene mcr-1 was observed in E. coli from humans [1,6,7], food [1,8] and companion animals [9,10,11] in other countries. In the Republic of Korea (Korea), after our first report of mcr-1-carrying E. coli in livestock [12], the mcr-1 gene was reported in E. coli isolated from humans, food animals, and fresh vegetables [13,14,15]. Despite the close interaction between companion animals and humans, no attempt has been made to identify mcr-1-carrying E. coli from companion animals in Korea to date. Here, we report the first detection of mcr-1 in colistin-resistant E. coli isolated from a dog.
2. Materials and Methods
2.1. Identification of Colistin-Resistant E. coli
E. coli isolates were recovered from fecal (diarrheic and non-diarrheic) and urine samples of dogs (1374) and cats (441) from seven metropolitan cities in Korea in 2018 and 2019 (Table S1). Isolation and identification of E. coli isolates were performed using Eosin methylene blue agar (EMB, Becton Dickinson, Sparks, Baltimore, MD, USA) and MacConkey agar plates (MAC, BD, Spark, Baltimore, MD, USA). Isolates were then confirmed by matrix-assisted laser desorption ionization‒time-of-flight mass spectrometry (MALDI-TOF, Biomerieux, Marcy L’Etoile, France). Only a single isolate per sample was considered for susceptibility study. The minimum inhibitory concentration (MIC) of colistin was determined by the broth microdilution method [16] in KRNV5F Sensititre Panel following the manufacturer’s instruction (Trek Diagnostic Systems, Cleveland, OH, USA). The MIC values were interpreted according to the EUCAST breakpoint (>2 µg/mL) [17]. Additionally, PCR amplification was performed to investigate the mcr-1 gene carriage of isolates demonstrating colistin resistance using primer pairs and PCR conditions described previously [1].
2.2. Conjugation Assay
Conjugation was performed by the filter mating method at 37 °C using azide-resistant E. coli J53 as the recipient strain and colistin-resistant isolates as donor strains. The experiments were conducted in triplicate onto Luria–Bertani agar plate, using a 1:10 donor to recipient ratio. Transconjugant bacteria were selected on Mueller-Hinton agar plates containing sodium azide (100 mg/mL) and colistin (1 μg/mL) [18]. Transfer frequencies were calculated based on the number of transconjugants obtained per donor. The transconjugant was confirmed by PCR detection of the mcr-1 gene as described above.
2.3. Molecular Characterization of mcr-1 Carrying E. coli
Pulsed-field gel electrophoresis (PFGE) of mcr-1 positive isolates was conducted using genomic DNA prepared in agarose blocks, digested with Xbal enzyme (TaKaRa, Shiga, Japan), as described previously [19]. The banding profiles were analyzed using Bionumerics software and the genetic relatedness of the isolates was calculated using the unweighted pair-group method. Additionally, molecular typing of mcr-1 carrying isolates was carried out according to the protocols specified at the E. coli multilocus sequence typing website [20].
2.4. Comparative Analysis of mcr-1 Carrying Plasmids
The mcr-1 carrying plasmid pK19EC149 was sequenced (Pacific Biosciences, Menlo Park, CA, USA; http://pacificbiosciences.com/) (GenBank accession number. CP050290) and compared with those of previously reported strains (Table 1). Briefly, nucleotide sequences of mcr-1 carrying plasmids were downloaded from the GenBank nucleotide database. As the sequences have different starting points, sequences were rotated for accurate alignment so that start sites of sequences were set as RepA using GAMOLA2 [21]. Average Nucleotide Identity (ANI) values were calculated with pairwise genome alignment of sequences by using the ANI-blast method implemented in PYANI (v.0.2.9) [22] and the phylogenetic tree is reconstructed based on the ANI values. The sequence of each plasmid was aligned using Blastn (v 2.8.1) [23] and compared using EasyFig (v.2.2.3) [24].

Table 1.
Lists of plasmids identified in isolates recovered from humans, dogs, and food animals.
3. Results and Discussion
Overall, 1202 E. coli isolates (288 cat and 914 dog isolates) were identified from fecal and urine samples of dogs and cats. Susceptibility testing demonstrated two colistin-resistant isolates from diarrheic dogs in Seoul city (MIC = 8 µg/mL). PCR and sequencing analysis identified the mcr-1 gene in one of the colistin-resistant isolates. The mcr-1 prevalence in E. coli isolates in this study was lower than those previously reported in E. coli recovered from companion animals in Argentina (1.9%) [25], Beijing, China (2.3%) [27], and Guangzhou, China (6.25%) [28]. However, it was almost similar to our previously described mcr-1 detection in food animals in Korea [12]. This is the first report of mcr-1-carrying E. coli from a dog in Korea. Considering the close relationship between humans and dogs, the observation of mcr-1-carrying E. coli in dog feces is a potential risk to public health.
The mcr-1-carrying strain belonged to ST162 and commensal subgroup B1, which concurred with our previous finding in chickens [12]. However, the PFGE pattern of our mcr-1-carrying ST162 E. coli strain differed from those of chicken strains (Figure 1) [12,15]. Zhang et al. [29] identified ST162 E. coli harboring multiple antibiotic-resistant genes, including mcr-1 from a dog in China. ST162 E. coli that belonged to subgroup B1 possessed high virulence and was linked with extraintestinal pathogenic E. coli-associated human infections [30]. The mcr-1-carrying E. coli exhibited additional resistance to multiple antimicrobials, including fluoroquinolones and third-generation cephalosporins (Figure 1). While, the other colistin-resistant isolate (mcr-1-negative isolate) was susceptible to all of the tested antimicrobials including aminoglycosides, cephalosporins, and fluoroquinolones. The broth mating assay indicated that the strain was capable of transferring its gene to the recipient E. coli J53 strain. Indeed, the conjugation efficiency of our isolate (2.8 × 10−4) was at least ten times higher than those of previous isolates from humans in Korea [15]. Nonetheless, resistance determinants other than colistin were not observed in the transconjugant.

Figure 1.
Xbal-digested pulsed-field gel electrophoresis patterns of mcr-1-carrying E. coli strains isolated from a diarrheic dog and chickens in Korea. AMP, ampicillin; CPD, cefpodoxime; CEF, cephalothin; CHL; chloramphenicol; CIP, ciprofloxacin; COL, colistin; CZO, cefazolin; DOX, doxycycline; ENR, enrofloxacin; FIS, sulfisoxazole; FOV, cefovecin; GEN, gentamicin; MAR, marbofloxacin; NAL, nalidixic acid; STR, streptomycin; SXT, trimethoprim/sulfamethoxazole; TET, tetracycline; TIC, ticarcillin; XNL, ceftiofur.
According to the 95% ANI threshold, the mcr-1-carrying plasmid pK19EC149 from this study was closely related to those from patients in Korea (GenBank accession no. KY657476 and KY657478) (Figure 2). Additionally, the mcr-1 carrying plasmid IncI2 pK19EC149 (60,864 bp) had a similar size and highly conserved backbone (>96%) to other IncI2 plasmids detected in E. coli strains from humans (KY657476 and KY657478) and chickens (KY471144, KY471145) in Korea (Figure 3). The findings indicate that the plasmids might have evolved from a single ancestor, or one might have evolved from the other. The IncI2 plasmids, which have a broad host range, are commonly associated with the acquisition and dissemination of new antibiotic-resistant genes. As well, they are known to adapt to new bacterial hosts [31,32].
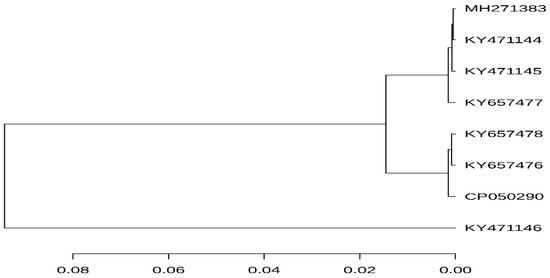
Figure 2.
ANI analysis was performed using the ANI-blast method implemented in PYANI (v.0.2.9) and the tree was generated based on the ANI values. The horizontal lines represent the 95% threshold value. The scale bar represents sequence divergence. Detailed information on the plasmids source is summarized in Table 1.
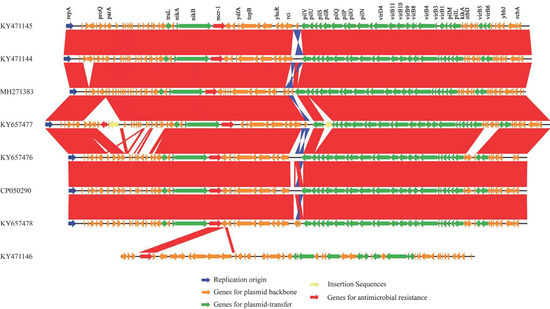
Figure 3.
Comparative analyses of the mcr-1-carrying plasmids from E. coli strains isolated from food animals, humans, and a dog. The sequence of each plasmid (Table 1) was aligned using Blastn (v 2.8.1) and compared using EasyFig (v.2.2.3). Highly conserved regions (>96% identity value) with normal alignment are indicated in red, and regions with inverted alignment are indicated in blue.
Colistin is not commonly used to treat companion animals in Korea; however, it is widely used in the livestock industry. Additionally, mcr-1 carrying E. coli was mainly identified in food animals in the country [12,14]. Thus, food animals are considered responsible for spreading the mcr-1 gene. Nonetheless, we isolated the mcr-1 carrying E. coli from a dog in the urban areas of the Seoul metropolitan. This implies that the dog had minimal or no contact with food animals. Considering the close and frequent contact between humans and companion animals, our results might suggest that mcr-1-carrying E. coli could be transferred between dogs and humans. Consistent with this study, the transmission of mcr-carrying E. coli between humans and companion animals, especially dogs, was reported in China [9,10]. Additionally, E. coli harboring the mcr-1 gene has been frequently identified from environmental samples [33,34,35]. Guenther et al. [36] showed a clear link between mcr-1-carrying E. coli isolated from the environment and those from humans and dogs. Therefore, the mcr-1-carrying E. coli in this study might also originate from the natural environment.
In conclusion, dogs can serve as a reservoir of mcr-1 carrying E. coli, adding another layer of intricacy to the rapidly evolving epidemiology of plasmid-mediated colistin resistance in the community. Thus, a “One Health” based strategy and a detailed knowledge of antimicrobial usage in humans and companion animals are needed to reduce the dissemination of colistin-resistant E. coli.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/9/11/768/s1. Table S1: The number of fecal samples, urine samples, and E. coli isolates collected from dogs and cats in 2018 and 2019 in Korea.
Author Contributions
Conceptualization, S.-K.L., and D.C.M.; methodology, H.Y.K., A.F.M., and D.C.M.; software, H.Y.K., J.-H.C., and S.-J.K.; validation, A.F.M., M.H.K., and J.-H.C.; formal analysis, H.-J.S., S.-S.Y., and M.H.K.; investigation, D.M.C., A.F.M., H.Y.K., H.-J.S., M.H.K., J.-H.C., and S.-J.K.; data curation, M.D.C., S.-S.Y., and M.H.K.; writing—original draft preparation, D.C.M., and A.F.M.; writing—review and editing, S.-S.Y., and S.-K.L.; supervision, S.-S.Y., and S.-K.L.; project administration, S.-S.Y., and H.Y.K.; funding acquisition; S.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded from the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food, and Rural Affairs, Korea, grant number B-1543081-2019-20-03.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Wang, C.; Feng, Y.; Sokerya, S.; Liu, L.; Wei, L.; Kang, M.; Zhong, Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes. Infect. 2020, 9, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, H.; Liu, Y.H.; Feng, Y. Towards understanding mcr-like colistin resistance. Trends Microbiol. 2018, 26, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Hu, Y.; Li, Z.; Sun, J.; Wang, Q.; Lin, J.; Ye, H.; Liu, F.; Srinivas, S.; Li, D.; et al. Dissemination and mechanism for the mcr-1 colistin resistance. PLoS Pathog. 2016, 12, e1005957. [Google Scholar] [CrossRef]
- Poirel, L.; Nordmann, P. Emerging plasmid-encoded colistin resistance: The animal world as the culprit? J. Antimicrob. Chemother. 2016, 8, 2626–2627. [Google Scholar] [CrossRef] [PubMed]
- Doumith, M.; Godbole, G.; Ashton, P.; Larkin, L.; Dallman, T.; Day, M.; Day, M.; Muller-Peboy, B.; Ellington, M.J.; de Pinna, E.; et al. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J. Antimicrob. Chemother. 2016, 71, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Stoesser, N.; Mathers, A.J.; Moore, C.E.; Nicholas, P.J.D.; Crook, D.W. Colistin resistance gene mcr-1 and pHNSHP45 plasmid in human isolates of Escherichia coli and Klebsiella pneumoniae. Lancet Infect. Dis. 2016, 16, 285–286. [Google Scholar] [CrossRef]
- Anjum, M.F.; Duggett, N.A.; AbuOun, M.; Randall, L.; Nunez-Gracia, J.; Ellis, R.J.; Rogers, J.; Horton, R.; Brena, C.; Williamson, S.; et al. Colistin resistance in Salmonella and Escherichia coli isolates from a pig farm in Great Britain. J. Antimicrob. Chemother. 2016, 71, 2306–2313. [Google Scholar] [CrossRef]
- Zhang, X.F.; Doi, Y.; Huang, X.; Li, H.Y.; Zhong, L.L.; Zeng, K.J.; Zhang, Y.F.; Patil, S.; Tian, G.B. Possible transmission of mcr-1-harboring Escherichia coli between companion animals and human. Emerg. Infect. Dis. 2016, 22, 1679–1681. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Y.; Schwarz, S.; Walsh, T.R.; Ou, Y.; Wu, Y.; Li, M.; Shen, Z. mcr-1 in Enterobacteriaceae from companion animals, Beijing, China, 2012–2016. Emerg. Infect. Dis. 2017, 23, 710–711. [Google Scholar] [CrossRef]
- Ortega-Paredes, D.; Haro, M.; Leoro-Garzón, P.; Barba, P.; Loaiza, K.; Mora, F.; Fors, M.; Vinueza-Burgos, C.; Fernández-Moreira, E. Multidrug-resistant Escherichia coli isolated from canine feces in a public park in Quito, Ecuador. J. Glob. Antimicrob. Resist. 2019, 18, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Kang, H.Y.; Lee, K.; Moon, D.C.; Lee, H.S.; Jung, S.C. First detection of the mcr-1 gene in Escherichia coli isolated from livestock between 2013 and 2015 in South Korea. Antimicrob. Agents Chemother. 2016, 60, 6991–6993. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belaynehe, K.M.; Shin, S.W.; Park, K.Y.; Jang, J.Y.; Won, H.G.; Yoon, I.J.; Yoo, H.S. Emergence of mcr-1 and mcr-3 variants coding for plasmid-mediated colistin resistance in Escherichia coli isolates from food-producing animals in South Korea. Int. J. Infect. Dis. 2018, 72, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.S.; Song, J.; Kim, J.; Shin, J. Increasing prevalence of multidrug-resistant mcr-1-positive Escherichia coli isolates from fresh vegetables and healthy food animals in South Korea. Int. J. Infect. Dis. 2020, 92, 53–55. [Google Scholar] [CrossRef]
- Yoon, E.J.; Hong, J.S.; Yang, J.W.; Lee, K.J.; Lee, H.; Jeong, S.H. Detection of mcr-1 plasmids in Enterobacteriaceae isolates from human specimens: Comparisons with those of human isolates from livestock in Korea. Ann. Lab. Med. 2018, 38, 555–562. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement; CLSI Document M100: Wayne, PA, USA, 2018. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. EUCAST, Version 8.1. 2018. Available online: http://www.eucast.org (accessed on 17 July 2020).
- Tendon, V.D.; Poirel, L.; Nordman, P. Transferability of the mcr-1 colistin resistance gene. Antimicrob. Resist. Infect. Control. 2017, 23, 813–815. [Google Scholar]
- Gautom, R.K. Rapid pulse-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in day 1. J. Clin. Microbiol. 1997, 35, 2977–2980. [Google Scholar] [CrossRef]
- Escherichia coli MLST Database. Available online: http://enterobase.warwick.ac.uk/species/ecoli/allele_st_search (accessed on 15 August 2020).
- Altermann, E.; Lu, J.; McCulloch, A. GAMOLA2, a comprehensive software package for the annotation and curation of draft and complete microbial genomes. Front. Microbiol. 2017, 8, 346. [Google Scholar] [CrossRef][Green Version]
- Pritchard, L.; Glover, R.H.; Humphris, S.; Elphinstone, J.G.; Toth, I.K. Genomics and taxonomy in diagnostics for food security: Soft-rotting enterobacterial plant pathogens. Anal. Methods. 2016, 8, 12–24. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopolus, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Rumi, M.V.; Mas, J.; Elena, A.; Cerdeira, L.; Muñoz, M.E.; Lincopan, N.; Gentilini, É.R.; di Conza, J.; Gutkind, G. Co-occurrence of clinically relevant β-lactamases and mcr-1 encoding genes in Escherichia coli from companion animals in Argentina. Vet. Microbiol. 2019, 230, 228–234. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lim, S.K.; Moon, Y.C.; Shin, J.; Ko, K.S. Whole sequences and characteristics of mcr-1- harboring plasmids of Escherichia coli strains isolated from livestock in South Korea. Microb. Drug. Resist. 2018, 24, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.; Zhang, Y.; Zhang, Z.; Lei, L.; Xia, Z. Increasing prevalence of ESBL-producing multidrug resistance Escherichia coli from diseased pets in Beijing, China From 2012 to 2017. Front. Microbiol. 2019, 10, 2852. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.Y.; Xia, Y.B., Guo; Guo, Z.W.; Ma, Z.B.; Yi, M.Y.; Lv, L.C.; Lu, P.L.; Yan, J.C.; Huang, J.W.; et al. Clonal spread of Escherichia coli ST93 carrying mcr-1-harboring IncN1-IncHI2/ST3 plasmid among companion animals, China. Front. Microbiol. 2018, 9, 2989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, J.; Bai, X.; Ma, J.; Xiong, Y.; Fanning, S.; Bai, L.; Yang, Z. Characterization of five Escherichia coli isolates co-expressing ESBL and mcr-1 resistance mechanisms from different origins in China. Front. Microbiol. 2019, 10, 1994. [Google Scholar] [CrossRef]
- Zhuge, X.; Jiang, M.; Tang, F.; Sun, Y.; Ji, Y.; Xue, F.; Ren, J.; Zhu, W.; Dai, J. Avian-source mcr-1-positive Escherichia coli is phylogenetically diverse and shares virulence characteristics with E. coli causing human extra-intestinal infections. Vet. Microbiol. 2019, 239, 108483. [Google Scholar] [CrossRef]
- Wong, M.H.; Liu, L.; Yan, M.; Chan, E.W.; Chen, S. Dissemination of IncI2 plasmids that harbor the blaCTX-M element among clinical Salmonella isolates. Antimicrob. Agents Chemother. 2015, 59, 5026. [Google Scholar] [CrossRef]
- Lv, L.; Partridge, S.R.; He, L.; Zeng, Z.; He, D.; Ye, J.; Liu, J.H. Genetic characterization of IncI2 plasmids carrying blaCTX-M-55 spreading in both pets and food animals in China. Antimicrob. Agents Chemother. 2013, 57, 2824–2827. [Google Scholar] [CrossRef]
- Han, H.; Liu, W.; Cui, X.; Cheng, X.; Jiang, X. Co-Existence of mcr-1 and blaNDM-5 in an Escherichia coli strain isolated from the pharmaceutical industry, WWTP. Infect. Drug Resist. 2020, 13, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Rahman, Z.; Monira, S.; Rahman, M.A.; Camilli, A.; George, C.M.; Ahmed, N.; Alam, M. Colistin-resistant Escherichia coli carrying mcr-1 in urban sludge samples: Dhaka, Bangladesh. Gut Pathog. 2017, 9, 77. [Google Scholar] [CrossRef]
- Sun, P.; Bi, Z.; Nilsson, M.; Zheng, B.; Berglund, B.; Lundborg, C.S.; Borjesson, S.; Li, X.; Chen, B.; Yin, H.; et al. Occurrence and genomic characterization of ESBL-producing, mcr-1-harboring Escherichia coli in farming soil. Antimicrob. Agents Chemother. 2017, 61, e02569-16. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Falgenhauer, L.; Semmler, T.; Imirzalioglu, C.; Chakraborty, T.; Roesler, U.; Roschanski, N. Environmental emission of multiresistant Escherichia coli carrying the colistin resistance gene mcr-1 from German swine farms. J. Antimicrob. Chemother. 2017, 72, 1289–1292. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).