Clinical Significance of Manuka and Medical-Grade Honey for Antibiotic-Resistant Infections: A Systematic Review
Abstract
1. Introduction
2. Results
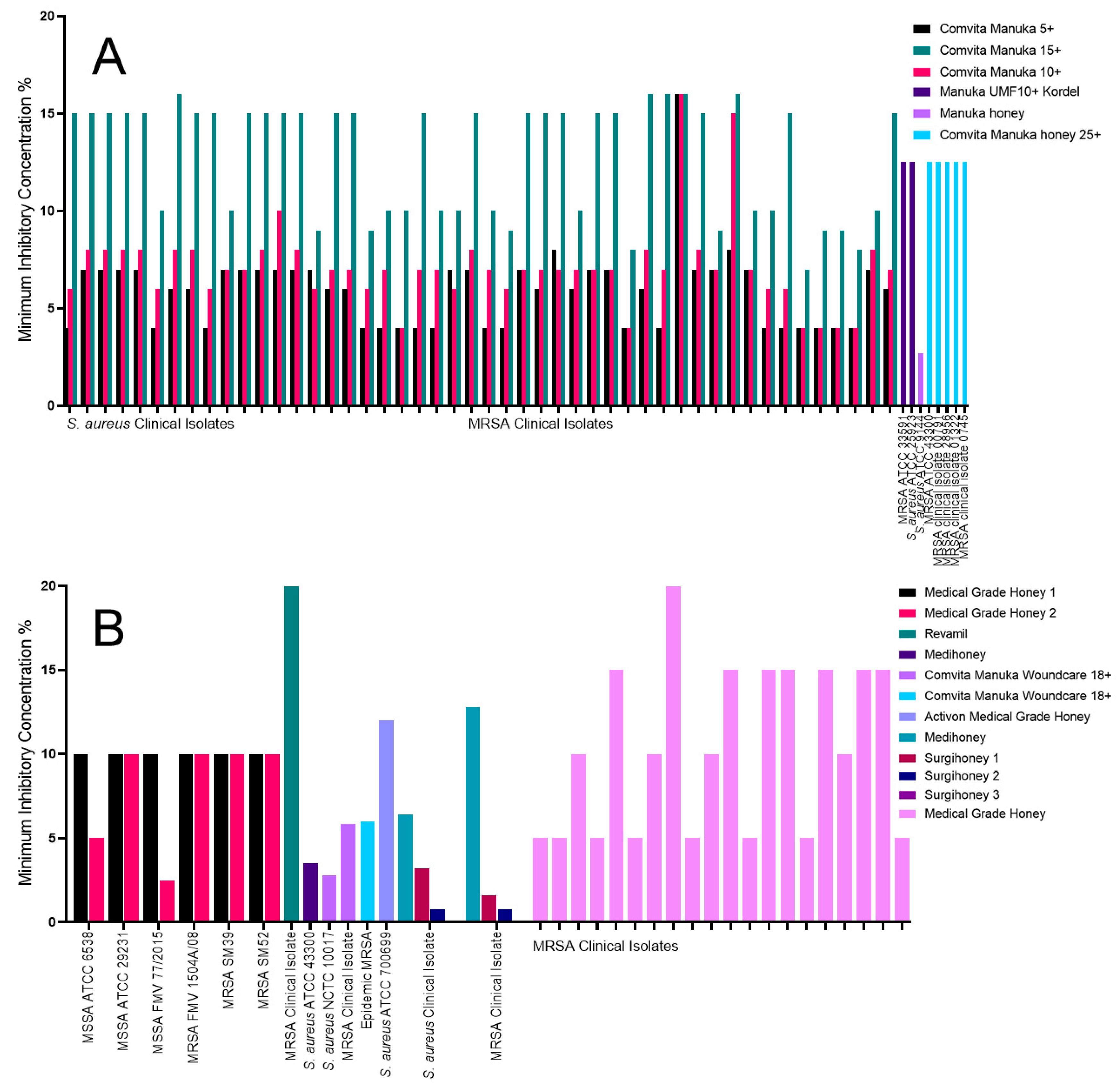
2.1. The Impact of Honey on Staphylococcus aureus
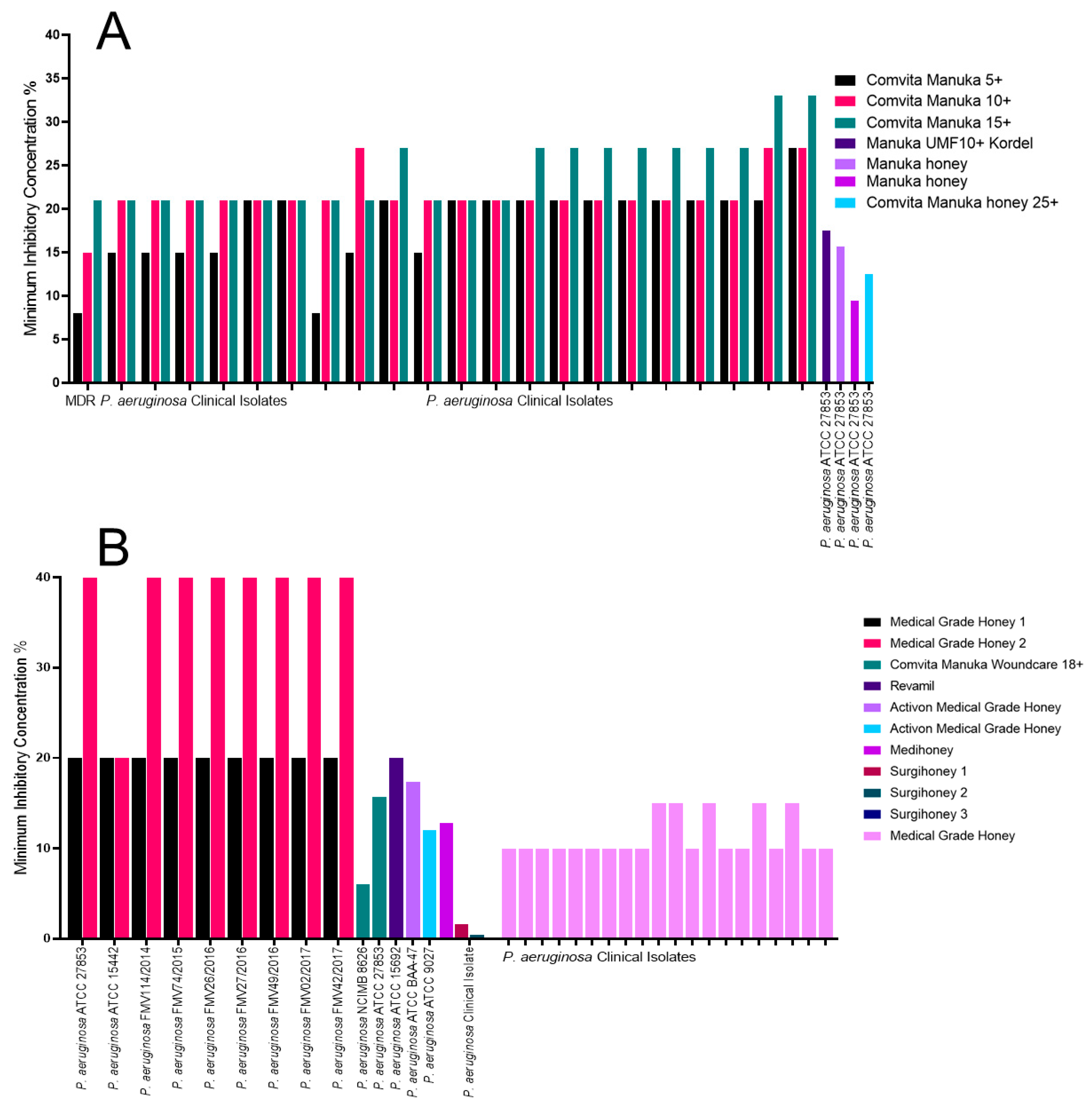
2.2. The Impact of Honey on Pseudomonas aeruginosa
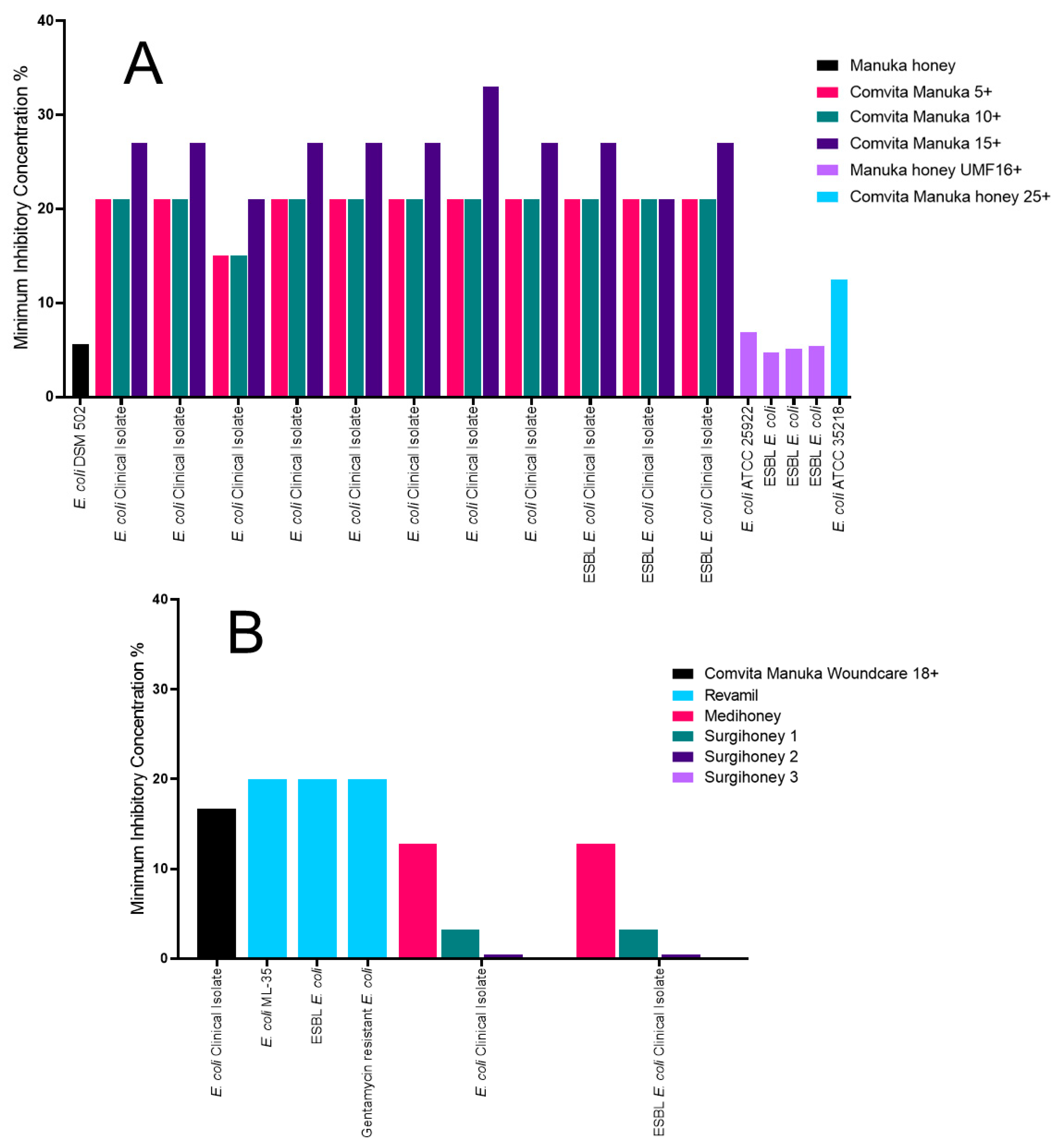
2.3. The Impact of Honey on Escherichia coli
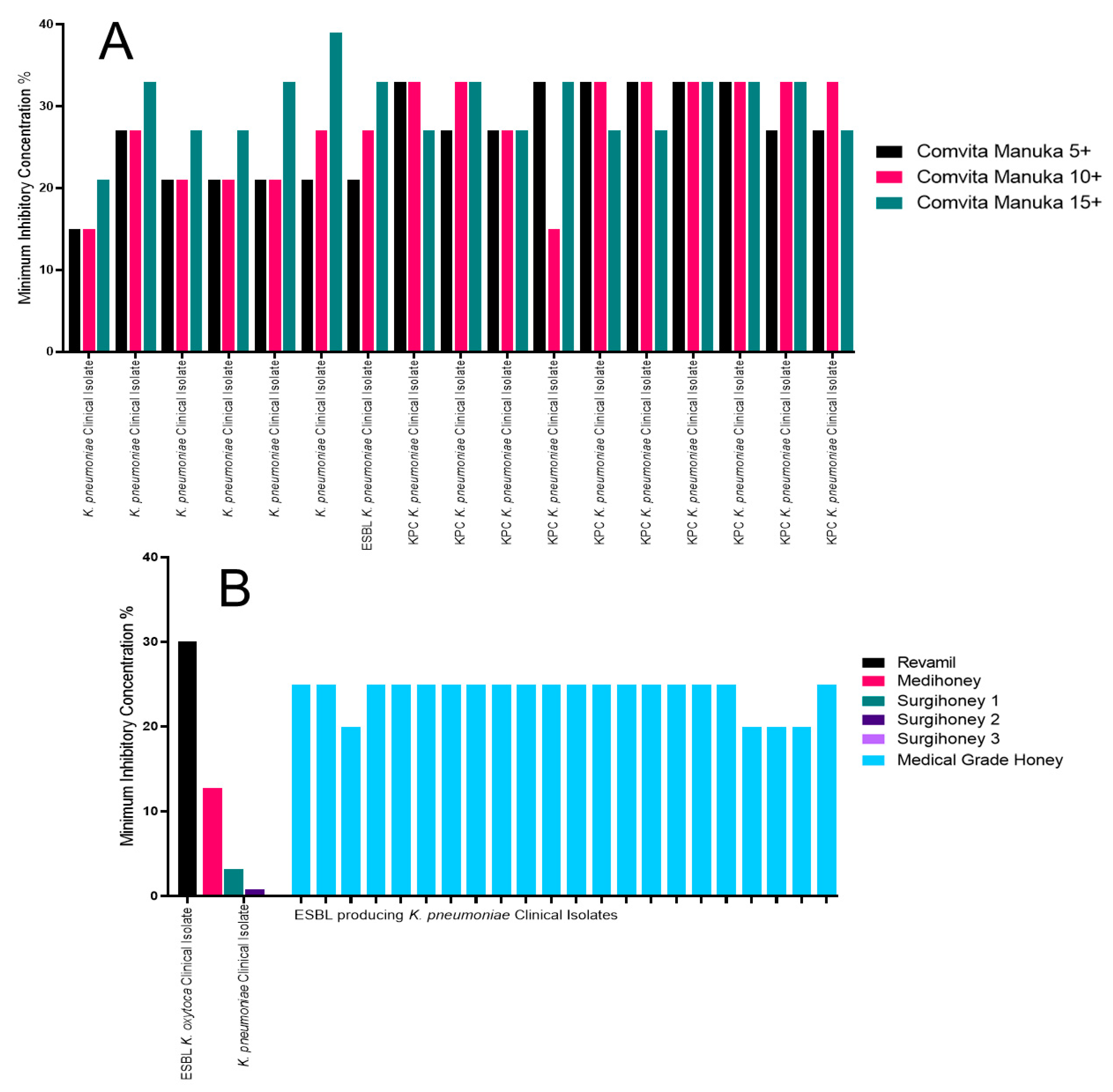
2.4. The Impact of Honey on Klebsiella pneumoniae
2.5. The Impact of Honey on a Variety of Staphylococcus spp.
2.6. The Impact of Honey on a Variety of Other Enterobacteriaceae
2.7. The Impact of Honey on all Other Organisms Identified
2.8. Bacterial Pathogen Overall Susceptibility
2.9. Bacterial Resistance Does Not Impact the Efficacy of Honey
3. Discussion
3.1. Manuka Honey
3.2. Medical-Grade Honey
3.3. Clinical Application of Honey
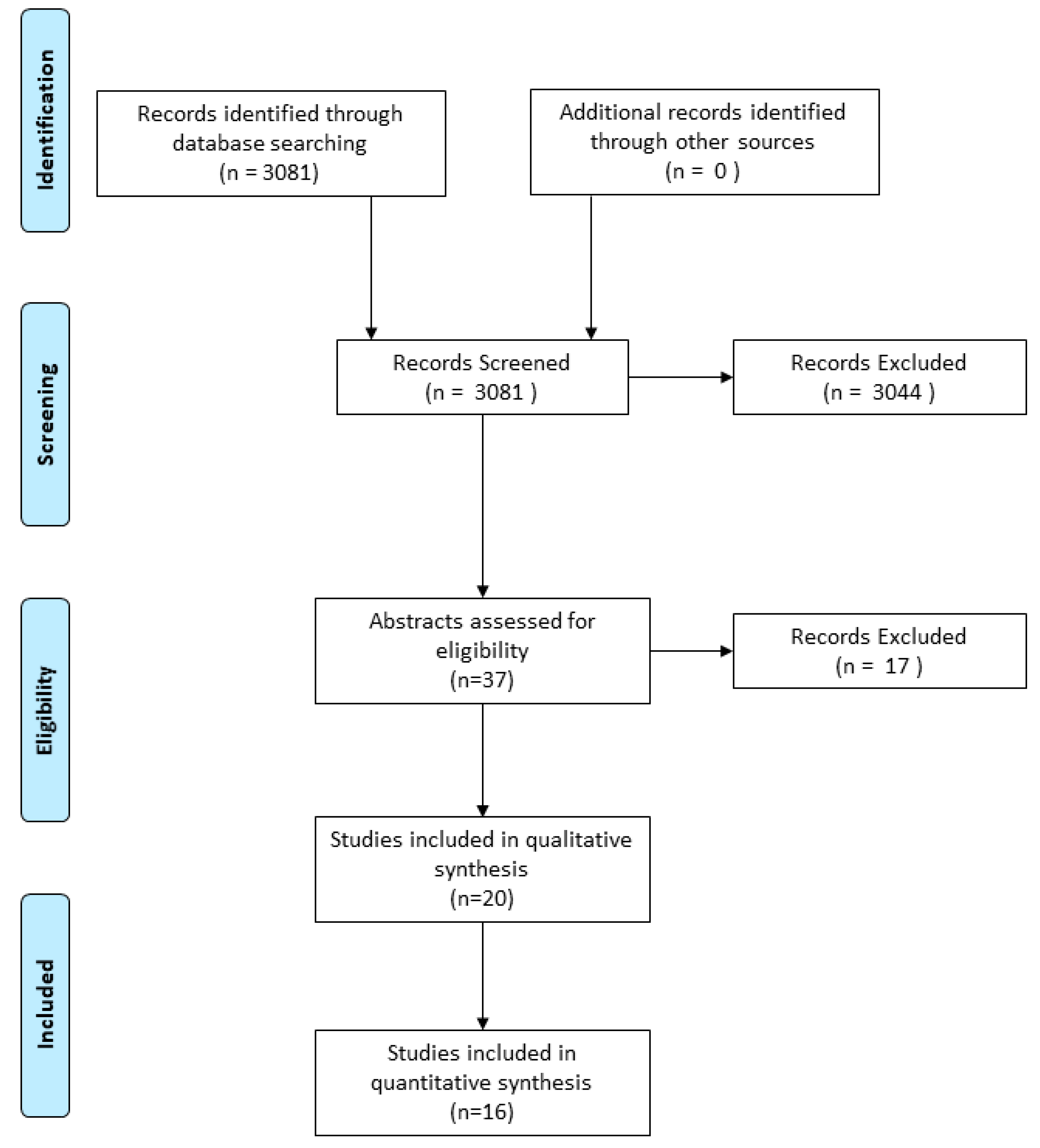
4. Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacertial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the antimicrobial composition of honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and % moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef]
- John-isa, J.F.; Adebolu, T.T.; Oyetayo, V.O. Antibacterial Effects of Honey in Nigeria on Selected Diarrhoeagenic Bacteria. South Asian J. Res. Microbiol. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Alvarez-suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Isla, M.I.; Craig, A.; Ordoñez, R.; Zampini, C.; Sayago, J.; Bedascarrasbure, E.; Alvarez, A.; Salomón, V.; Maldonado, L. Physico chemical and bioactive properties of honeys from Northwestern Argentina. LWT Food Sci. Technol. 2011, 44, 1922–1930. [Google Scholar] [CrossRef]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; Mcdougall, G.J. Compositional analysis of Scottish honeys with antimicrobial activity against antibiotic-resistant bacteria reveals novel antimicrobial components. LWT Food Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Escuredo, O.; Silva, L.R.; Valentão, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012, 130, 671–678. [Google Scholar] [CrossRef]
- Matzen, R.D.; Leth-Espensen, J.Z.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The Antibacterial Effect in Vitro of Honey Derived from Various Danish Flora. Dermatol. Res. Pract. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef]
- Hegazi, A.G. Antimicrobial Activity of Different Egyptian H.pdf. Res. J. Microbiol. 2011, 6, 488–495. [Google Scholar]
- Laallam, H.; Boughediri, L.; Bissati, S.; Menasria, T.; Mouzaoui, M.S.; Hadjadj, S.; Hammoudi, R.; Chenchouni, H. Modeling the synergistic antibacterial effects of honey characteristics of different botanical origins from the Sahara Desert of Algeria. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Johnston, M.; Mcbride, M.; Dahiya, D.; Owusu-apenten, R. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Cokcetin, N.N.; Pappalardo, M.; Campbell, L.T.; Brooks, P.; Carter, D.A.; Blair, S.E.; Harry, E.J. The antibacterial activity of Australian Leptospermum honey correlates with methylglyoxal levels. PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Grainger, M.N.C.; Manley-harris, M.; Lane, J.R.; Field, R.J. Kinetics of the conversion of dihydroxyacetone to methylglyoxal in New Zealand mā nuka honey: Part II–Model systems. Food Chem. 2016, 202, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C.; Betts, J.A. Clinical usage of honey as a wound dressing: An update. J. Wound Care 2004, 13, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The Effect of New Zealand Kanuka, Manuka and Clover Honeys on Bacterial Growth Dynamics and Cellular Morphology Varies According to the Species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef] [PubMed]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Resistance of gram-positive bacteria to current antibacterial agents and overcoming approaches. Molecules 2020, 25, 2888. [Google Scholar] [CrossRef]
- O’Neil, J. Review on Antibiotic resisitance. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Heal. Wealth Nations 2014, 1–16. [Google Scholar] [CrossRef]
- Datta, R.; Juthani-Mehta, M. Burden and management of multidrug-resistant organisms in palliative care. Palliat. Care 2017, 10, 10. [Google Scholar] [CrossRef]
- Dunford, C.; Cooper, R.; Molan, P.; White, R. The use of honey in wound treatment. Nurs. Stand. 2000, 15, 63–68. [Google Scholar] [CrossRef]
- Mashhood, A.A.; Khan, T.A.; Sami, A.N. Honey compared with 1% silver sulfadiazine cream in the treatment of superficial and partial thickness burns. J. Pak. Assoc. Dermatol. 2006, 16, 14–19. [Google Scholar]
- Subrahmanyam, M. Honey Dressing Accelerates Split-Thickness Skin Graft Donor Site Healing. Indian J. Surg. 2015, 77, 261–263. [Google Scholar] [CrossRef]
- Girma, A.; Seo, W.; She, R.C. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS ONE 2019, 14, e0224495. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.T.; Rahman, R.A.; Gan, S.H.; Halim, A.S.; Hassan, S.A.; Sulaiman, S.A.; Singh, K.-K.B. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement. Altern. Med. 2009, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Willix, D.J.; Molan, P.C.; Harfoot, C.G. A comparison of the sensitivity of wound-infecting species of bacteria to the antibacterial activity of manuka honey and other honey. J. Appl. Bacteriol. 1992, 73, 388–394. [Google Scholar] [CrossRef]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 10, 47. [Google Scholar] [CrossRef]
- Lin, S.M.; Molan, P.C.; Cursons, R.T. The controlled in vitro susceptibility of gastrointestinal pathogens to the antibacterial effect of manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 569–574. [Google Scholar] [CrossRef]
- Cremers, N.; Belas, A.; Santos Costa, S.; Couto, I.; de Rooster, H.; Pomba, C. In vitro antimicrobial efficacy of two medical grade honey formulations against common high-risk meticillin-resistant staphylococci and Pseudomonas spp. pathogens. Vet. Dermatol. 2020, 31, 90–96. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Van den Akker, J.P.C.; Güçlü, A.; Aslami, H.; Binnekade, J.M.; de Boer, L.; Boszhard, L.; Paulus, F.; Middelhoek, P.; te Velde, A.A.; et al. Medical-Grade Honey Kills Antibiotic-Resistant Bacteria In Vitro and Eradicates Skin Colonization. Clin. Infect. Dis. 2008, 46, 1677–1682. [Google Scholar] [CrossRef]
- Dryden, M.; Lockyer, G.; Saeed, K.; Cooke, J. Engineered honey: In vitro antimicrobial activity of a novel topical wound care treatment. J. Glob. Antimicrob. Resist. 2014, 2, 168–172. [Google Scholar] [CrossRef]
- Tirado, D.J.; Hudson, N.R.; Maldonado, C.J. Efficacy of medical grade honey against multidrug-resistant organisms of operational significance: Part i. J. Trauma Acute Care Surg. 2014, 77, 204–207. [Google Scholar] [CrossRef]
- Jenkins, R.; Cooper, R. Improving Antibiotic Activity against Wound Pathogens with Manuka Honey In Vitro. PLoS ONE 2012, 7, e45600. [Google Scholar] [CrossRef]
- Cooper, R.A.; Jenkins, L.; Henriques, A.F.M.; Duggan, R.S.; Burton, N.F. Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.N.; Donkor, E.S. Antibacterial effect of Manuka honey on Clostridium difficile. BMC Res. Notes 2013, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.L.; Maddocks, S.E.; Cooper, R.A. Manuka honey is bactericidal against Pseudomonas aeruginosa and results in differential expression of oprF and algD. Microbiology 2012, 158, 3005–3013. [Google Scholar] [CrossRef]
- Sindi, A.; Chawn, M.V.B.; Hernandez, M.E.; Green, K.; Islam, M.K.; Locher, C.; Hammer, K. Anti-biofilm effects and characterisation of the hydrogen peroxide activity of a range of Western Australian honeys compared to Manuka and multifloral honeys. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Glasser, J.S.; Guymon, C.H.; Mende, K.; Wolf, S.E.; Hospenthal, D.R.; Murray, C.K. Activity of topical antimicrobial agents against multidrug-resistant bacteria recovered from burn patients. Burns 2010, 36, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.A.; Blair, S.E.; Cokcetin, N.N.; Bouzo, D.; Brooks, P.; Schothauer, R.; Harry, E.J. Therapeutic manuka honey: No longer so alternative. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Cooke, J.; Dryden, M.; Patton, T.; Brennan, J.; Barrett, J. The antimicrobial activity of prototype modified honeys that generate reactive oxygen species (ROS) hydrogen peroxide. BMC Res. Notes 2015, 8, 4–8. [Google Scholar] [CrossRef]
- Mohd Zohdi, R.; Abu Bakar Zakaria, Z.; Yusof, N.; Mohamed Mustapha, N.; Abdullah, M.N.H. Gelam (Melaleuca spp.) honey-based hydrogel as burn wound dressing. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a topical treatment for wounds (Review). Cochrane Libr. 2015, 6, CD005083. [Google Scholar] [CrossRef]
- Molan, P.C. Re-introducing honey in the management of wounds and ulcers-theory and practice. Ostomy Wound Manage. 2002, 48, 28–40. [Google Scholar] [PubMed]
- Molan, P.C. The evidence supporting the use of honey as a wound dressing. Int. J. Low. Extrem. Wounds 2006, 5, 40–54. [Google Scholar] [CrossRef]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How methylgyloxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The intracellular effects of manuka honey on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Saranraj, P.; Sivasakthi, S. Comprehensive Review on Honey: Biochemical and Medicinal Properties. J. Acad. Ind. Res. 2018, 6, 165–181. [Google Scholar]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Abubaker, K.; Miotto, D. Unraveling a mechanism of honey antibacterial action: Polyphenol/H2O2-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 2012, 133, 329–336. [Google Scholar] [CrossRef]
- McLoone, P.; Warnock, M.; Fyfe, L. Honey: A realistic antimicrobial for disorders of the skin. J. Microbiol. Immunol. Infect. 2016, 49, 161–167. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; Harakeh, S. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi J. Biol. Sci. 2017, 24, 1255–1261. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Velde, A.A.; Boer, L.; Speijer, D.; Vandenbrouke-Grauls, C.M.J.E.; Zaat, S.A.J. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef]
- Halstead, F.D.; Webber, M.A.; Oppenheim, B.A. Use of an engineered honey to eradicate preformed biofilms of important wound pathogens: An in vitro study. J. Wound Care 2017, 26, 442–450. [Google Scholar] [CrossRef] [PubMed]
- NHS. Medical Honey Simplified. A Patient Guide to the Role of Honey in Wound Management; NHS: Oxford, UK, 2015. [Google Scholar]
- Landis, S.; Ryan, S.; Woo, K. Infections in chronic wounds. In Chronic Wound Care: A Clinical Source Book for Healthcare Professionals; HMP Communications: Malver, PA, USA, 2007. [Google Scholar]
- Cefalu, J.E.; Barrier, K.M.; Davis, A.H. Wound Infections in Critical Care. Crit. Care Nurs. Clin. N. Am. 2017, 29, 81–96. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Malic, S.; Hill, K.E.; Hayes, A.; Percival, S.L.; Thomas, D.W.; Williams, D.W. Detection and identification of specific bacteria in wound biofilms using peptide nucleic acid fluorescent in situ hybridization (PNA FISH). Microbiology 2009, 155, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ranall, M.V.; Butler, M.S.; Blaskovich, M.A.T.; Cooper, M.A. Resolving Biofilm Infections: Current Therapy and Drug Discovery Strategies. Curr. Drug Targets 2012, 13, 1375–1385. [Google Scholar] [CrossRef]
- Lu, J.; Turnbull, L.; Burke, C.M.; Liu, M.; Carter, D.A.; Schlothauer, R.C.; Whitchurch, C.B.; Harry, E.J. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ 2014, 2014, 1–25. [Google Scholar] [CrossRef]
- Subrahmanyam, M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns 1998, 24, 157–161. [Google Scholar] [CrossRef]
- Kingsley, A. The use of honey in the treatment of infected wounds: Case studies. Br. J. Nurs. 2001, 10, S13–S20. [Google Scholar] [CrossRef]
- AL-Waili, N.; Al Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Al-Mubarak, A.; Salom, K. Differences in composition of honey samples and their impact on the antimicrobial activities against drug multiresistant bacteria and pathogenic fungi. Arch. Med. Res. 2013, 44, 307–316. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. The bacterial challenge: Time to react. ECDA/EMEA Jt. Tech. Rep. 2009. [Google Scholar]
- Rhomberg, P.R.; Fritsche, T.R.; Sader, H.S.; Jones, R.N. Antimicrobial susceptibility pattern comparisons among intensive care unit and general ward Gram-negative isolates from the Meropenem Yearly Susceptibility Test Information Collection Program (USA). Diagn. Microbiol. Infect. Dis. 2006, 56, 57–62. [Google Scholar] [CrossRef]
- Samtani, M.N.; Flamm, R.; Kaniga, K.; Nandy, P. Pharmacokinetic-pharmacodynamic-model-guided doripenem dosing in critically ill patients. Antimicrob. Agents Chemother. 2010, 54, 2360–2364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Theuretzbacher, U. Pharmacokinetic and pharmacodynamic issues for antimicrobial therapy in patients with cancer. Clin. Infect. Dis. 2012, 54, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.E.; Cooper, R. Synergy between oxacillin and manuka honey Sensitizes methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 2012, 67, 1405–1407. [Google Scholar] [CrossRef]
- Müller, P.; Alber, D.G.; Turnbull, L.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Synergism between Medihoney and Rifampicin against Methicillin-Resistant Staphylococcus aureus (MRSA). PLoS ONE 2013, 8, e57679. [Google Scholar] [CrossRef]
- Liu, M.Y.; Cokcetin, N.N.; Lu, J.; Turnbull, L.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Rifampicin-manuka honey combinations are superior to other antibiotic-manuka honey combinations in eradicating Staphylococcus aureus biofilms. Front. Microbiol. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- NICE. Chronic Wounds: Advanced Wound Dressings and Antimicrobial Dressings; NICE: London, UK, 2016. [Google Scholar]
- NICE. Diabetic Foot Problems: Prevention and Management; NICE: London, UK, 2019. [Google Scholar]
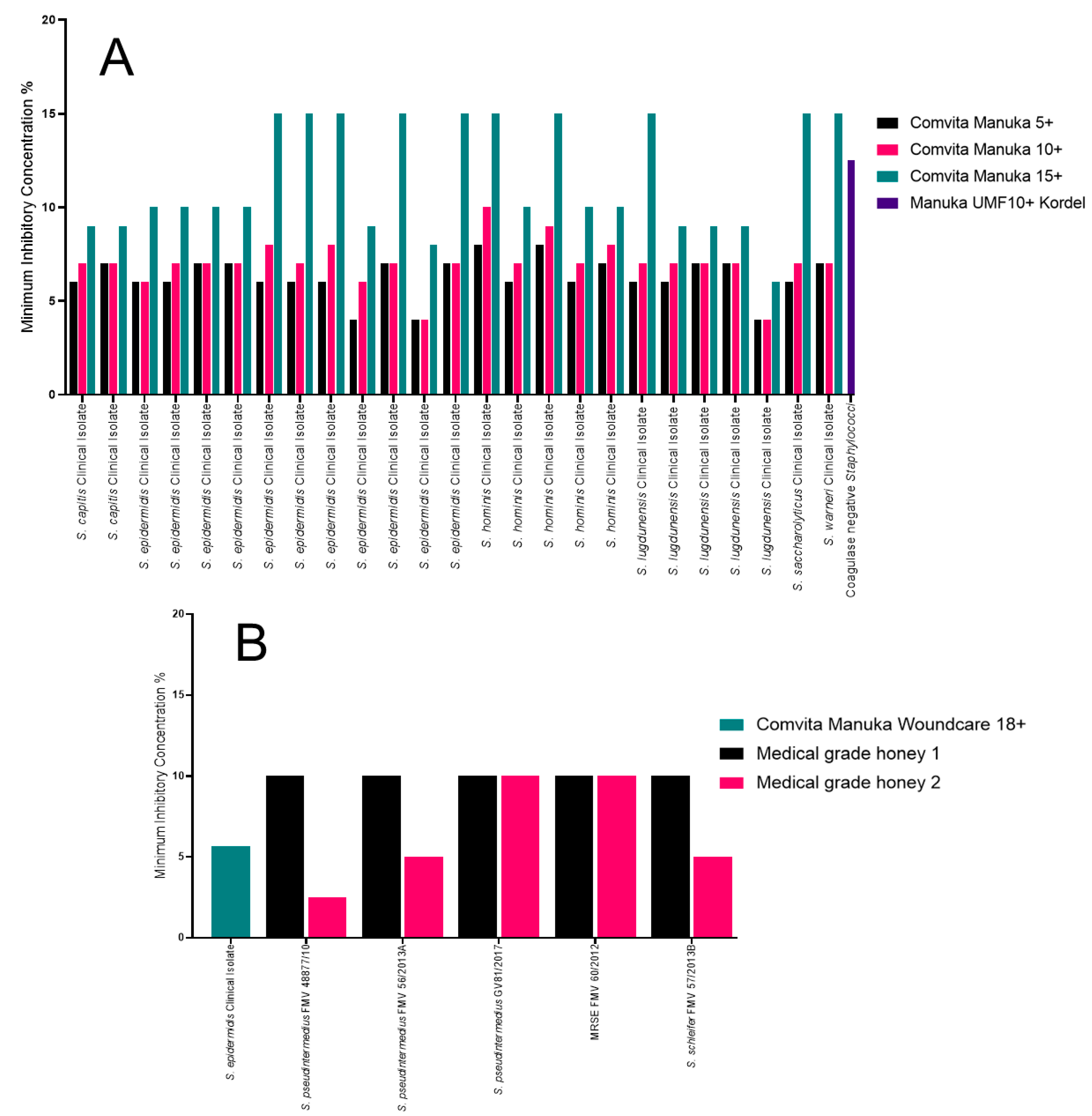
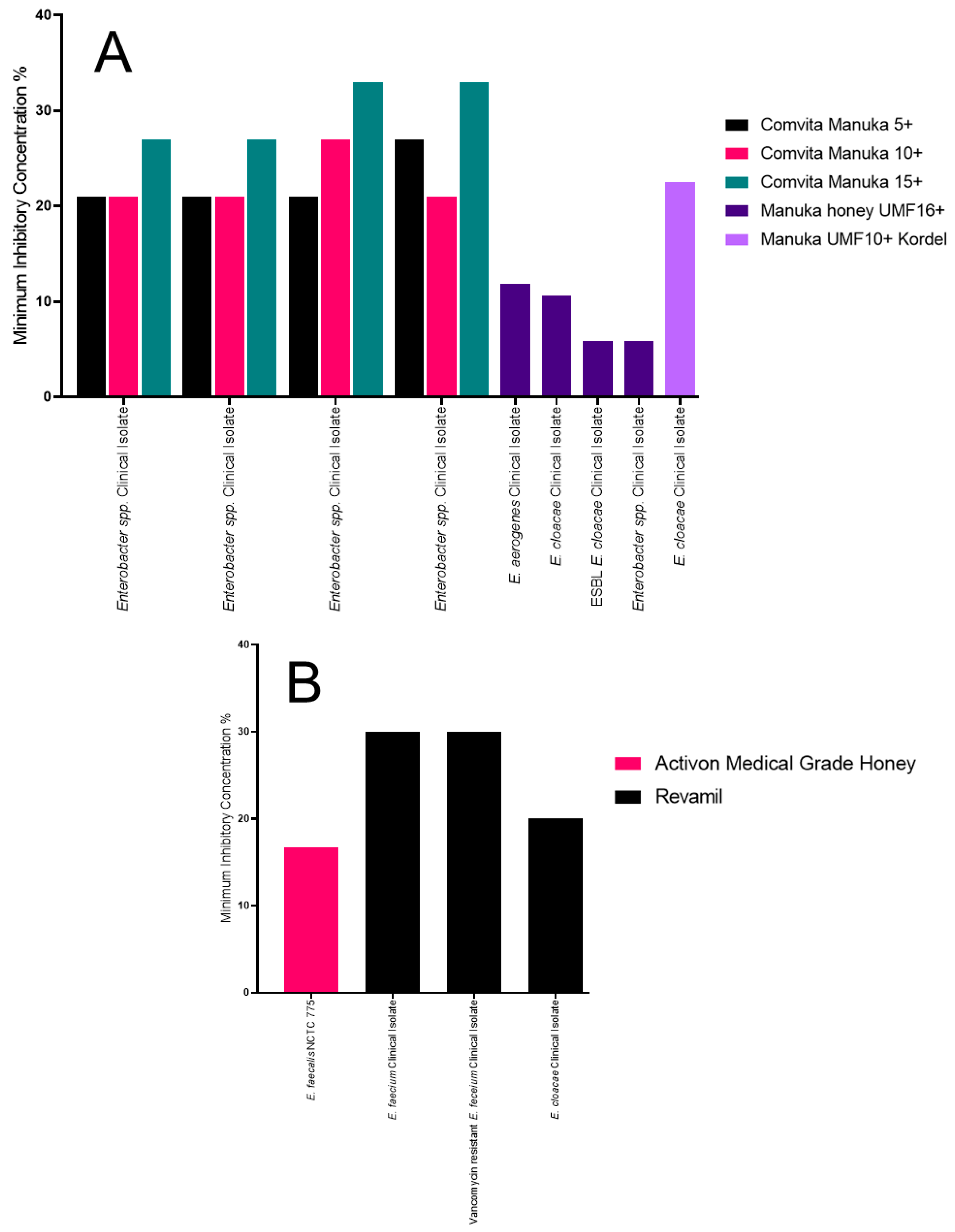
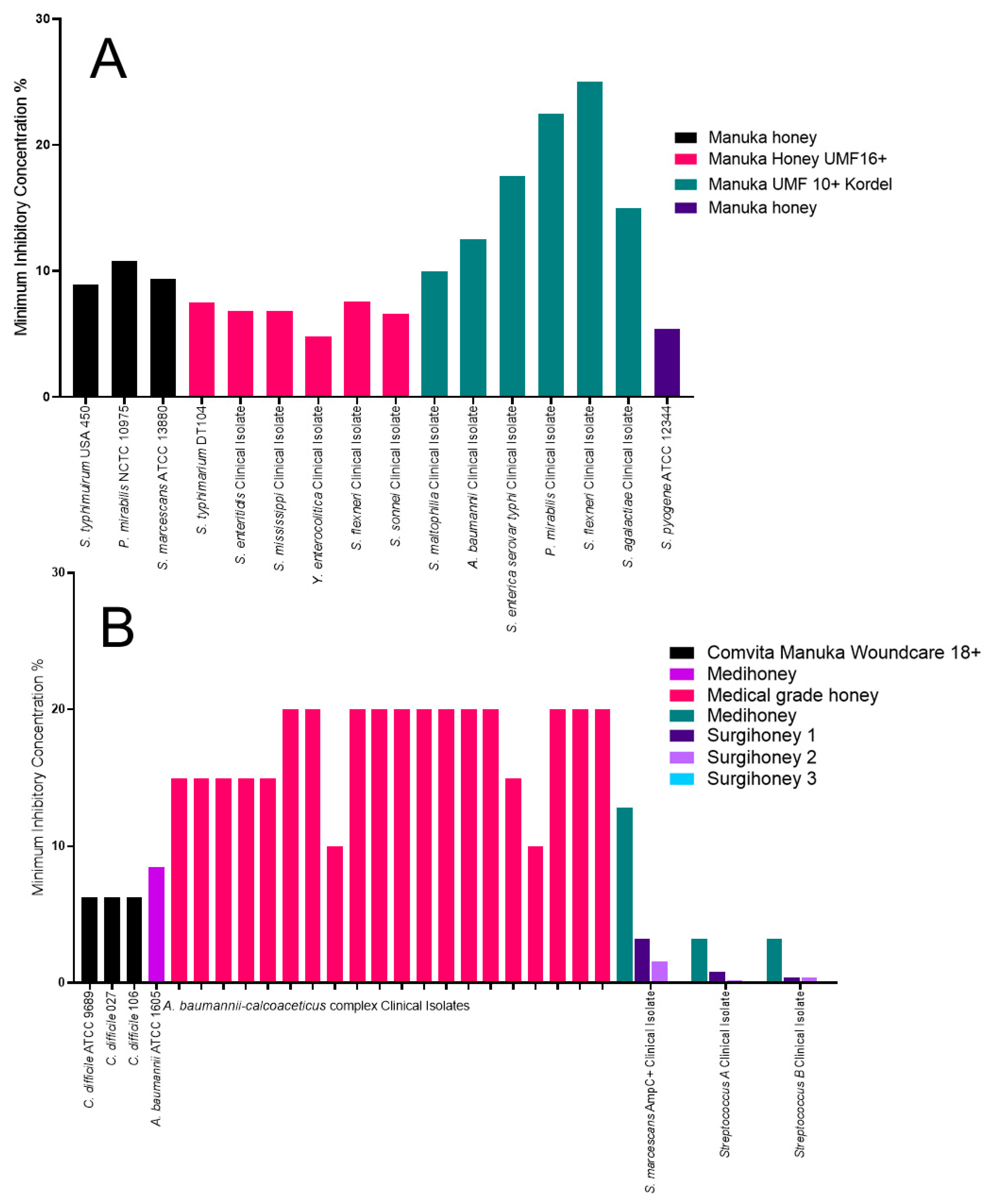
| Honey | Manuka or Medical Grade | Organisms Tested | Antibiotic Resistance |
|---|---|---|---|
| Comvita Manuka 5+, Comvita Manuka 10+ and Comvita Manuka 15+ [31] | Manuka honey | Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Staphylococcus epidermidis, Staphylococcus lugdunensis, Staphylococcus capitis, Staphylococcus hominis, Staphylococcus saccharolyticus, Staphylococcus warneri and Enterobacter spp. | Methicillin resistant, multidrug resistant, carbapenamase producers, extended-spectrum β-lactamase producers. |
| Manuka honey UMF10+ Kordel [32] | Manuka honey | S. aureus, P. aeruginosa, Coagulase negative Staphylococcus, Enterobacter cloacae, Stenotrophomonas maltophilia, Shigella flexneri, Acinetobacter baumannii, Streptococcus agalactiae, Salmonella enterica serovar typhi and Proteus mirabilis | Methicillin resistance. |
| Ungraded Manuka honey [33] | Manuka honey | S. aureus, P. aeruginosa, E. coli, S. typhimurium, P. mirabilis and Staphylococcus pyogenes | N/A |
| Ungraded Manuka honey [34] | Manuka honey | P. aeruginosa | N/A |
| Comvita Manuka 25+ [35] | Manuka honey | S. aureus, P. aeruginosa and E. coli | Methicillin resistance and oxacillin resistance. |
| Manuka honey UMF 16+ [36] | Manuka honey | E. coli, E. cloacae, Enterobacter spp., Enterobacter aerogenes, S. typhimurium, Salmonella enteritidis, Yersinia enterocolitica, S. flexneri and Shigella sonnei | Extended spectrum β-lactamase producers. |
| Medical-grade honey 1 and Medical-grade honey 2 [37] | Medical-grade honey | S. aureus, P. aeruginosa, Staphylococcus pseudintermedius, Staphylococcus epidermidis and Staphylococcus schleiferi | Methicillin resistance. |
| Revamil [38] | Medical-grade honey | S. aureus, P. aeruginosa, E. coli, Klebsiella oxytoca, Enterococcus faecium, E. cloacae and S. epidermidis | Methicillin resistance, gentamycin resistance and extended spectrum β-lactamase producers. |
| Surgihoney 1, Surgihoney 2 and Surgihoney 3 [39] | Medical-grade honey | S. aureus, P. aeruginosa, E. coli, K. pneumoniae, Sterptococcus A, Streptococcus B, and Serratia marcescans | Methicillin resistance, vancomycin resistance and extended spectrum β-lactamase producers. |
| Medihoney [39,40] | Medical-grade honey | S. aureus, A. baumannii, P. aeruginosa, E. coli, K. pneumoniae, Sterptococcus A, Streptococcus and Serratia marcescans | Methicillin resistance, vancomycin resistance, extended spectrum β-lactamase producers, oxacillin resistance and multidrug resistance. |
| Comvita Manuka Woundcare 18+ [41,42,43] | Medical-grade honey | S. aureus and P. aeruginosa, E. coli, S. epidermidis and Clostridium difficile. | Methicillin resistance, clindamycin, moxifloxacin, fluoroquinolone and rifampicin resistance. |
| Activon medical-grade honey [44,45] | Medical-grade honey | S. aureus, P. aeruginosa, E. coli and E. faecalis | N/A |
| Medical-grade honey [46] | Medical-grade honey | S. aureus, P. aeruginosa, E. coli, K. pneumoniae, E. faecalis and Acinetobacter baumannii–A. calcoaceticus complex | Methicillin resistance and extended spectrum β-lactamase producers. |
| Bacterial Pathogen | Most Effective Honey | Least Effective Honey | ||
|---|---|---|---|---|
| Manuka | Medical Grade | Manuka | Medical Grade | |
| S. aureus | Manuka honey ungraded, MIC of 2.7% [33] | Surgihoney 3, MIC of 0.01% [39] | Comvita Manuka 15+, MIC of 15% [31] | Activon medical-grade honey, MIC of 12% [45] |
| MRSA | Comvita Manuka 5+, MIC of <5% [31] | Surgihoney 3, MIC of 0.01% [39] | Comvita Manuka 15+, MIC of >15% [31] | Revamil honey, MIC of 20% [38] |
| P. aeruginosa | Manuka honey ungraded, MIC of 9.5% [34] | Surgihoney 3, MIC of 0.1% [39] | Comvita Manuka 15+, MIC of 33% [31] | Medical-grade honey 2, MIC of 40% [37] |
| MDR P. aeruginosa | Comvita Manuka 5+, MIC of <9% [31] | Surgihoney 3, MIC of 0.1% [39] | Comvita Manuka 15+, MIC of 27% [31] | N/A |
| E. coli | Manuka honey ungraded, MIC of 5.6% [33] | Surgihoney 3, MIC of 0.1% [39] | Comvita Manuka 15+, MIC of 33% [31] | Revamil honey, MIC of 20% [38] |
| ESBL producing E. coli | Manuka honey 16+, MIC of 5.08% [36] | Surgihoney 3, MIC of 0.1% [39] | Comvita Manuka 15+, MIC of 27% [31] | Revamil honey, MIC of 20% [38] |
| K. pneumoniae | Comvita Manuka 10+, Mic of 15% [31] | Surgihoney 3, MIC of 0.1% [39] | Comvita Manuka 15+, MIC of 39% [31] | Revamil honey, MIC of 30% [38] |
| S. epidermidis | Comvita Manuka 5+, MIC of <5% [31] | N/A | Comvita Manuka 15+, MIC of 15% [31] | N/A |
| Enterobacter spp. | Manuka honey UMF16+, MIC of 5.88% [36] | N/A | Comvita Manuka 15+, MIC of 33% [31] | N/A |
| E. cloacae | Manuka honey UMF16+, MIC of 5.88% [36] | N/A | Manuka honey UMF10+ Kordel, MIC of 21% [32] | N/A |
| A. baumannii | N/A | Medihoney, MIC of 8.5% [40] | N/A | Medical-grade honey, MIC of 20% [46] |
| S. marcescans | N/A | Surgihoney 3, MIC of 0.1% [39] | N/A | Medihoney, MIC of 12.8% [39] |
| Organism | Antibiotic Resistance | Honey and MIC |
|---|---|---|
| MRSA [46] | Clindamycin, erythromycin, levofloxacin and moxifloxacin | Medical-grade honey, 5% |
| MRSA [46] | Erythromycin, levofloxacin and moxifloxacin | Medical-grade honey, 20% |
| MRSA [46] | Gentamycin, levofloxacin, tetracycline and trimethoprim | Medical-grade honey, 5% |
| MRSA [46] | Erythromycin | Medical-grade honey, 15% |
| P. aeruginosa [46] | Amikacin, gentamycin, tobramycin, ampicillin/sulbactam, cefepime, ceftazidime, pipericillin/tazobactam, levofloxacin, ciprofloxacin, imipenem and meropenem | Medical-grade honey, 10% |
| P. aeruginosa [46] | Ampicillin/sulbactam | Medical-grade honey, 15% |
| ABC complex [46] | Amikacin, gentamycin, tobramycin, ampicillin/sulbactam, cefepime, ceftazidime, pipericillin/tazobactam, levofloxacin, ciprofloxacin and meropenem | Medical-grade honey, 10% |
| ABC complex [46] | Pipericillin/tazobactam | Medical-grade honey, 20% |
| Multidrug-resistant P. aeruginosa [31] | Not Specified | Comvita Manuka 5+, <9% Comvita Manuka 10+, 15% Comvita Manuka 15+, 21% |
| P. aeruginosa [31] | No resistance | Comvita Manuka 5+, 27% Comvita Manuka 10+, 27% Comvita Manuka 15+, 33% |
| E. coli [36] | Amoxycillin, Amoxy/Clavulanate, Cefaclor, Trimethoprim, Ceftriaxone, Cefuroxime, Ceprofloxacin, Cotrimoxazole and Gentamicin | Manuka honey UMF16+, 5.08% |
| E. coli ATCC 25923 [36] | Not Specified | Manuka honey UMF16+, 6.87% |
| E. cloacae [36] | Amoxycillin, Amoxy/Clavulanate, Cefaclor | Manuka honey UMF16+, 5.88% |
| E. cloacae [36] | Not Specified | Manuka honey UMF16+, 10.65% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nolan, V.C.; Harrison, J.; Wright, J.E.E.; Cox, J.A.G. Clinical Significance of Manuka and Medical-Grade Honey for Antibiotic-Resistant Infections: A Systematic Review. Antibiotics 2020, 9, 766. https://doi.org/10.3390/antibiotics9110766
Nolan VC, Harrison J, Wright JEE, Cox JAG. Clinical Significance of Manuka and Medical-Grade Honey for Antibiotic-Resistant Infections: A Systematic Review. Antibiotics. 2020; 9(11):766. https://doi.org/10.3390/antibiotics9110766
Chicago/Turabian StyleNolan, Victoria C., James Harrison, John E. E. Wright, and Jonathan A. G. Cox. 2020. "Clinical Significance of Manuka and Medical-Grade Honey for Antibiotic-Resistant Infections: A Systematic Review" Antibiotics 9, no. 11: 766. https://doi.org/10.3390/antibiotics9110766
APA StyleNolan, V. C., Harrison, J., Wright, J. E. E., & Cox, J. A. G. (2020). Clinical Significance of Manuka and Medical-Grade Honey for Antibiotic-Resistant Infections: A Systematic Review. Antibiotics, 9(11), 766. https://doi.org/10.3390/antibiotics9110766






