Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria?
Abstract
1. Introduction
2. Factors Involved in Antibacterial Activity of Honey
2.1. Phisicochemical Characteristics with Antibacterial Effects
2.1.1. Osmotic Pressure and Low Water Activity
2.1.2. Acidity and Low pH
2.2. Compounds with Antibacterial Effects
2.2.1. Hydrogen Peroxide
2.2.2. Phenolic Acids and Flavonoids
2.2.3. Methylglyoxal
2.2.4. Bee Defensin-1 Peptide
3. How Honey Acts against Bacteria: Antibacterial Action Mechanisms
3.1. Structural and Morphological Changes
3.2. Alterations of Bacterial Membrane Potential
3.3. Bacterial Cell Cycle and Cell Growth Changes
3.4. Disruption of Bacterial Metabolism
3.5. Effect on Efflux Pumps Activity
3.6. Bacterial Quorum Sensing Alterations
3.7. Biofilm Inhibition
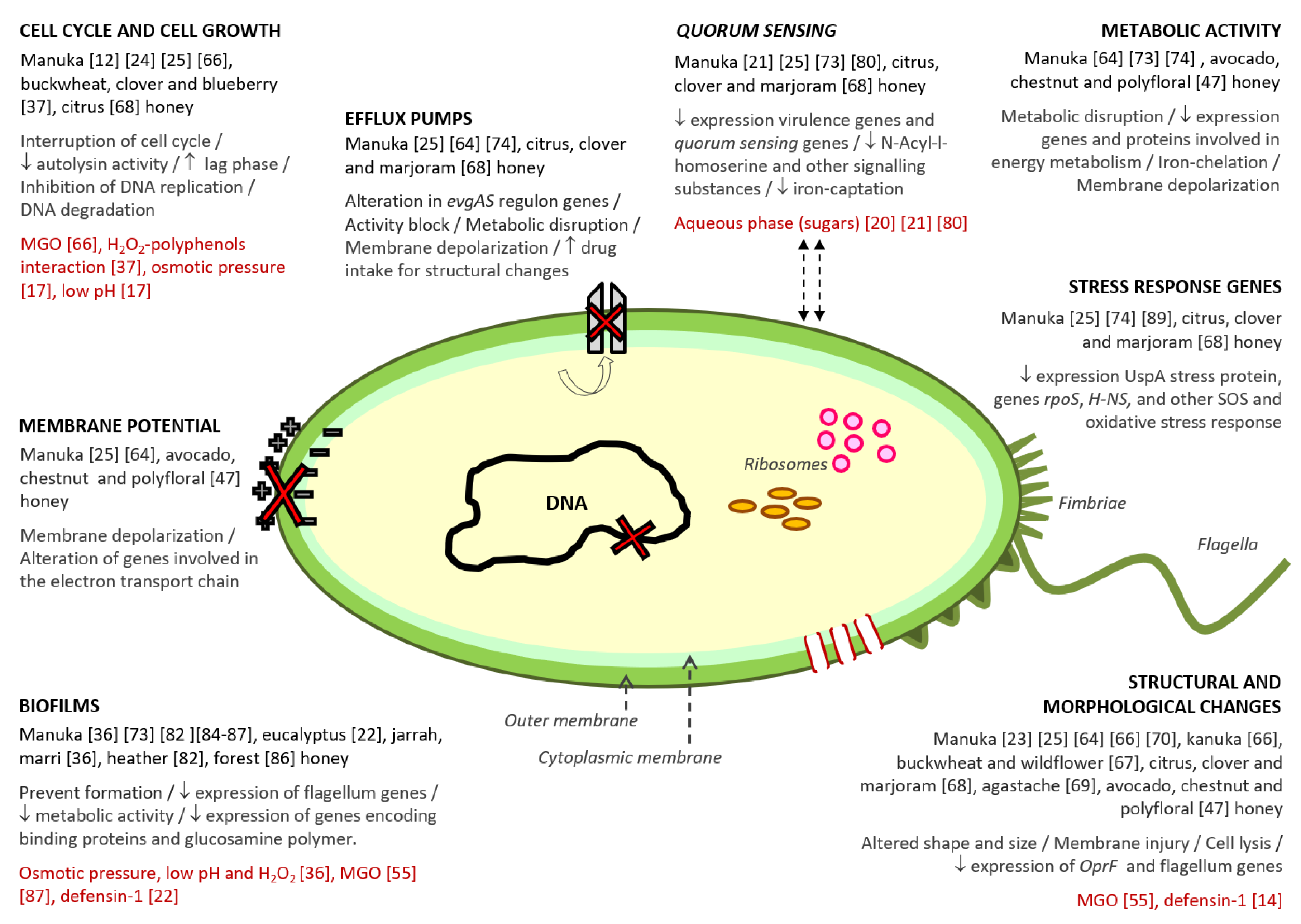
3.8. Effects on Bacterial Stress Response
4. Honey against Antibiotic-Resistant Bacteria
| Honey Type. | Microorganism | Type of Study | Reference |
|---|---|---|---|
| Manuka | MRSA | in vivo hydroxyurea-induced leg ulcer leg ulcer | [93] [101] |
| in vitro | [24,69,94,95,96,97,98,99,100,113] | ||
| VRSA | in vitro | [91] | |
| VRE | in vitro | [100] | |
| ESBL-E. coli | in vitro | [94,102] | |
| ESBL-K. pneumoniae | in vitro | [94,103,104] | |
| CARB-K. pneumoniae | in vitro | [94] | |
| MDR-P. aeruginosa | |||
| Ureaplasma parvum U. urealyticum | in vitro | [105] | |
| Medihoney | Bacteria related to catheterization infections | in vivo catheterization infections | [106] |
| Revamil | MRSA MRSE ESBL-E. coli ESBL-P. aeruginosa ESBL-E. cloacae ESBL-K. oxytoca VREF | in vitro | [60,61,107] |
| Colonizing skin bacteria | in vivo skin colonization | [107] | |
| L-Mesitran | MRSA | in vitro | [99] |
| MDR-P. aeruginosa MDR-streptococci AR-polymicrobial infection PR-polymicrobial infection MDR-E. coli | in vivo chronic diabetic ulcers | [108] | |
| Aromatic-and-medicinal-plants honey | MDR-E. coli | in vitro | [109] |
| MDR-S. aureus | |||
| MDR-Bacillus spp. | |||
| MDR-P. aeruginosa | |||
| Ulmo tree | MRSA | in vitro | [110] |
| Sweet clover Blueberry Buckwheat | MRSA VREF | in vitro | [111] |
| Black seed Beri Shain | MRSA | in vitro | [112] |
| Blossom Heather Highland Portobello | PR-A. calcoaceticus PR-S. aureus PR-P. aeruginosa PR-E. coli | in vitro | [38] |
| Sage Chestnut Locust tree Lime tree Indigo bush Rapeseed Maple Mint Spring and Autumn pasture Maple Fir honeydew | MRSA MDR-P. aeruginosa ESBL-E. coli MDR-A. baumannii | in vitro | [114] |
| Agastache Tea-tree Jelly bush Jarrah | MRSA | in vitro | [69] |
| Avocado Chestnut Eucalyptus Rosemary Thyme Polyfloral | MRSA MDR-S. pyogenes MDR-E. coli MDR-P. aeruginosa | in vitro | [30] |
| Germania | MRSA | in vitro | [113] |
5. What about Honey-Resistance Acquisition?
6. Limitations of the Use of Honey in Therapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sir Alexander Fleming-Nobel Lecture: Penicillin. Available online: https://www.nobelprize.org/prizes/medicine/1945/fleming/lecture/ (accessed on 27 August 2020).
- ECDC. Surveillance of Antimicrobial Resistance in Europe Annual Report of the European Antimicrobial Resistance Surveillance Network (2018); ECDC: Stockholm, Sweden, 2019.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 28 August 2020).
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Antibiotic Resistance: Moving from Individual Health Norms to Social Norms in One Health and Global Health. Front. Microbiol. 2020, 11, 1914. [Google Scholar] [CrossRef] [PubMed]
- Oniciuc, E.A.; Likotrafiti, E.; Alvarez-Molina, A.; Prieto, M.; López, M.; Alvarez-Ordóñez, A. Food processing as a risk factor for antimicrobial resistance spread along the food chain. Curr. Opin. Food Sci. 2019, 30, 21–26. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef]
- Al Alsheikh, H.M.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Wozniak, T.M.; Barnsbee, L.; Lee, X.J.; Pacella, R.E. Using the best available data to estimate the cost of antimicrobial resistance: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.M.; Lindahl, O.; Harbarth, S.; de Kraker, M.E.A.; Edwards, S.; Hollis, A. Industry incentives and antibiotic resistance: An introduction to the antibiotic susceptibility bonus. J. Antibiot. 2020, 73, 421–428. [Google Scholar] [CrossRef]
- Carter, D.A.; Blair, S.E.; Cokcetin, N.N.; Bouzo, D.; Brooks, P.; Schothauer, R.; Harry, E.J. Therapeutic Manuka Honey: No Longer So Alternative. Front. Microbiol. 2016, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The intracellular effects of manuka honey on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 45–50. [Google Scholar] [CrossRef]
- Maddocks, S.E.; Jenkins, R.E. Honey: A sweet solution to the growing problem of antimicrobial resistance? Future Microbiol. 2013, 8, 1419–1429. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the antimicrobial composition of honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Valencia-Barrera, R.M.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tornadijo, M.E.; Fresno, J.M. Spanish honeys with quality brand: A multivariate approach to physicochemical parameters, microbiological quality and floral origin. J. Apic. Res. 2019, 58, 92–103. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 4507. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.C.; Chirife, J. Determination of water activity change due to crystallization in honeys from Argentina. Food Control 2006, 17, 59–64. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Truchado, P.; López-Gálvez, F.; Gil, M.I.; Tomás-Barberán, F.A.; Allende, A. Quorum sensing inhibitory and antimicrobial activities of honeys and the relationship with individual phenolics. Food Chem. 2009, 115, 1337–1344. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Kim, J.A.; Neupane, G.P.; Cho, M.H.; Lee, C.S.; Lee, J. Low concentrations of honey reduce biofilm formation, quorum sensing, and virulence in Escherichia coli O157:H7. Biofouling 2011, 27, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Proaño, A.; Coello, D.; Villacrés-Granda, I.; Ballesteros, I.; Debut, A.; Vizuete, K.; Brenciani, A.; Álvarez-Suarez, J.M. The osmotic action of sugar combined with hydrogen peroxide and bee-derived antibacterial peptide Defensin-1 is crucial for the antibiofilm activity of eucalyptus honey. LWT 2021, 136, 110379. [Google Scholar] [CrossRef]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 167–171. [Google Scholar] [CrossRef]
- Jenkins, R.; Burton, N.; Cooper, R. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 2536–2542. [Google Scholar] [CrossRef]
- Bouzo, D.; Cokcetin, N.N.; Li, L.; Ballerin, G.; Bottomley, A.L.; Lazenby, J.; Whitchurch, C.B.; Paulsen, I.T.; Hassan, K.A.; Harry, E.J. Characterizing the mechanism of action of an ancient antimicrobial, manuka honey, against pseudomonas aeruginosa using modern transcriptomics. mSystems 2020, 5, e00106-20. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Lu, Y.; Tan, S.T. Extractives from New Zealand Honeys. 5. Aliphatic Dicarboxylic Acids in New Zealand Rewarewa (Knightea excelsa) Honey. J. Agric. Food Chem. 1995, 43, 3021–3025. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M. Bee Products—Chemical and Biological Properties; Springer International Publishing: Basel, Switzerland, 2017; ISBN 9783319596891. [Google Scholar]
- Karabagias, I.K.; Vavoura, M.V.; Nikolaou, C.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Floral authentication of Greek unifloral honeys based on the combination of phenolic compounds, physicochemical parameters and chemometrics. Food Res. Int. 2014, 62, 753–760. [Google Scholar] [CrossRef]
- Olaitan, P.B.; Adeleke, O.E.; Ola, I.O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007, 7, 159–165. [Google Scholar] [PubMed]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019, 67, 688–698. [Google Scholar] [CrossRef]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Brudzynski, K.; Miotto, D.; Kim, L.; Sjaarda, C.; Maldonado-Alvarez, L.; Fukś, H. Active macromolecules of honey form colloidal particles essential for honey antibacterial activity and hydrogen peroxide production. Sci. Rep. 2017, 7, 7637. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; Wang, T. Powerful bacterial killing by buckwheat honeys is concentration-dependent, involves complete DNA degradation and requires hydrogen peroxide. Front. Microbiol. 2012, 3, 242. [Google Scholar] [CrossRef]
- Poli, J.P.; Guinoiseau, E.; Luciani, A.; Yang, Y.; Battesti, M.J.; Paolini, J.; Costa, J.; Quilichini, Y.; Berti, L.; Lorenzi, V. Key role of hydrogen peroxide in antimicrobial activity of spring, Honeydew maquis and chestnut grove Corsican honeys on Pseudomonas aeruginosa DNA. Lett. Appl. Microbiol. 2018, 66, 427–433. [Google Scholar] [CrossRef]
- Sindi, A.; Chawn, M.V.B.; Hernandez, M.E.; Green, K.; Islam, M.K.; Locher, C.; Hammer, K. Anti-biofilm effects and characterisation of the hydrogen peroxide activity of a range of Western Australian honeys compared to Manuka and multifloral honeys. Sci. Rep. 2019, 9, 17666. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; Miotto, D. Unraveling a mechanism of honey antibacterial action: Polyphenol/H2O2-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 2012, 133, 329–336. [Google Scholar] [CrossRef]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; McDougall, G.J. Compositional analysis of Scottish honeys with antimicrobial activity against antibiotic-resistant bacteria reveals novel antimicrobial components. LWT—Food Sci. Technol. 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef]
- Sousa, J.M.; de Souza, E.L.; Marques, G.; Meireles, B.; de Magalhães Cordeiro, Â.T.; Gullón, B.; Pintado, M.M.; Magnani, M. Polyphenolic profile and antioxidant and antibacterial activities of monofloral honeys produced by Meliponini in the Brazilian semiarid region. Food Res. Int. 2016, 84, 61–68. [Google Scholar] [CrossRef]
- Liu, J.R.; Ye, Y.L.; Lin, T.Y.; Wang, Y.W.; Peng, C.C. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013, 139, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; Martos, I.; Bortolotti, L.; Sabatini, A.G.; Ferreres, F.; Tomas-Barberan, F.A. Use of Quinoline Alkaloids as Markers of the Floral Origin of Chestnut Honey. J. Agric. Food Chem. 2009, 57, 5680–5686. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Natural Occurrence of Abscisic Acid in Heather Honey and Floral Nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Teixeira-Santos, R.; Rodrigues, A.G.; Pina-Vaz, C.; Fresno, J.M.; Eugenia Tornadijo, M. Antibacterial action mechanisms of honey: Physiological Effects of Avocado, Chestnut, and Polyfloral Honey upon Staphylococcus aureus and Escherichia coli. Molecules 2020, 25, 1252. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; King, J.; Revell, M.; Manley-Harris, M.; Balks, M.; Janusch, F.; Kiefer, M.; Clearwater, M.; Brooks, P.; Dawson, M. Regional, annual, and individual variations in the dihydroxyacetone content of the nectar of Manuka (Leptospermum scoparium) in New Zealand. J. Agric. Food Chem. 2014, 62, 10332–10340. [Google Scholar] [CrossRef]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.S.; Compton, B.J.; Marshall, A.; Anderson, R.; Li, Y.; van der Woude, H.; Hermans, I.F.; Painter, G.F.; Gasser, O. Mānuka honey-derived methylglyoxal enhances microbial sensing by mucosal-associated invariant T cells. Food Funct. 2020, 11, 5782–5787. [Google Scholar] [CrossRef]
- Salonen, A.; Virjamo, V.; Tammela, P.; Fauch, L.; Julkunen-Tiitto, R. Screening bioactivity and bioactive constituents of Nordic unifloral honeys. Food Chem. 2017, 237, 214–224. [Google Scholar] [CrossRef]
- Arena, E.; Ballistreri, G.; Tomaselli, F.; Fallico, B. Survey of 1,2-Dicarbonyl Compounds in Commercial Honey of Different Floral Origin. J. Food Sci. 2011, 76, C1203–C1210. [Google Scholar] [CrossRef]
- Oinaala, D.; Lehesvaara, M.; Lyhs, U.; Tikkanen-Kaukanen, C. Antimicrobial activity of organic honeys against food pathogenic bacterium Clostridium perfringens. Org. Agric. 2015, 5, 153–159. [Google Scholar] [CrossRef][Green Version]
- Karabagias, I.K.; Maia, M.; Karabagias, V.K.; Gatzias, I.; Badeka, A.V. Quality and origin characterisation of Portuguese, Greek, Oceanian, and Asian honey, based on poly-parametric analysis hand in hand with dimension reduction and classification techniques. Eur. Food Res. Technol. 2020, 246, 987–1006. [Google Scholar] [CrossRef]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How methylglyoxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef]
- Casteels-Josson, K.; Zhang, W.; Capaci, T.; Casteels, P.; Tempst, P. Acute transcriptional response of the honeybee peptide-antibiotics gene repertoire and required post-translational conversion of the precursor structures. J. Biol. Chem. 1996, 269, 28569–28575. [Google Scholar]
- Ilyasov, R.A.; Gaifullina, L.R.; Saltykova, E.S.; Poskryakov, A.V.; Nikolenko, A.G. Review of the expression of antimicrobial peptide defensin in honey bees Apis Mellifera L. J. Apic. Sci. 2012, 56, 115–124. [Google Scholar] [CrossRef]
- Erban, T.; Shcherbachenko, E.; Talacko, P.; Harant, K. The Unique Protein Composition of Honey Revealed by Comprehensive Proteomic Analysis: Allergens, Venom-like Proteins, Antibacterial Properties, Royal Jelly Proteins, Serine Proteases, and Their Inhibitors. J. Nat. Prod. 2019, 82, 1217–1226. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Velde, A.A.T.; Boer, L.; Speijer, D.; Christina Vandenbroucke-Grauls, M.J.; Zaat, S.A.J. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; De Boer, L.; Ruyter-Spira, C.P.; Creemers-Molenaar, T.; Helsper, J.P.F.G.; Vandenbroucke-Grauls, C.M.J.E.; Zaat, S.A.J.; Te Velde, A.A. Medical-grade honey enriched with antimicrobial peptides has enhanced activity against antibiotic-resistant pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 251–257. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Velde, A.A.T.; de Boer, L.; Vandenbroucke-Grauls, C.M.J.E.; Zaat, S.A.J. Two Major Medicinal Honeys Have Different Mechanisms of Bactericidal Activity. PLoS ONE 2011, 6, e17709. [Google Scholar] [CrossRef]
- Valachová, I.; Bučeková, M.; Majtán, J. Quantification of bee-derived peptide. Czech J. Food Sci. 2016, 34, 233–243. [Google Scholar]
- Liu, M.; Lu, J.; Müller, P.; Turnbull, L.; Burke, C.M.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Antibiotic-specific differences in the response of Staphylococcus aureus to treatment with antimicrobials combined with manuka honey. Front. Microbiol. 2015, 5, 779. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Teixeira-Santos, R.; Rodrigues, A.G.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Evaluation of Physiological Effects Induced by Manuka Honey Upon Staphylococcus aureus and Escherichia coli. Microorganisms 2019, 7, 258. [Google Scholar] [CrossRef]
- Roberts, A.E.L.; Maddocks, S.E.; Cooper, R.A. Manuka honey is bactericidal against Pseudomonas aeruginosa and results in differential expression of oprF and algD. Microbiology 2012, 158, 3005–3013. [Google Scholar] [CrossRef]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The Effect of New Zealand Kanuka, Manuka and Clover Honeys on Bacterial Growth Dynamics and Cellular Morphology Varies According to the Species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C. Antibacterial compounds of canadian honeys target bacterial cell wall inducing phenotype changes, growth inhibition and cell lysis that resemble action of β-lactam antibiotics. PLoS ONE 2014, 9, e106967. [Google Scholar] [CrossRef]
- Wasfi, R.; Elkhatib, W.F.; Khairalla, A.S. Effects of selected egyptian honeys on the cellular ultrastructure and the gene expression profile of Escherichia coli. PLoS ONE 2016, 11, e0150984. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial activity of Agastache honey and characterization of its bioactive compounds in comparison with important commercial honeys. Front. Microbiol. 2019, 10, 263. [Google Scholar] [CrossRef]
- Roberts, A.E.L.; Maddocks, S.E.; Cooper, R.A. Manuka honey reduces the motility of Pseudomonas aeruginosa by suppression of flagella-associated genes. J. Antimicrob. Chemother. 2015, 70, 716–725. [Google Scholar] [CrossRef]
- Sträuber, H.; Müller, S. Viability states of bacteria-Specific mechanisms of selected probes. Cytom. Part A 2010, 77, 623–634. [Google Scholar] [CrossRef]
- Strahl, H.; Hamoen, L.W. Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. USA 2010, 107, 12281–12286. [Google Scholar] [CrossRef]
- Jenkins, R.; Burton, N.; Cooper, R. Proteomic and genomic analysis of methicillin-resistant Staphylococcus aureus (MRSA) exposed to manuka honey in vitro demonstrated down-regulation of virulence markers. J. Antimicrob. Chemother. 2014, 69, 603–615. [Google Scholar] [CrossRef]
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. [Google Scholar] [CrossRef]
- Ankley, L.M.; Monteiro, M.P.; Camp, K.M.; O’Quinn, R.; Castillo, A.R. Manuka honey chelates iron and impacts iron regulation in key bacterial pathogens. J. Appl. Microbiol. 2020, 128, 1015–1024. [Google Scholar] [CrossRef]
- Kronda, J.M.; Cooper, R.A.; Maddocks, S.E. Manuka honey inhibits siderophore production in Pseudomonas aeruginosa. J. Appl. Microbiol. 2013, 115, 86–90. [Google Scholar] [CrossRef]
- Lu, W.-J.; Lin, H.-J.; Hsu, P.-H.; Lin, H.-T.V. Determination of Drug Efflux Pump Efficiency in Drug-Resistant Bacteria Using MALDI-TOF MS. Antibiotics 2020, 9, 639. [Google Scholar] [CrossRef]
- Muniz, D.F.; dos Santos Barbosa, C.R.; de Menezes, I.R.A.; de Sousa, E.O.; Pereira, R.L.S.; Júnior, J.T.C.; Pereira, P.S.; de Matos, Y.M.L.S.; da Costa, R.H.S.; de Morais Oliveira-Tintino, C.D.; et al. In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2021, 337, 127776. [Google Scholar] [CrossRef]
- Jenkins, R.; Cooper, R. Improving Antibiotic Activity against Wound Pathogens with Manuka Honey In Vitro. PLoS ONE 2012, 7, e45600. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Starkey, M.; Hazan, R.; Rahme, L.G. Honey’s ability to counter bacterial infections arises from both bactericidal compounds and QS inhibition. Front. Microbiol. 2012, 3, 144. [Google Scholar] [CrossRef]
- Jadaun, V.; Prateeksha, P.; Singh, B.R.; Paliya, B.S.; Upreti, D.K.; Rao, C.V.; Rawat, A.K.S.; Singh, B.N. Honey enhances the anti-quorum sensing activity and anti-biofilm potential of curcumin. RSC Adv. 2015, 5, 71060–71070. [Google Scholar] [CrossRef]
- Fernandes, L.; Oliveira, A.; Henriques, M.; Rodrigues, M.E. Honey as a strategy to fight candida tropicalis in mixed-biofilms with pseudomonas aeruginosa. Antibiotics 2020, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Prateeksha, S.B.; Shoeb, M.; Sharma, S.; Naqvi, A.H.; Gupta, V.K.; Singh, B.N. Scaffold of Selenium Nanovectors and Honey Phytochemicals for Inhibition of Pseudomonas aeruginosa Quorum Sensing and Biofilm Formation. Front. Cell. Infect. Microbiol. 2017, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Jenkins, L.; Hooper, S. Inhibition of biofilms of Pseudomonas aeruginosa by Medihoney in vitro. J. Wound Care 2014, 23, 93–104. [Google Scholar] [CrossRef]
- Maddocks, S.E.; Lopez, M.S.; Rowlands, R.S.; Cooper, R.A. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology 2012, 158, 781–790. [Google Scholar] [CrossRef]
- Merckoll, P.; Jonassen, T.Ø.; Vad, M.E.; Jeansson, S.L.; Melby, K.K. Bacteria, biofilm and honey: A study of the effects of honey on “planktonic” and biofilm-embedded chronic wound bacteria. Scand. J. Infect. Dis. 2009, 41, 341–347. [Google Scholar] [CrossRef]
- Lu, J.; Cokcetin, N.N.; Burke, C.M.; Turnbull, L.; Liu, M.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Kot, B.; Sytykiewicz, H.; Sprawka, I.; Witeska, M. Effect of manuka honey on biofilm-associated genes expression during methicillin-resistant Staphylococcus aureus biofilm formation. Sci. Rep. 2020, 10, 13552. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Burton, N.; Cooper, R. Effect of manuka honey on the expression of universal stress protein A in meticillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2011, 37, 373–376. [Google Scholar] [CrossRef]
- Packer, J.M.; Irish, J.; Herbert, B.R.; Hill, C.; Padula, M.; Blair, S.E.; Carter, D.A.; Harry, E.J. Specific non-peroxide antibacterial effect of manuka honey on the Staphylococcus aureus proteome. Int. J. Antimicrob. Agents 2012, 40, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Wootton, M.; Howe, R.; Cooper, R. Susceptibility to manuka honey of Staphylococcus aureus with varying sensitivities to vancomycin. Int. J. Antimicrob. Agents 2012, 40, 88–89. [Google Scholar] [CrossRef]
- Cooper, R.; Jenkins, R. Are there feasible prospects for manuka honey as an alternative to conventional antimicrobials? Expert Rev. Anti. Infect. Ther. 2012, 10, 623–625. [Google Scholar] [CrossRef]
- Natarajan, S.; Williamson, D.; Grey, J.; Harding, K.G.; Cooper, R.A. Healing of an MRSA-colonized, hydroxyurea-induced leg ulcer with honey. J. Dermatol. Treat. 2001, 12, 33–36. [Google Scholar] [CrossRef]
- Girma, A.; Seo, W.; SheI, R.C. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS ONE 2019, 14, e0224495. [Google Scholar] [CrossRef]
- Jenkins, R.E.; Cooper, R. Synergy between oxacillin and manuka honey sensitizes methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 2012, 67, 1405–1407. [Google Scholar] [CrossRef]
- George, N.M.; Cutting, K.F. Antibacterial Honey: In-vitro Activity Against Clinical Isolates of MRSA, VRE, and Other Multiresistant Gram-negative Organisms | Wounds Research. Wounds 2007, 19, 231–236. [Google Scholar]
- Mokhtar, J.A.; McBain, A.J.; Ledder, R.G.; Binsuwaidan, R.; Rimmer, V.; Humphreys, G.J. Exposure to a Manuka Honey Wound Gel Is Associated With Changes in Bacterial Virulence and Antimicrobial Susceptibility. Front. Microbiol. 2020, 11, 2036. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Alber, D.G.; Turnbull, L.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Synergism between Medihoney and Rifampicin against Methicillin-ResistantStaphylococcus aureus (MRSA). PLoS ONE 2013, 8, e57679. [Google Scholar]
- Cremers, N.; Belas, A.; Santos Costa, S.; Couto, I.; de Rooster, H.; Pomba, C. In vitro antimicrobial efficacy of two medical grade honey formulations against common high-risk meticillin-resistant staphylococci and Pseudomonas spp. pathogens. Vet. Dermatol. 2020, 31, 90. [Google Scholar] [CrossRef]
- Cooper, R.A.; Molan, P.C.; Harding, K.G. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 2002, 93, 857–863. [Google Scholar] [CrossRef]
- Gethin, G.; Cowman, S. Bacteriological changes in sloughy venous leg ulcers treated with manuka honey or hydrogel: An RCT. J. Wound Care 2008, 17, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.R.; Afegbua, S.L. Single and joint antibacterial activity of aqueous garlic extract and Manuka honey on extended-spectrum beta-lactamase-producing Escherichia coli. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 472–478. [Google Scholar] [CrossRef]
- Shah Pratibha, J.; Williamson Manita, T. Antibacterial activity of honey against ESBL producing Klebsiella pneumoniae from burn wound infections. Int. J. Curr. Pharm. Res. 2015, 7, 32–36. [Google Scholar]
- Qamar, M.U.; Saleem, S.; Toleman, M.A.; Saqalein, M.; Waseem, M.; Nisar, M.A.; Khurshid, M.; Taj, Z.; Jahan, S. In vitro and in vivo activity of Manuka honey against NDM-1-producing Klebsiella pneumoniae ST11. Future Microbiol. 2018, 13, 13–26. [Google Scholar] [CrossRef]
- Hillitt, K.L.; Jenkins, R.E.; Spiller, O.B.; Beeton, M.L. Antimicrobial activity of Manuka honey against antibiotic-resistant strains of the cell wall-free bacteria Ureaplasma parvum and Ureaplasma urealyticum. Lett. Appl. Microbiol. 2017, 64, 198–202. [Google Scholar] [CrossRef]
- Johnson, D.W.; Van Eps, C.; Mudge, D.W.; Wiggins, K.J.; Armstrong, K.; Hawley, C.M.; Campbell, S.B.; Isbel, N.M.; Nimmo, G.R.; Gibbs, H. Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.S.; Van Den Akker, J.P.C.; Güçlü, A.; Aslami, H.; Binnekade, J.M.; De Boer, L.; Boszhard, L.; Paulus, F.; Middelhoek, P.; Te Velde, A.A.; et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin. Infect. Dis. 2008, 46, 1677–1682. [Google Scholar] [CrossRef]
- Nair, H.K.R.; Tatavilis, N.; Pospíšilová, I.; Kučerová, J.; Cremers, N.A.J. Medical-grade honey kills antibiotic-resistant bacteria and prevents amputation in diabetics with infected ulcers: A prospective case series. Antibiotics 2020, 9, 529. [Google Scholar] [CrossRef]
- Malika, N.O.A.M.A.N.; Mohamed, F.; Chakib, E.A. Antimicrobial Activities of Natural Honey from Aromatic and Medicinal Plants on Antibio-resistant Strains of Bacteria. Int. J. Agric. Biol. 2004, 6, 289–293. [Google Scholar]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 10, 47. [Google Scholar] [CrossRef]
- Brudzynski, K.; Lannigan, R. Mechanism of Honey Bacteriostatic Action against MRSA and VRE Involves Hydroxyl Radicals Generated from Honey’s Hydrogen Peroxide. Front. Microbiol. 2012, 3, 36. [Google Scholar] [CrossRef]
- Hussain, M.B.; Hannan, A.; Absar, M.; Butt, N.S. In-vitro susceptibility of methicillin-resistant Stayphylococcus aureus to honey. Complement. Ther. Clin. Pract. 2017, 27, 57–60. [Google Scholar] [CrossRef]
- Bazzi, A.M.; Rabaan, A.A.; Al-Tawfiq, J.A.; Shannak, B.M. Comparison of Effectiveness of Germania Honey Compared to Manuka Honey in Methicillin-Resistant Staphylococcus aureus (MRSA) Killing. Open Microbiol. J. 2019, 13, 21–27. [Google Scholar] [CrossRef]
- Gobin, I.; Crnković, G.; Magdalenić, M.; Begić, G.; Babić, A.; Lušić, D.; Vučković, D. Antibacterial potential of Croatian honey against antibiotic resistant pathogenic bacteria. Med. Glas. 2018, 15, 139–144. [Google Scholar]
- Cooper, R.A.; Jenkins, L.; Henriques, A.F.M.; Duggan, R.S.; Burton, N.F. Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1237–1241. [Google Scholar] [CrossRef]
- Camplin, A.L.; Maddocks, S.E. Manuka honey treatment of biofilms of Pseudomonas aeruginosa results in the emergence of isolates with increased honey resistance. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 19. [Google Scholar] [CrossRef]
- Campeau, M.E.M.; Patel, R. Antibiofilm Activity of Manuka Honey in Combination with Antibiotics. Int. J. Bacteriol. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Hayes, G.; Wright, N.; Gardner, S.L.; Telzrow, C.L.; Wommack, A.J.; Vigueira, P.A. Manuka honey and methylglyoxal increase the sensitivity of Staphylococcus aureus to linezolid. Lett. Appl. Microbiol. 2018, 66, 491–495. [Google Scholar] [CrossRef]
- Liu, M.Y.; Cokcetin, N.N.; Lu, J.; Turnbull, L.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Rifampicin-Manuka Honey Combinations Are Superior to Other Antibiotic-Manuka Honey Combinations in Eradicating Staphylococcus aureus Biofilms. Front. Microbiol. 2018, 8, 2653. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Salom, K.; Butler, G.; Al Ghamdi, A.A. Honey and microbial infections: A review supporting the use of honey for microbial control. J. Med. Food 2011, 14, 1079–1096. [Google Scholar] [CrossRef]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef]
- Gunduz, A.; Turedi, S.; Russell, R.M.; Ayaz, F.A. Clinical review of grayanotoxin/mad honey poisoning past and present. Clin. Toxicol. 2008, 46, 437–442. [Google Scholar] [CrossRef]
- Gunduz, A.; Turedi, S.; Oksuz, H. The honey, the poison, the weapon. Wilderness Environ. Med. 2011, 22, 182–184. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Mahendiran, B.; Gopalakrishnan, S.; Muthusamy, S.; Malarkodi Elangovan, S. Honey based treatment strategies for infected wounds and burns: A systematic review of recent pre-clinical research. Wound Med. 2020, 30, 100188. [Google Scholar] [CrossRef]
- Simon, A.; Traynor, K.; Santos, K.; Blaser, G.; Bode, U.; Molan, P. Medical honey for wound care-still the ‘latest resort’? Evid. Based Complement. Altern. Med. 2009, 6, 165–173. [Google Scholar] [CrossRef]
- Roberts, A.E.L.; Brown, H.L.; Jenkins, R. On the antibacterial effects of manuka honey: Mechanistic insights. Res. Rep. Biol. 2015, 6, 215–224. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. https://doi.org/10.3390/antibiotics9110774
Combarros-Fuertes P, Fresno JM, Estevinho MM, Sousa-Pimenta M, Tornadijo ME, Estevinho LM. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics. 2020; 9(11):774. https://doi.org/10.3390/antibiotics9110774
Chicago/Turabian StyleCombarros-Fuertes, Patricia, José M. Fresno, Maria Manuela Estevinho, Mário Sousa-Pimenta, M. Eugenia Tornadijo, and Leticia M. Estevinho. 2020. "Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria?" Antibiotics 9, no. 11: 774. https://doi.org/10.3390/antibiotics9110774
APA StyleCombarros-Fuertes, P., Fresno, J. M., Estevinho, M. M., Sousa-Pimenta, M., Tornadijo, M. E., & Estevinho, L. M. (2020). Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics, 9(11), 774. https://doi.org/10.3390/antibiotics9110774







