Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens
Abstract
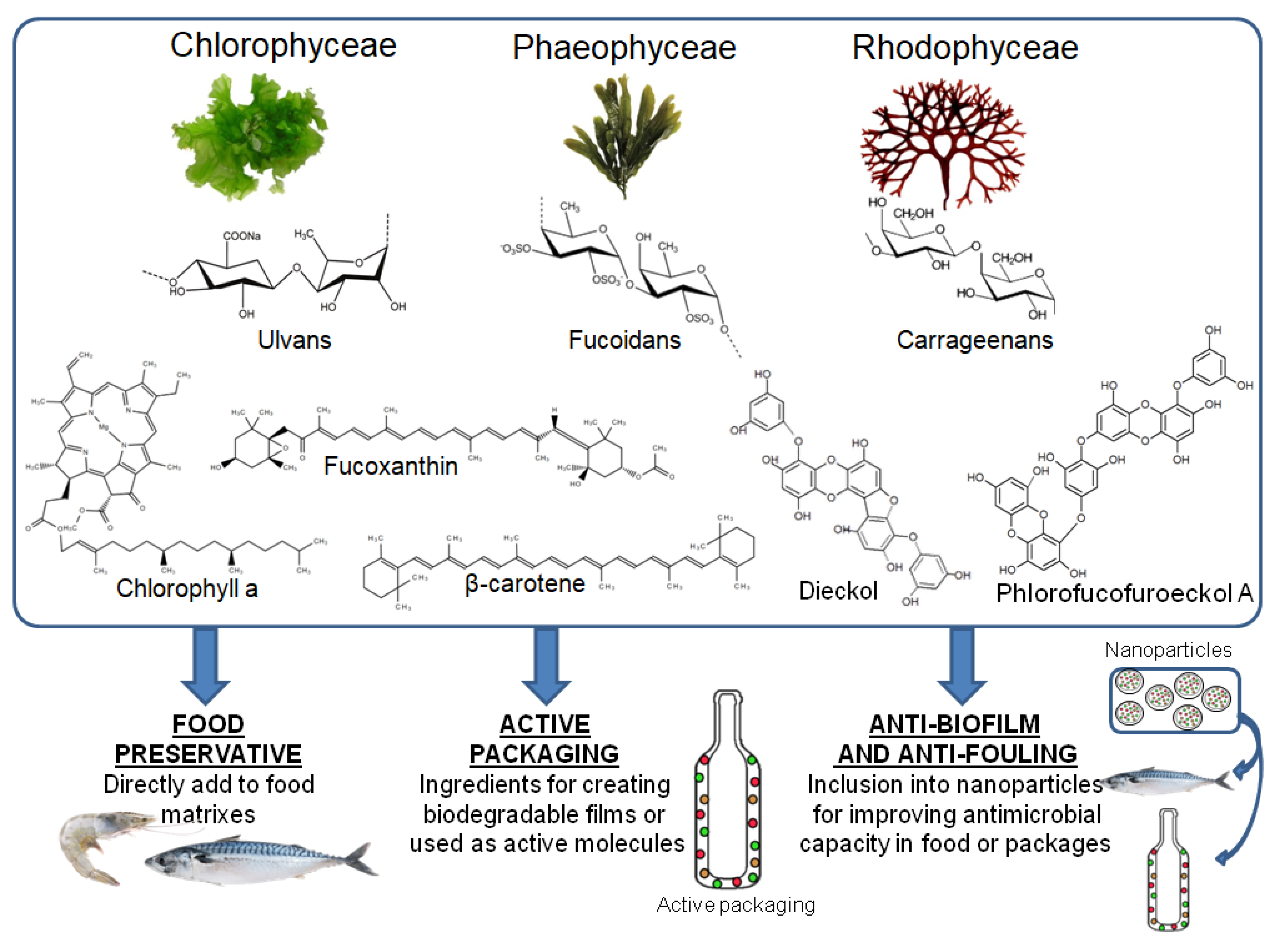
1. General View on Algae Compounds
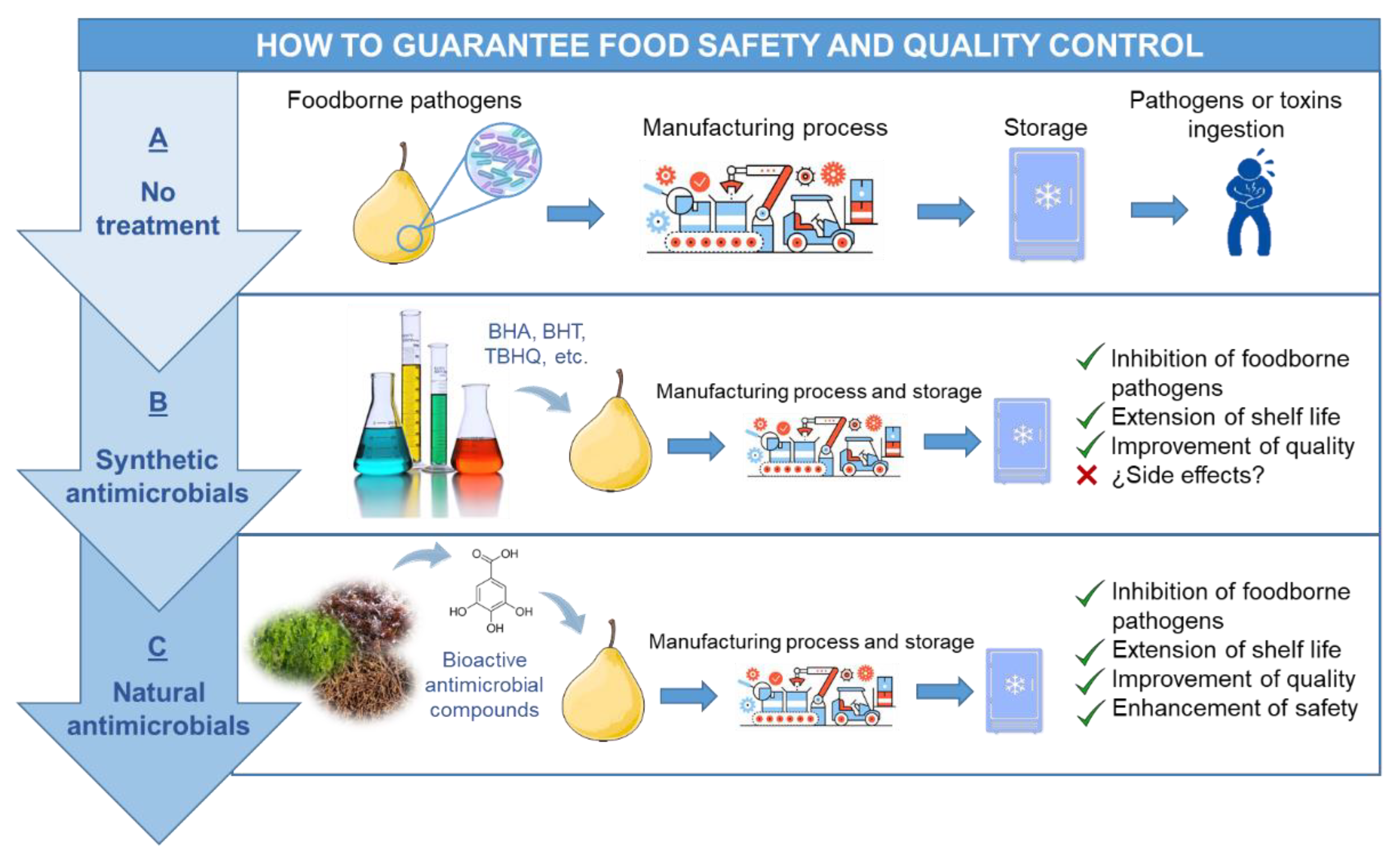
2. Microbial Contamination in Food Industry
3. Antibacterial Potential of Bioactive Compounds from Macroalgae against Pathogens and Spoilage Microorganisms
3.1. Evaluation Criteria
3.2. Gram Negative
3.2.1. Enterobacteriaceae
Escherichia coli
Enterobacter spp.
Shigella flexneri
Proteus spp.
Salmonella spp.
3.2.2. Pseudomonadaceae: Pseudomonas aeruginosa
3.3. Gram Positive
3.3.1. Staphylococcus aureus
3.3.2. Listeria monocytogenes
3.3.3. Enterococus spp.
3.3.4. Bacillus cereus
3.3.5. Streptococcus spp.
4. Incorporation to the Food Industry
4.1. Food Preservative
4.2. Active Packaging
4.3. Anti-Biofilm and Anti-Fouling
5. Food Safety and Quality Control Enhancement Using Antimicrobials from Seaweeds
6. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Generic | |
| ATCC | American Type Culture Collection |
| B | brown algae (Phaeophyta) |
| BAA | Asian Bacterial Bank |
| CLSI | Clinical and Laboratory Standards Institute |
| CFU | Colony Formation Unix |
| CMCC | China Medical Culture Collection Center |
| DSM | Deutsche Sammlung von Mikroorganismen |
| f | fraction |
| G | green algae (Chlorophyta) |
| FRAP | Ferric Antioxidant Power |
| MBC | minimal bactericide concentration |
| MIC | minimal inhibition concentration |
| MRSA | methicillin-resistant S. aureus |
| MTCC | Microbial Type Culture Collection and Gene Bank |
| NCIMB | National Collection of Industrial, Food and Marine Bacteria |
| nd | not determined |
| nt | not tested |
| NI | no Inhibition |
| PLA | Polylactic acid |
| PTCC | Persian Type Culture Collection |
| GMP | good manufacturing practices |
| HACCP | Hazard Analysis and Critical Control Point |
| I | intermediate 15–19 mm |
| IC50 | Half maximal inhibitory concentration |
| RCM | The Republic Collection of Microogrganisms |
| Re | resistant ≤ 14 mm |
| SSOP | sanitation standard operating procedure |
| R | red algae (Rhodophyta) |
| S | susceptible ≥ 20 mm |
| Compounds | |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AcO | acetone |
| n-BuOH | butanol |
| ClHx | ciclohexane |
| CHCl3 | chloroform |
| CO2 | carbon dioxide |
| DCM | dichloromethane |
| DMSO | dimethilsulfoxide |
| DIEt | diethyl ether |
| DPPH | 1,1-diphenyl-2-picryl hydrazyl |
| EtAc | ethylacetate |
| EtOAc | ethyl ethanoate |
| EtOH | ethanol |
| Hex | hexane |
| H2O | water |
| MeOH | methanol |
| n-Hex | n-hexane |
| PeEt | petroleum ether |
| PUFA | poly-unsaturated fatty acids |
| PolySA | polysaccharides agar |
References
- Ibrahim, M.; Salman, M.; Kamal, S.; Rehman, S.; Razzaq, A.; Akash, S.H. Algae-Based Biologically Active Compounds. In Algae Based Polymers, Blends, and Composites: Chemistry, Biotechnology and Materials Science; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 155–271. ISBN 9780128123607. [Google Scholar]
- Kandale, A.; Meena, A.K.; Rao, M.M.; Panda, P.; Mangal, A.K.; Reddy, G.; Babu, R. Marine Algae: An Introduction, Food Value and Medicinal Uses. J. Pharm. Res. 2011, 4, 219–221. [Google Scholar]
- Malhotra, S.; Singh, A.P. Algae, traditional medicine, and pharmacological advances. Int. J. Algae 2008, 10, 299–308. [Google Scholar] [CrossRef]
- Aknin, M.; Dogbevi, K.; Samb, A.; Kornprobst, J.M.; Gaydou, E.M.; Miralles, J. Fatty acid and sterol composition of eight brown algae from the Senegalese coast. Comp. Biochem. Physiol. Part B Biochem. 1992, 102B, 841–843. [Google Scholar] [CrossRef]
- Frikha, F.; Kammoun, M.; Hammami, N.; Mchirgui, R.; Belbahri, L.; Gargouri, Y.; Miled, N.; Ben, R.F. Chemical composition and some biological activities of marine algae collected in Tunisia. Cienc. Mar. 2011, 37, 113–124. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Patra, J.K.; Rath, S.K.; Jena, K.; Rathod, V.K.; Thatoi, H. Evaluation of antioxidant and antimicrobial activity of seaweed (Sargassum sp.) extract: A study on inhibition of glutathione-S-transferase activity. Turk. J. Biol. 2008, 32, 119–125. [Google Scholar]
- Agatonovic-kustrin, S.; Ramenskaya, G.; Kustrin, E.; Ortakand, D.B.; Morton, D.W. A new integrated HPTLC—ATR/FTIR approach in marine algae bioprofiling. J. Pharm. Biomed. Anal. 2020, 113488. [Google Scholar] [CrossRef]
- Andriani, Z.; Fasya, A.G.; Hanapi, A. Antibacterial Activity of the Red Algae Eucheuma cottonii Extract from Tanjung Coast, Sumenep Madura. Alchemy 2016, 4, 93–100. [Google Scholar] [CrossRef]
- Bittkau, K.S.; Neupane, S.; Alban, S. Initial evaluation of six different brown algae species as source for crude bioactive fucoidans. Algal Res. 2020, 45, 101759. [Google Scholar] [CrossRef]
- de Borba Gurpilhares, D.; Moreira, T.R.; da Luz Bueno, J.; Cinelli, L.P.; Mazzola, P.G.; Pessoa, A.; Sette, L.D. Algae’s sulfated polysaccharides modifications: Potential use of microbial enzymes. Process Biochem. 2016, 51, 989–998. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Wijesekara, I.; Kim, S.K.; Li, Y. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Desnoyers, M.; Gilbert, K.; Rousseau, G. Cardioprotective Effects of Omega-3 Polyunsaturated Fatty Acids: Dichotomy between Experimental and Clinical Studies. Mar. Drugs 2018, 16, 234. [Google Scholar] [CrossRef]
- Bhagavathy, S.; Sumathi, P.; Jancy Sherene Bell, I. Green algae Chlorococcum humicola- a new source of bioactive compounds with antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011, 1, S1. [Google Scholar] [CrossRef]
- Taskin, E.; Ozturk, M.; Taskin, E.; Kurt, O. Antibacterial activities of some marine algae from the Aegean Sea (Turkey). Afr. J. Biotechnol. 2007, 6, 2746–2751. [Google Scholar]
- Robinson, I.; Junqua, G.; Van Coillie, R.; Thomas, O. Trends in the detection of pharmaceutical products, and their impact and mitigation in water and wastewater in North America. Anal. Bioanal. Chem. 2007, 387, 1143–1151. [Google Scholar] [CrossRef]
- Kini, S.; Divyashree, M.; Mani, M.K.; Mamatha, B.S. Algae and cyanobacteria as a source of novel bioactive compounds for biomedical applications. In Advances in Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 173–194. [Google Scholar]
- Plaza, M.; Santoyo, S.; Jaime, L.; García-Blairsy Reina, G.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Prathima, A.; Periyasamy, M. Characteristics studies on Stoechospermum marginatum, brown marine algae with Al2O3 nanofluid. Mater. Today Proc. 2020, 2–6. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, W.I.; Lee, S.J. Fault tree analysis as a quantitative hazard analysis with a novel method for estimating the fault probability of microbial contamination: A model food case study. Food Control 2020, 110, 107019. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Microbial Contamination, Prevention, and Early Detection in Food Industry. In Microbial Contamination and Food Degradation; Elsevier: Cambridge, UK, 2018; pp. 21–47. [Google Scholar]
- Mani-López, E.; Palou, E.; López-Malo, A. Biopreservatives as Agents to Prevent Food Spoilage. In Microbial Contamination and Food Degradation; Elsevier: Cambridge, UK, 2018; pp. 235–270. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Akremi, N.; Cappoen, D.; Anthonissen, R.; Verschaeve, L.; Bouraoui, A. Phytochemical and in vitro antimicrobial and genotoxic activity in the brown algae Dictyopteris membranacea. S. Afr. J. Bot. 2017, 108, 308–314. [Google Scholar] [CrossRef]
- Peihang, X.U.; Huaqiang, T.A.N.; Weiguang, J.I.N. Antioxidative and antimicrobial activities of intertidal seaweeds and possible effects of abiotic factors on these bioactivities. J. Oceanol. Limnol. 2018, 36, 2243–2256. [Google Scholar]
- Rajauria, G.; Jaiswal, A.K.; Abu-gannam, N.; Gupta, S. Antioxidant and free radical-scavenging capacity of brown seaweed himanthalia elongata from western coast of Ireland. J. Food Biochem. 2012, 37, 1–14. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hiji, M. Antioxidant, antibacterial and in vivo wound healind properties of laminaran purified from Cystoseira barbata seaweed. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- CLSI M02-A12. Performance Standards for Antimicrobial Disk; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2015; Volume 32, ISBN 1562389858. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Tenover, F.C. Antibiotic Susceptibility Testing. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 67–77. [Google Scholar]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent Advances in Understanding Enteric Pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed]
- Ripolles-Avila, C.; Martínez-Garcia, M.; Capellas, M.; Yuste, J.; Fung, D.Y.C.; Rodríguez-Jerez, J. From hazard analysis to risk control using rapid methods in microbiology: A practical approach for the food industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1877–1907. [Google Scholar] [CrossRef]
- Authority, E.F.S. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Heiman, K.E.; Mody, R.K.; Johnson, S.D.; Griffin, P.M.; Gould, L.H. Escherichia coli O157 Outbreaks in the United States, 2003–2012. Emerg. Infect. Dis. 2015, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- De Alencar, D.B.; de Carvalho, F.C.T.; Rebouças, R.H.; dos Santos, D.R.; dos Santos Pires-Cavalcante, K.M.; de Lima, R.L.; Baracho, B.M.; Bezerra, R.M.; Viana, F.A.; dos Fernandes Vieira, R.H.S.; et al. Bioactive extracts of red seaweeds Pterocladiella capillacea and Osmundaria obtusiloba (Floridophyceae: Rhodophyta) with antioxidant and bacterial agglutination potential. Asian Pac. J. Trop. Med. 2016, 9, 372–379. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. J. Food Meas. Charact. 2019, 13, 330–338. [Google Scholar] [CrossRef]
- Stanojkovic, T.P.; Ranković, B.; Kosanić, M. Evaluation of antioxidant, antimicrobial and anticancer effects of three selected marine macroalgae. Rom. Biotechnol. Lett. 2018, 23, 13804–13813. [Google Scholar]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; Garc, R. Pressurized Liquid Extraction (PLE) as an Innovative Green Technology for the Effective Enrichment of Galician Algae Extracts with High Quality Fatty Acids and Antimicrobial and Antioxidant Properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef]
- Meillisa, A.; Siahaan, E.A.; Park, J.N.; Woo, H.C.; Chun, B.S. Effect of subcritical water hydrolysate in the brown seaweed Saccharina japonica as a potential antibacterial agent on food-borne pathogens. J. Appl. Phycol. 2013, 25, 763–769. [Google Scholar] [CrossRef]
- Trigui, M.; Gasmi, L.; Zouari, I. Seasonal variation in phenolic composition, antibacterial and antioxidant activities of Ulva rigida (Chlorophyta) and assessment of antiacetylcholinesterase potential. J. Appl. Phycol. 2013, 319–328. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological potential of marine macroalgae of the genus Cystoseira. Acta Biol. Hung. 2015, 66, 374–384. [Google Scholar] [CrossRef]
- Salem, W. Screening for antibacterial activities in some marine algae from the red sea (Hurghada, Egypt). Afr. J. Microbiol. Res. 2011, 5, 2160–2167. [Google Scholar] [CrossRef]
- Chong, C.W.; Hii, S.L.; Wong, C.L. Antibacterial activity of Sargassum polycystum C. Agardh and Padina australis Hauck (Phaeophyceae). Afr. J. Biotechnol. 2011, 10, 14125–14131. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Ilkhani, M.; Rustaiyan, A.; Larijani, K.; Sartavi, K.; Tahmasebi, R.; Asayesh, G. Antibacterial effect of the brown alga Cystoseira trinodis. J. Med. Plant Res. 2011, 5, 4654–4657. [Google Scholar]
- Ertürk, Ö.; Ta, B. Antibacterial and Antifungal Effects of Some Marine Algae Makale Kodu. Kafkas Univ. Vet. Fak. Derg. 2011, 17, 121–124. [Google Scholar]
- Milović, S.; Kundaković, T.; Mačić, V.; Stanković, J.A.; Grozdanić, N.; Đuričić, I.; Stanković, I.; Stanojković, T. Anti α-glucosidase, antitumour, antioxidative, antimicrobial activity, nutritive and health protective potential of some seaweeds from the Adriatic coast of Montenegro. Farmacia 2017, 65, 731–740. [Google Scholar]
- Osman, N.A.H.K.; Siam, A.A.; El-manawy, I.M.; Jeon, Y. Anti-microbial and Anti-diabetic Activity of Six Seaweeds Collected from the Red Sea, Egypt. Catrina Int. J. Environ. Sci. 2019, 19, 55–60. [Google Scholar]
- Alves, C.; Pinteus, S.; Simões, T.; Horta, A.; Silva, J.; Tecelão, C.; Pedrosa, R. Bifurcaria bifurcata: A key macro-alga as a source of bioactive compounds and functional ingredients. Int. J. Food Sci. Technol. 2016, 51, 1638–1646. [Google Scholar] [CrossRef]
- Taskin, E.; Caki, Z.; Ozturk, M.; Taskin, E. Assessment of in vitro antitumoral and antimicrobial activities of marine algae harvested from the eastern Mediterranean sea. Afr. J. Biotechnol. 2010, 9, 4272–4277. [Google Scholar] [CrossRef]
- Zeid, A.H.A.; Aboutabl, E.A.; Sleem, A.A.; El-rafie, H.M. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carbohydr. Polym. 2014, 113, 62–66. [Google Scholar] [CrossRef]
- Al Khazan, M.M.; Omar, H.H.; Gumgumjee, N.M.; Shiekh, H.M.; El-Gendy, A.M. Marine macroalgae as a potential source of bioactive natural products with antibacterial activity. Main Gr. Chem. 2016, 15, 139–151. [Google Scholar] [CrossRef]
- Ambreen, A.; Hira, K.; Ruqqia, A.; Sultana, V. Evaluation of biochemical component and antimicrobial activity of some seaweeeds occurring at Karachi coast. Pak. J. Bot. 2012, 44, 1799–1803. [Google Scholar]
- Omar, H.H.; Shiekh, H.M.; Gumgumjee, N.M. Antibacterial activity of extracts of marine algae from the Red Sea of Jeddah, Saudi Arabia. Afr. J. Biotechnol. 2012, 11, 13576–13585. [Google Scholar] [CrossRef]
- Aoun, Z.B.; Said, R.B.; Farhat, F. Anti-inflammatory, antioxidant and antimicrobial activities of aqueous and organic extracts from Dictyopteris membranacea. Bot. Mar. 2010, 53, 259–264. [Google Scholar] [CrossRef]
- Karima, S.; Fatiha, B. Study of the antimicrobial activity of four algerian marin algae species. In Microbes in Applied Research; World Scientific: Singapore, 2012; pp. 578–581. [Google Scholar]
- Arulkumar, A.; Rosemary, T.; Paramasivam, S.; Rajendran, R.B. Phytochemical composition, in vitro antioxidant, antibacterial potential and GC-MS analysis of red seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay, India. Biocatal. Agric. Biotechnol. 2018, 15, 63–71. [Google Scholar] [CrossRef]
- Omar, H.H.; Al-Judaibiand, A.; El-Gendy, A. Antimicrobial, antioxidant, anticancer activity and phytochemical analysis of the red alga, laurencia papillosa. Int. J. Pharmacol. 2018, 14, 572–583. [Google Scholar] [CrossRef]
- Manilal, A.; Sujith, S.; Sabarathnam, B.; Kiran, G.S.; Selvin, J.; Shakir, C.; Lipton, A.P. Biological activity of the red alga Laurencia brandenii. Acta Bot. Croat. 2011, 70, 81–90. [Google Scholar] [CrossRef]
- Dos Santos Amorim, R.D.N.; Rodrigues, J.A.G.; Holanda, M.L.; Quinderé, A.L.G.; de Paula, R.C.M.; Melo, V.M.M.; Benevides, N.M.B. Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed gracilaria ornata. Braz. Arch. Biol. Technol. 2012, 55, 171–181. [Google Scholar] [CrossRef]
- Assaw, S.; Rosli, N.L.; Azmi, N.A.M.; Mazlan, N.W.; Ismail, N. Antioxidant and antibacterial activities of polysaccharides and methanolic crude extracts of local edible red seaweed Gracilaria sp. Malays. Appl. Biol. 2018, 47, 135–144. [Google Scholar]
- Mashjoor, S.; Yousefzadi, M.; Esmaeili, M.A.; Rafiee, R. Cytotoxicity and antimicrobial activity of marine macro algae (Dictyotaceae and Ulvaceae) from the Persian Gulf. Cytotechnology 2016, 68, 1717–1726. [Google Scholar] [CrossRef]
- Sirbu, R.; Stanciu, G.; Tomescu, A.; Ionescu, A.M.; Cadar, E. Evaluation of antioxidant and antimicrobial activity in relation to total phenolic content of green algae from Black Sea. Rev. Chim. 2019, 70, 1197–1203. [Google Scholar] [CrossRef]
- Abdel-Latif, H.H.; Shams El-Din, N.G.; Ibrahim, H.A.H. Antimicrobial activity of the newly recorded red alga Grateloupia doryphora collected from the Eastern Harbor, Alexandria, Egypt. J. Appl. Microbiol. 2018, 125, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Goecke, F.; Labes, A.; Wiese, J.; Imhoff, J.F. Dual effect of macroalgal extracts on growth of bacteria in Western Baltic Sea. Rev. Biol. Mar. Oceanogr. 2012, 47, 75–86. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Mohamed, H.; Mostafa, S.; Ibraheem, I. Controlling of Microbial Growth by Using Cystoseira barbata Extract. Egypt. J. Bot. 2017, 57, 469–477. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Antioxidant and antibacterial activity of red seaweed; Kappaphycus alvarezii against pathogenic bacteria. Glob. J. Environ. Sci. Manag. 2020, 6, 47–58. [Google Scholar] [CrossRef]
- Munir, N.; Rafique, M.; Altaf, I.; Sharif, N.; Naz, S. Antioxidant and antimicrobial activities of extracts from selected algal species. Bangladesh J. Bot. 2018, 47, 53–61. [Google Scholar]
- Jassbi, A.R.; Mohabati, M.; Eslami, S.; Sohrabipour, J.; Miri, R. Biological activity and chemical constituents of red and brown algae from the Persian Gulf. Iran. J. Pharm. Res. 2013, 12, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.K.; Kumar, Y.; Yar, K.M.S.; Gupta, V.; de Clercq, E. Antimicrobial and cytotoxic activities of Turbinaria conoides (J.Agardh) Kuetz. Iran. J. Pharm. Res. 2010, 9, 411–416. [Google Scholar] [CrossRef]
- Selim, S.; Amin, A.; Hassan, S.; Hagazey, M. Antibacterial, cytotoxicity and anticoagulant activities from Hypnea esperi and Caulerpa prolifera marine algae. Pak. J. Pharm. Sci. 2015, 28, 525–530. [Google Scholar]
- Rahelivao, M.P.; Gruner, M.; Andriamanantoanina, H.; Bauer, I.; Knölker, H.-J. Brown Algae (Phaeophyceae) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products. Nat. Prod. Bioprospect. 2015, 5, 223–235. [Google Scholar] [CrossRef]
- Rahelivao, M.P.; Gruner, M.; Andriamanantoanina, H.; Andriamihaja, B.; Bauer, I.; Knölker, H. Red Algae (Rhodophyta) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products. Mar. Drugs 2015, 13, 4197–4216. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Truong, H.B.; Ha, N.; Tran, V.; Thu, T.M. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2017, 6419, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zouaoui, B.; Raho, B. The Phenolic Contents and Antimicrobial Activities of Some Marine Algae from the Mediterranean Sea (Algeria) 1. Russ. J. Mar. Biol. 2017, 43, 491–495. [Google Scholar] [CrossRef]
- Ismail, A.; Ktari, L.; Ben Redjem Romdhane, Y.; Aoun, B.; Sadok, S.; Boudabous, A.; El Bour, M. Antimicrobial Fatty Acids from Green Alga Ulva rigida (Chlorophyta). Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Rhimou, B.; Hassane, R.; José, M.; Nathalie, B. The antibacterial potential of the seaweeds (Rhodophyaceae) of the Strait of Gibraltar and the Mediterranean coast of Morocco. Afr. J. Biotechnol. 2010, 9, 6365–6372. [Google Scholar] [CrossRef]
- Chnadra, S. Textbook of Microbiology & Immunology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Igo, M.; Schaffner, D. Quantifying the Influence of Relative Humidity, Temperature, and Diluent on the Survival and Growth of Enterobacter aerogenes. J. Food Prot. 2019, 82, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Yamane, R.; Dang, V.C.; Nguyen, D.P.; Nguyen, T.A.D.; Jinnai, M.; Yonogi, S.; Kawahara, R.; Kanki, M.; Kawai, T.; et al. Prevalence and Antimicrobial Susceptibility of Enterobacteriaceae Isolated from Retail Pepper in Vietnam. J. Food Prot. 2016, 80, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.R.; Parish, M.E.; Schneider, K.R. Shigella as a Foodborne Pathogen and Current Methods for Detection in Food. Crit. Rev. Food Sci. Nutr. 2006, 46, 551–567. [Google Scholar] [CrossRef]
- Taneja, N.; Mewara, A. Shigellosis: Epidemiology in India. Indian J. Med. Res. 2016, 143, 565. [Google Scholar] [CrossRef]
- Alizadeh-Hesar, M.; Bakhshi, B.; Najar-Peerayeh, S. Clonal dissemination of a single Shigella sonnei strain among Iranian children during Fall 2012 in Tehran, I.R. Iran. Infect. Genet. Evol. 2015, 34, 260–266. [Google Scholar] [CrossRef]
- El Shafay, S.M.; Ali, S.S.; El-Sheekh, M.M. Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 2016, 42, 65–74. [Google Scholar] [CrossRef]
- Różalski, A.; Torzewska, A.; Moryl, M.; Kwil, I.; Maszewska, A.; Ostrowska, K.; Drzewiecka, D.; Zabłotni, A.; Palusiak, A.; Siwińska, M.; et al. Proteus sp.—An opportunistic bacterial pathogen—Classification, swarming growth, clinical significance and virulence factors. Folia Biol. Oecol. 2012, 8, 1–17. [Google Scholar] [CrossRef]
- Drzewiecka, D. Significance and Roles of Proteus spp. Bacteria in Natural Environments. Microb. Ecol. 2016, 72, 741–758. [Google Scholar] [CrossRef]
- Gong, Z.; Shi, X.; Bai, F.; He, X.; Zhang, H.; Li, Y.; Wan, Y.; Lin, Y.; Qiu, Y.; Chen, Q.; et al. Characterization of a Novel Diarrheagenic Strain of Proteus mirabilis Associated With Food Poisoning in China. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.-W.; Zhang, A.-Y.; Liu, B.-H.; Wang, H.-N.; Guan, Z.-B.; Xu, C.-W.; Xia, Q.-Q.; Cheng, H.; Zhang, D.-D. Molecular Characteristics of Salmonella Genomic Island 1 in Proteus mirabilis Isolates from Poultry Farms in China. Antimicrob. Agents Chemother. 2014, 58, 7570–7572. [Google Scholar] [CrossRef]
- Lei, C.-W.; Zhang, A.-Y.; Wang, H.-N.; Liu, B.-H.; Yang, L.-Q.; Yang, Y.-Q. Characterization of SXT/R391 Integrative and Conjugative Elements in Proteus mirabilis Isolates from Food-Producing Animals in China. Antimicrob. Agents Chemother. 2016, 60, 1935–1938. [Google Scholar] [CrossRef]
- Oni, V.; Oni, A.; Esumeh, F. Prevalence of Bacteria food poison from vegetable salads. Internet J. Nnutr. Wellness. 2009, 10, 1–5. [Google Scholar]
- Li, X.; Du, Y.; Du, P.; Dai, H.; Fang, Y.; Li, Z.; Lv, N.; Zhu, B.; Kan, B.; Wang, D. SXT/R391 integrative and conjugative elements in Proteus species reveal abundant genetic diversity and multidrug resistance. Sci. Rep. 2016, 6, 37372. [Google Scholar] [CrossRef]
- Anibijuwon, I.I.; Gbala, I.D.; Abioye, J.A.; Ogunlade, P.O. Antibiotic Sensitivity and Evaluation of Plasmid Profile of Major Foodborne Pathogens. J. Health Allied Sci. NU 2016, 6, 4–9. [Google Scholar] [CrossRef]
- Al-Enazi, N.M.; Awaad, A.S.; Alqasoumi, S.I.; Alwethairi, M.F. Biological Activities of the Red Algae Galaxaura Rugosa and Liagora Hawaiiana Butters. Saudi Pharm. J. 2018, 26, 25–32. [Google Scholar] [CrossRef]
- Moorthi, P.V. Antimicrobial properties of marine seaweed, Sargassum muticum against human pathogens. J. Coast. Life Med. 2015. [Google Scholar] [CrossRef]
- Boonchum, W.; Peerapornpisal, Y.; Kanjanapothi, D.; Pekkoh, J.; Amornlerdpison, D.; Pumas, C.; Sangpaiboon, P.; Vacharapiyasophon, P. Antimicrobial and anti-inflammatory properties of various seaweeds from the Gulf of Thailand. Int. J. Agric. Biol. 2011, 13, 100–104. [Google Scholar]
- Maheswari, M.U.; Reena, A.; Sivaraj, C. GC-MS analysis, antioxidant and antibacterial activity of the brown algae, Padina tetrastromatica. Int. J. Pharm. Sci. Res. 2017, 41, 84–93. [Google Scholar] [CrossRef]
- Manivannan, K.; Karthikai, G.; Anantharaman, P.; Balasubramanian, T. Antimicrobial potential of selected brown seaweeds from Vedalai coastal waters, Gulf of Mannar. Asian Pac. J. Trop. Biomed. 2011, 1, 114–120. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Lines, C.C. Anti-Proliferative Activity of Meroditerpenoids Isolated from the Brown Alga Stypopodium flabelliforme against Several cancer cell lines. Mar. Drugs 2011, 852–862. [Google Scholar] [CrossRef]
- Richardson, H.; Smaill, F. Recent advances: Medical microbiology. BMJ 1998, 317, 1060–1062. [Google Scholar] [CrossRef]
- Lund, B.M. Provision of microbiologically safe food for vulnerable people in hospitals, care homes and in the community. Food Control 2019, 96, 535–547. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Q.; Zhang, J.; Huang, J.; Chen, L.; Wu, S.; Zeng, H.; Wang, J.; Chen, M.; Wu, H.; et al. Prevalence, Bacterial Load, and Antimicrobial Resistance of Salmonella Serovars Isolated From Retail Meat and Meat Products in China. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Plumb, I.D.; Schwensohn, C.A.; Gieraltowski, L.; Tecle, S.; Schneider, Z.D.; Freiman, J.; Cote, A.; Noveroske, D.; Kolsin, J.; Brandenburg, J.; et al. Outbreak of Salmonella Newport Infections with Decreased Susceptibility to Azithromycin Linked to Beef Obtained in the United States and Soft Cheese Obtained in Mexico—United States, 2018–2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 713–717. [Google Scholar] [CrossRef]
- Asfaw Ali, D.; Tadesse, B.; Ebabu, A. Prevalence and Antibiotic Resistance Pattern of Salmonella Isolated from Caecal Contents of Exotic Chicken in Debre Zeit and Modjo, Ethiopia. Int. J. Microbiol. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Kang, O.; Brice, O.; Lee, Y.; Chae, H. Antibacterial Activity of Ecklonia cava Against. Foodborne Pathog. Dis. 2010, 7, 435–441. [Google Scholar] [CrossRef]
- Stoler, J.; Ahmed, H.; Asantewa Frimpong, L.; Bello, M. Presence of Pseudomonas aeruginosa in coliform-free sachet drinking water in Ghana. Food Control 2015, 55, 242–247. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019; pp. 1–113. [CrossRef]
- Oludairo, O.O.; Aiyedun, J.O. Contamination of Commercially Packaged Sachet Water and the Public. Bangl. J. Vet. Med 2015, 13, 73–81. [Google Scholar] [CrossRef]
- Banu, N.; Menakuru, H. Enumeration of microbial contaminants in sachet water: A public health challenge. Health (Irvine. Calif). 2010, 2, 582–588. [Google Scholar] [CrossRef]
- Study, A.C.; Ibemesim, B.; Obinna, A. Comparative Study of Qualities of Sachet and Bottle Water Sold on the Streets of Abuja, Nigeria. S. Am. J. Public Health 2014, 2, 308–328. [Google Scholar]
- Mgbakor, C.; Ojiegbe, G.C.; Okonko, I.O.; Odu, N.N.; Alli, J.A.; Nwanze, J.C.; Onoh, C.C. Bacteriological evaluation of some sachet water on sales in Owerri metropolis, Imo State, Nigeria. Malays. J. Microbiol. 2011, 7, 217–225. [Google Scholar] [CrossRef]
- Brown, N.M.; Arbon, J.; Redpath, C. Contamination of milk-bank samples with Pseudomonas aeruginosa during pasteurization by penetration of organisms through the screw lid during cooling. J. Hosp. Infect. 2000, 46, 321–322. [Google Scholar] [CrossRef]
- Maggs, C.A.; Gilmore, B.F. Antibiofilm Activity of the Brown Alga Halidrys siliquosa against Clinically Relevant Human Pathogens. Mar. Drugs 2015, 13, 3581–3605. [Google Scholar] [CrossRef]
- Moubayed, N.M.S.; Al Houri, H.J.; Al Khulaifi, M.M.; Al Farraj, D.A. Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf). Saudi J. Biol. Sci. 2017, 24, 162–169. [Google Scholar] [CrossRef]
- Sasidharan, S.; Darah, I.; Noordin, M.K.M.J. In Vitro antimicrobial activity against Pseudomonas aeruginosa and acute oral toxicity of marine algae Gracilaria changii. New Biotechnol. 2010, 27, 390–396. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Cecere, E.; Petrocelli, A. Biotechnological potential of the seaweed Cladophora rupestris (Chlorophyta, Cladophorales) lipidic extract. New Biotechnol. 2014, 31, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Eom, S.-H.; Lee, E.-H.; Jung, Y.-J.; Kim, H.-J.; Jo, M.-R.; Son, K.-T.; Lee, H.-J.; Kim, J.H.; Lee, M.-S.; et al. In Vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Loir, Y.L.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2003, 7–28. [Google Scholar] [CrossRef]
- Atanassova, V.; Meindl, A.; Ring, C. Prevalence of Staphylococcus aureus and staphylococcal enterotoxins in raw pork and uncooked smoked ham—A comparison of classical culturing detection and RFLP-PCR. Int. J. Food Microbiol. 2001, 68, 105–113. [Google Scholar] [CrossRef]
- Fisher, E.L.; Otto, M.; Cheung, G.Y.C. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front. Microbiol. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today. 2018, 23, 434–447. [Google Scholar] [CrossRef]
- Benassai, E.; Del Bubba, M.; Ancillotti, C.; Colzi, I.; Gonnelli, C.; Calisi, N.; Salvatici, M.C.; Casalone, E.; Ristori, S. Green and cost-effective synthesis of cooper nanoparticles by extract of non-edible and waste plant material from Vaccinium species: Characterization and antimicrobial activity. Mater. Aci. Eng. C 2021, 119, 111453. [Google Scholar] [CrossRef]
- Águila-Ramírez, R.N.; Arenas-González, A.; Hernández-Guerrero, C.J.; González-Acosta, B.; Borges-Souza, J.M.; Véron, B.; Pope, J.; Hellio, C. Antimicrobial and antifouling activities achieved by extracts of seaweeds from Gulf of California, Mexico. Hidrobiologica 2012, 22, 8–15. [Google Scholar]
- Massironi, A.; Morelli, A.; Grassi, L.; Puppi, D.; Braccini, S.; Maisetta, G.; Esin, S.; Batoni, G.; Della Pinta, C.; Chiellini, F. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: Application to green synthesis of sillver nanoparticles. Carbohydr. Polym. 2019, 203, 310–321. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Pouyan, M.; Zandi, K.; Bahramian, P.; Sartavi, K.; Fouladvand, M.; Asayesh, G.; Barazesh, A. In Vitro study of antibacterial activity of the alga Sargassum oligocystum from the Persian Gulf. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 293–298. [Google Scholar]
- Ghania, A.; Nabila, B.B.; Larbi, B.; Elisabeth, M.; Philippe, G.; Mariem, B.; Khadidja, K.K.; Wacila, B.R.; Fawzia, A.B. Antimicrobial and antiparasitic activities of three algae from the northwest coast of Algeria. Nat. Prod. Res. 2019, 33, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Hoare, A.H.; Tan, S.P.; Mcloughlin, P.; Mulhare, P.; Hughes, H. The Screening and Evaluation of Fucus serratus and Fucus vesiculosus Extracts against Current Strains of MRSA Isolated from a Clinical Hospital Setting. Sci. Rep. 2019, 1–9. [Google Scholar] [CrossRef]
- Baliano, A.P.; Pimentel, E.F.; Buzin, A.R.; Vieira, T.Z.; Romão, W.; Tose, L.V.; Lenz, D.; de Andrade, T.U.; Fronza, M.; Kondratyuk, T.P.; et al. Brown seaweed Padina gymnospora is a prominent natural wound-care product. Braz. J. Pharmacogn. 2016, 26, 714–719. [Google Scholar] [CrossRef]
- Iannetti, L.; Schirone, M.; Neri, D.; Visciano, P.; Acciari, V.A.; Centorotola, G.; Mangieri, M.S.; Torresi, M.; Santarelli, G.A.; Di Marzio, V.; et al. Listeria monocytogenes in poultry: Detection and strain characterization along an integrated production chain in Italy. Food Microbiol. 2020, 91, 103533. [Google Scholar] [CrossRef] [PubMed]
- McLauchlin, J.; Mitchell, R.T.; Smerdon, W.J.; Jewell, K. Listeria monocytogenes and listeriosis: A review of hazard characterisation for use in microbiological risk assessment of foods. Int. J. Food Microbiol. 2004, 92, 15–33. [Google Scholar] [CrossRef]
- Szymczak, B.; Szymczak, M.; Trafiałek, J. Prevalence of Listeria species and L. monocytogenes in ready-to-eat foods in the West Pomeranian region of Poland: Correlations between the contamination level, serogroups, ingredients, and producers. Food Microbiol. 2020, 91, 103532. [Google Scholar] [CrossRef]
- Jackson, K.A.; Gould, L.H.; Hunter, J.C.; Kucerova, Z.; Jackson, B. Listeriosis Outbreaks Associated with Soft Cheeses, United States, 1998–20141. Emerg. Infect. Dis. 2018, 24, 1116–1118. [Google Scholar] [CrossRef]
- Cox, S. An Assessment of the Antioxidant and Antimicrobial Activity of Six Species of Edible Irish Seaweeds. Int. Food Res. J. 2010. [Google Scholar] [CrossRef]
- Terra, M.R.; Costa, L.C.; Dardaque, R.M.; Furlaneto, M.C.; Furlaneto-maia, L. Food as a potencial reservoir of enterococcus that host dererminants of virulence and resistence. Braz. J. Surg. Clin. Res. BJSCR 2018, 22, 86–93. [Google Scholar]
- Nami, Y.; Bakhshayesh, R.V.; Jalaly, H.M.; Lotfi, H.; Eslami, S.; Hejazi, M.A. Probiotic properties of enterococcus isolated from artisanal dairy products. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Murray, B.E. The life and times of the enterococcus. Clin. Microbiol. Rev. 1990, 3, 46–65. [Google Scholar] [CrossRef]
- Giraffa, G. Enterococci from foods. FEMS Microbiol. Rev. 2002, 26, 163–171. [Google Scholar] [CrossRef]
- Ismail, A.; Ktari, L.; Ahmed, M.; Bolhuis, H.; Bouhaouala-Zahar, B.; Stal, L.J.; Boudabbous, A.; El Bour, M. Heterotrophic bacteria associated with the green alga Ulva rigida: Identification and antimicrobial potential. J. Appl. Phycol. 2018, 30, 2883–2899. [Google Scholar] [CrossRef]
- Chorin, E.; Thuault, D.; Cléret, J.-J.; Bourgeois, C.-M. Modelling Bacillus cereus growth. Int. J. Food Microbiol. 1997, 38, 229–234. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus spores and toxins—The potential role of biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, J.; Fei, P.; Feng, H.; Wang, Y.; Ali, M.A.; Li, S.; Jing, H.; Yang, W. Prevalence, molecular characterization, and antibiotic susceptibility of Bacillus cereus isolated from dairy products in China. J. Dairy Sci. 2020, 103, 3994–4001. [Google Scholar] [CrossRef]
- Yu, P.; Yu, S.; Wang, J.; Guo, H.; Zhang, Y.; Liao, X.; Zhang, J.; Wu, S.; Gu, Q.; Xue, L.; et al. Bacillus cereusIsolated from vegetables in China: Incidence, genetic diversity, virulence genes, and antimicrobial resistance. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Antibacterial activity and action mechanism of the essential oil from enteromorpha linza L. against foodborne pathogenic bacteria. Molecules 2016, 21, 388. [Google Scholar] [CrossRef] [PubMed]
- Alves-Barroco, C.; Roma-Rodrigues, C.; Raposo, L.R.; Brás, C.; Diniz, M.; Caço, J.; Costa, P.M.; Santos-Sanches, I.; Fernandes, A.R. Streptococcus dysgalactiae subsp. dysgalactiae isolated from milk of the bovine udder as emerging pathogens: In Vitro and in vivo infection of human cells and zebrafish as biological models. Microbiologyopen 2019, 8, e00623. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kawahara, R.; Katsukawa, C.; Kanki, M.; Harada, T.; Yonogi, S.; Iwasaki, S.; Uehara, H.; Okajima, S.; Nishimura, H.; et al. Foodborne Outbreak of Group G Streptococcal Pharyngitis in a School Dormitory in Osaka, Japan. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, A.A.; Rocha, C.M.B.M.; Bruhn, F.R.P.; Custódio, D.A.C.; Braz, M.S.; Pinto, S.M.; Silva, D.B.; Costa, G.M. Staphylococcus aureus and Streptococcus agalactiae: Prevalence, resistance to antimicrobials, and their relationship with the milk quality of dairy cattle herds in Minas Gerais state, Brazil. Pesqui. Vet. Bras. 2019, 39, 308–316. [Google Scholar] [CrossRef]
- Riekerink, R.G.M.O.; Barkema, H.W.; Veenstra, S.; Poole, D.E.; Dingwell, R.T.; Keefe, G.P. Prevalence of contagious mastitis pathogens in bulk tank milk in Prince Edward Island. Can. Vet. J. 2006, 46, 567–572. [Google Scholar]
- Jans, C.; Kaindi, D.W.M.; Böck, D.; Njage, P.M.K.; Kouamé-Sina, S.M.; Bonfoh, B.; Lacroix, C.; Meile, L. Prevalence and comparison of Streptococcus infantarius subsp. infantarius and Streptococcus gallolyticus subsp. macedonicus in raw and fermented dairy products from East and West Africa. Int. J. Food Microbiol. 2013, 167, 186–195. [Google Scholar] [CrossRef]
- Manilal, A.; Sujith, S.; Kiran, G.S.; Selvin, J.; Shakir, C.; Gandhimathi, R.; Lipton, A.P. Antimicrobial potential and seasonality of red algae collected from the southwest coast of India tested against shrimp, human and phytopathogens. Ann. Microbiol. 2009, 59, 207–219. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Petrocelli, A.; Cecere, E. The lipidic extract of the seaweed Gracilariopsis longissima (Rhodophyta, Gracilariales): A potential resource for biotechnological purposes? New Biotechnol. 2012, 29, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Berri, M.; Slugocki, C.; Olivier, M.; Helloin, E.; Jacques, I.; Salmon, H.; Demais, H.; Le Goff, M.; Collen, P.N. Marine-sulfated polysaccharides extract of Ulva armoricana green algae exhibits an antimicrobial activity and stimulates cytokine expression by intestinal epithelial cells. J. Appl. Phycol. 2016, 28, 2999–3008. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abushady, A.M.; Elshobary, M.E. In Vitro screening of antimicrobial activity of extracts of some macroalgae collected from Abu-Qir bay Alexandria, Egypt. Afr. J. Biotechnol. 2010, 9, 7203–7208. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Safafar, H.; van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviosson, G.O.; Karlsson, E.N. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Vuong, D.; Kaplan, M.; Lacey, H.J.; Crombie, A.; Lacey, E.; Piggott, A.M. A study of the chemical diversity of macroalgae from South Eastern Australia. Fitoterapia 2018, 126, 53–64. [Google Scholar] [CrossRef] [PubMed]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2008, 65, 535–543. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Li, J. Shelf-life extension of Pacific white shrimp using algae extracts during refrigerated storage. J. Sci. Food Agric. 2017, 97, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Arulkumar, A.; Paramasivam, S.; Miranda, J.M. Combined Effect of Icing Medium and Red Alga Gracilaria verrucosa on Shelf Life Extension of Indian Mackerel (Rastrelliger kanagurta). Food Bioprocess Technol. 2018, 11, 1911–1922. [Google Scholar] [CrossRef]
- Oucif, H.; Miranda, J.M.; Mehidi, S.A.; Abi-Ayad, S.-M.E.-A.; Barros-Velázquez, J.; Aubourg, S.P. Effectiveness of a combined ethanol–aqueous extract of alga Cystoseira compressa for the quality enhancement of a chilled fatty fish species. Eur. Food Res. Technol. 2018, 244, 291–299. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Campos, C.A.; Aubourg, S.P. Preservative Effect of Algae Extracts on Lipid Composition and Rancidity Development in Brine-Canned Atlantic Chub Mackerel (Scomber colias). Eur. J. Lipid Sci. Technol. 2019, 121, 1900129. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Gómez-Guillén, M.C.; Montero, M.P. Integral Mastocarpus stellatus use for antioxidant edible film development. Food Hydrocoll. 2014, 40, 128–137. [Google Scholar] [CrossRef]
- Alemán, A.; Blanco-Pascual, N.; Montero, M.P.; Gómez-Guillén, M.C. Simple and efficient hydrolysis procedure for full utilization of the seaweed Mastocarpus stellatus to produce antioxidant films. Food Hydrocoll. 2016, 56, 277–284. [Google Scholar] [CrossRef]
- García-Soto, B.; Miranda, J.M.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Rodríguez-Martínez, A.V.; Barros-Velázquez, J.; Aubourg, S.P. Effect of biodegradable film (lyophilised alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lepidorhombus whiffiagonis). Int. J. Food Sci. Technol. 2015, 50, 1891–1900. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, A.V.; Sendón, R.; Abad, M.J.; González-Rodríguez, M.V.; Barros-Velázquez, J.; Aubourg, S.P.; Paseiro-Losada, P.; Rodríguez-Bernaldo de Quirós, A. Migration kinetics of sorbic acid from polylactic acid and seaweed based films into food simulants. LWT Food Sci. Technol. 2016, 65, 630–636. [Google Scholar] [CrossRef]
- Gomaa, M.; Fawzy, M.A.; Hifney, A.F.; Abdel-Gawad, K.M. Use of the brown seaweed Sargassum latifolium in the design of alginate-fucoidan based films with natural antioxidant properties and kinetic modeling of moisture sorption and polyphenolic release. Food Hydrocoll. 2018, 82, 64–72. [Google Scholar] [CrossRef]
- Gomaa, M.; Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M. Use of seaweed and filamentous fungus derived polysaccharides in the development of alginate-chitosan edible films containing fucoidan: Study of moisture sorption, polyphenol release and antioxidant properties. Food Hydrocoll. 2018, 82, 239–247. [Google Scholar] [CrossRef]
- Doh, H.; Dunno, K.D.; Whiteside, W.S. Preparation of novel seaweed nanocomposite film from brown seaweeds Laminaria japonica and Sargassum natans. Food Hydrocoll. 2020, 105, 105744. [Google Scholar] [CrossRef]
- He, Q.; Huang, Y.; Lin, B.; Wang, S. A nanocomposite film fabricated with simultaneously extracted protein-polysaccharide from a marine alga and TiO2 nanoparticles. J. Appl. Phycol. 2017, 29, 1541–1552. [Google Scholar] [CrossRef]
- Augusto, A.; Dias, J.R.; Campos, M.J.; Alves, N.M.; Pedrosa, R.; Silva, S.F.J. Influence of Codium tomentosum Extract in the Properties of Alginate and Chitosan Edible Films. Foods 2018, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J. Development of antioxidant and antimicrobial packaging films based on chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and silicon dioxide nanoparticles. Food Packag. Shelf Life 2020, 24, 100503. [Google Scholar] [CrossRef]
- Junter, G.-A.; Thébault, P.; Lebrun, L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016, 30, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz Öztürk, B.; Yenice Gürsu, B.; Dağ, İ. Antibiofilm and antimicrobial activities of green synthesized silver nanoparticles using marine red algae Gelidium corneum. Process Biochem. 2020, 89, 208–219. [Google Scholar] [CrossRef]
- Dahms, H.U.; Dobretsov, S. Antifouling Compounds from Marine Macroalgae. Mar. Drugs 2017, 15, 265. [Google Scholar] [CrossRef]
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Rai, M.; Chikindas, M. Natural Antimicrobials in Food Safety and Quality; CABI: Wallingford, UK, 2011; ISBN 9781845937690. [Google Scholar]
- Aloui, H.; Khwaldia, K. Natural Antimicrobial Edible Coatings for Microbial Safety and Food Quality Enhancement. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef]
- Arshad, M.S.; Batool, S.A. Natural Antimicrobials, their Sources and Food Safety. Food Addit. 2017. [Google Scholar] [CrossRef]
- Fu, Y.; Sarkar, P.; Bhunia, A.K.; Yao, Y. Delivery systems of antimicrobial compounds to food. Trends Food Sci. Technol. 2016, 57, 165–177. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Abu-Ghannam, N.; Rajauria, G. Antimicrobial activity of compounds isolated from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 287–306. ISBN 9780857095121. [Google Scholar]
- Triandafyllidou, A.; McAuliffe, M. Report overview. Migr. Smuggling Data Res. 2019, 1–18. [Google Scholar] [CrossRef]
- Gillis, R.; Adams, G.; Besong, D.; Machova, E.; Ebringerova, A.; Harding, S.; Patel, T. Application of novel analytical ultracentrifuge analysis to solutions of fungal mannans. Eur. Biophys. J. 2017, 46, 235–245. [Google Scholar] [CrossRef][Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation Into Innovative Food Products With Potential Health Benefits. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef]
- Neetoo, H.; Ye, M.; Chen, H. Bioactive alginate coatings to control Listeria monocytogenes on cold-smoked salmon slices and fillets. Int. J. Food Microbiol. 2010, 136, 326–331. [Google Scholar] [CrossRef]
| Seaweed | Solvent | MIC/IC50/MBC (mg/mL) | Agar Diffusion | Ref. |
|---|---|---|---|---|
| Laurencia brandenii (R) | MeOH | Re | [63] | |
| G. ornata (R) | H2O/EtOH f | NI/NI/R | [64] | |
| Pterocladia capillacea (R) | H2O cold/H20 hot | NI/NI | [55] | |
| Dictyopteris membranacea (B) | I/NI | |||
| D. membranacea (B) | (MeOH:DCM)/MeOH f/AcO f/(MeOH:DCM) | Re/Re/I/NI | [28] | |
| P. capillacea (R)/Osmundaria obtusiloba (R) | EtOH/Hex | NI | [40] | |
| Dictyota dichotoma (B)/P. pavonica (B)/Sargassum vulgare (B) | AcO | MIC (2.5/2.5/5) | [41] | |
| Cystoseira amentacea (B)/Cystoseira barbata (B)/Cystoseira compressa (B) | AcO | MIC (5) | [46] | |
| Cystoesira myrica (B)/Cystoesira trinodis (B)/Padina gymnospora (B)/Sargassum dentifolium (B)/Sargassum hystrix (B)/Actinotrichia fragilis (R)/Caulerpa racemosa (G)/Codium fragile (G) | MeOH/EtAc | Re/Re | [47] | |
| Padina australis Hauck (B) | MeOH/DCM/n-Hex | MIC (0.88/1.04/1.25) | [48] | |
| Sargassum polycystum (B) | MIC (0.88/1.04/1.04) | |||
| S. polycystum (B) | MIC (0.73/0.83/0.73) | |||
| P. australis Hauck (B) | MIC (2.08/2.08/1.66) | |||
| S. polycystum (B) | MIC (0.83/1.04/0.625) | |||
| S. polycystum (B) | MIC (0.52/0.83/0.42) | |||
| Gracilaria sp. (R) | MeOH | Re | [65] | |
| L. papillosa (R) | DCM/(DCM:MeOH)/MeOH/H2O | MIC (0.8/0.2/0.8/0.4) MBC (1.4/0.2/1.6/0.6) | Re/Re/Re/Re | [62] |
| Padina antillarum (B) | MeOH/EtAc | MIC (3.75/3.75) | I/I | [66] |
| Padina boergeseni (B) | MIC (7.5/7.5) | I/I | ||
| Ulva flexuosa (G) | MIC (1.18/0.93) | S/S | ||
| Ulva lactuca (G)/Enteromorpha intestinales (G)/Cladophora vagabunda (G) | EtOH | R e/Re/I | [67] | |
| Grateloupia doryphora (R) | EtOH/MeOH/EtAc | NI | [68] | |
| Halimeda tuna (G)/Melanothamnus afaqhusainii (R)/Dictyota indica (B)/D. dichotoma var. intricata (B)/Sargassum lanceolatum (B) | EtOH | Re | [57] | |
| C. trinodis (B) | DiEt:EtOH:Hex | MIC (4.12) | [49] | |
| Cladophora glomerata (G) | EtOH | MIC (>1.25) | I | [50] |
| Enteromorpha linza (G) | MIC (>5) | Re | ||
| Ulva rigida (G) | MIC (>10) | Re | ||
| C. barbata (B) | MIC (>5) | Re | ||
| P. pavonica (B) | MIC (>1.25) | I | ||
| C. officinalis (R) | MIC (>1.25) | I | ||
| Ceramium ciliatum (R) | MIC (>5) | Re | ||
| D. membranacea (B) | H2O/EtAc/CHCl3 | NI | [59] | |
| Cymodocea nodosa (G)/H. tuna (G)/C. barbata (B)/Codium bursa (G) | DCM:MeOH | NI | [51] | |
| Chaetomorpha linum (G)/Cladophora rupestris (G)/Fucus serratus (B)/Halosiphon tomentosus (B)/Saccharina latissima (B)/Bonnemaisonia hamifera (R)/Callithamonion corymbosum (R)/Ceramium tenuicorne (R)/Dasya baillouviana (R)/Delesseria sanguinea (R)/Dumontia contorta (R)/Polysiphonia elongata (R)/Polysiphonia nigra (R)/R. confervoides (R) | DCM | NI | [69] | |
| G. corticata (R) | MeOH/DMSO | S/Re | [61] | |
| G. edulis (R) | MeOH | Re | ||
| S. lomentaria (B)/P. pavonica (B)/Cystoseira mediterranea (B)/Hypnea musciformis (R)/Spyridia filamentosa (R) | MeOH | I/I/NI/NI/Re | [54] | |
| A. fragilis (R)/C. myrica (B)/Hormophysa cuneiformis (B)/L. papillosa (R)/Sargassum cinereum (R)/Turbinaria turbinata (B) | MeOH | Re | [52] | |
| C. barbata (B) | EtOH | Re | [70] | |
| Kappaphycus alvarezii (R) | EtOH/Hot H2O | NI | [71] | |
| Oedogonium sp. (G) | MeOH/EtOH | NI | [72] | |
| Stigeoclonium sp. (G) | I/I | |||
| Ulothrix sp. (G) | I/I | |||
| Nitzschia sp. (G) | Re/I | |||
| Bifurcaria bifurcata (B) | MeOH/DCM | Re/NI | [53] | |
| Re/Re | ||||
| Hypnea flagelliformis (R) | MeOH/H2O/DCM | NI | [73] | |
| C. myrica (B) | NI | |||
| Sargassum boveanum(B) | NI | |||
| Turbinaria conoides (B) | n-Hex/ClHx/MeOH/(EtOH:H2O) | Re/Re/NI | [74] | |
| Cystoseira tamariscifolia (B)/P. pavonica (B)/R. confervoides (R)/U. lactuca (G) | MeOH | I/I/I/Re | [60] | |
| Laurencia obtusa (R)/Codium elongatum (G)/C. multifida (B) | AcO | MIC (0.17/2.5/0.16) | [42] | |
| Fucus vesiculosus (B)/Cystoseira baccata (B) | EtOH | IC50 (2.25/2.5) | [43] | |
| Saccharina japonica (B) | CO2 | MIC (3.4) | [44] | |
| Enteromorpha prolifera (G) | PeEt/DiEt/EtAc/MeOH | I/I/R e/I | [58] | |
| Ulva reticulata (G) | S/I/Re/I | |||
| C. myrica (B) | I | |||
| P. pavonica (B) | I | |||
| Turbinaria triquetra (B) | I | |||
| Sargassum portieriatum (B) | S/I/I/I | |||
| G. multipartite (R) | I/I/I/Re | |||
| Hypnea esperi (R)/Caulerpa prolifera (G) | MeOH | Re/NI | [75] | |
| Himanthalia elongata (B) | n-Hex/EtOH/H2O | MBC (7.2/6.0/12.5) | [21] | |
| Sargassum ilicifolium (B)/H. cuneiformis (B)/S. polycystum (B)/Sargassum sp. (B)/T. conoides (B)/Turbinaria decurrens (B)/Turbinaria ornata (B) | MeOH | Re | [76] | |
| Sargassum incisifolium(B) | EtAc | Re | [76] | |
| L. complanata (R) | MeOH/Hex f/(Hex:EtAc) f/MeOH f | Re/NI/NI/NI | [77] | |
| Grateloupia sp. (R)/G. corticata (R)/Spyridia sp. (R)/Meta-mastophora sp. (R)/Calloseris sp. (R)/Neurymenia fraxinifolia (R) | MeOH | Re | ||
| Halymenia sp. (R) | DiEt | Re | ||
| U. reticulata (G) | (MeOH:CHCl3:H2O) | I | [78] | |
| U. rigida (G) | (EtOH:H2O)/Hex f/CHCl3 f/EtAc f/ButOH f/H2O f | Re/NI/I/Re/NI/NI | [45] | |
| NI/Re/NI/NI/NI | ||||
| U. lactuca (G)/D. dichotoma (B) | MeOH/DiEt/CHCl3 | Re/Re/S | [79] | |
| Re/Re/Re | ||||
| U. rigida (G) | DCM/DCM:MeOH | NI/Re | [80] |
| Seaweed | Solvent | MIC/IC50/MBC (mg/mL) | Agar Diffusion | Ref. |
|---|---|---|---|---|
| Enterobacter spp. (E. cloacae) | ||||
| G. ornata (R) | H2O/EtOH f | Re | [64] | |
| Gracilaria sp. (R) | MeOH | NI | [65] | |
| H. cuneiformis (B)/S. ilicifolium (B)/S. incisifolium (B)/S. polycystum (B)/Sargassum sp. (B)/T. conoides (B)/T. decurrens (B)/T. ornata (B) | MeOH | Re | [76] | |
| L. complanata (R)/Grateloupia sp. (R)/G. corticata (R)/Halymenia sp. (R)/Spyridia sp. (R)/Meta-mastophora sp. (R)/Calloseris sp. (R)/N. fraxinifolia (R) | MeOH | Re | [77] | |
| U. reticulata (G) | MeOH:CHCl3:H2O | S | [78] | |
| Proteus spp. (P. mirabilis; P. vulgaris) | ||||
| L. papillosa (R) | DCM/DCM:MeOH/MeOH/H2O | Re/Re/Re/Re | ||
| Sargassum binderi (B) | H2O/EtOH | Re/Re | [99] | |
| Amphiroa sp. (R) | Re/Re | |||
| T. conoides (B) | Re/Re | |||
| Halimeda macroloba (G) | Re/NI | |||
| Galaxaura rugosa (R) | EtOH/CHCL3 f/n-BuOH f/H2O f | MIC (2.5/1.25/2.5/5) | I/S/S/I | [97] |
| L. hawaiiana (R) | MIC (-/2.5/0.625/10) | NI/I/SI | ||
| Polysiphonia hainanensis (R) | EtOH/AcO | Re | [29] | |
| Halidrys siliquosa (B) | MeOH/Hex;EtAc f | MIC (1.25) | [117] | |
| T. conoides (B) | MeOH/CHCl3/EtOH/DiEt/PeEt/EtAc/AcO | Re | [101] | |
| P. gymnospora (B) | Re | |||
| S. tenerrimum (B) | Re/I/Re/Re/I/I/I | |||
| Padina tetrastromatica (B) | MeOH | Re | [100] | |
| Sargassum muticum (B) | AcO/CHCl3/MeOH | Re/Re/Re | [98] | |
| Salmonella spp. (S. choleraesuis; S. typhi; S. typhimurium; S. enterica; S. gallinarum) | ||||
| L. brandenii (R) | MeOH | Re | [63] | |
| L. complanata (R)/Grateloupia sp. (R)/G. corticata (R)/Halymenia sp. (R)/Spyridia sp. (R)/Meta-mastophora sp. (R)/Calloseris sp. (R)/N. fraxinifolia (R) | MeOH | Re | [77] | |
| G. ornata (R) | H2O/EtOH f | Re | [64] | |
| Cladophora socialis (G) | AcO/MeOH | S/I | [118] | |
| Sargassum latifolium B (B) | I/I | |||
| Sargassum platycarpum A (B) | Re/Re | |||
| D. membranacea (B) | MeHO:DCM/EtOH f/AcO f | Re/I/Re | [28] | |
| S. hytrix (B) | EtAc/MeOH | Re/NI | [47] | |
| C. racemosa (G) | Re/NI | |||
| S. dentifolium (B) | Re/NI | |||
| C. myrica (G) | NI/Re | |||
| P. gymnospora (B) | Re/I | |||
| C. fragile (G) | Re | |||
| A. fragilis (R) | Re/NI | |||
| C. trinodis (B) | Re/Re | |||
| H. tuna (G)/D. dichotoma var intricate (B)/D. indica (B)/M. afaqhusainii (R)/S. lanceolatum (B) | EtOH:H2O | Re/I/I/Re/Re | [57] | |
| C. glomerata (G)/E. linza (G)/U. rigida (G)/C. barbata (B)/P. pavonica (B)/C. ciliatum (R)/C. officinalis (R) | EtOH | I/Re/I/Re/R e/Re/I | [50] | |
| D. membranacea (B) | H2O/CHCl3/EtOAc | NI/NI/NI | [59] | |
| S. lomentaria (B)/P. pavonica (B)/C. mediterranea (B)/H. musciformis (R)/S. filamentosa (R) | MeOH | I/NI/NI/NI/NI | [54] | |
| E. cava (B) | EtOH/n-Hex/DCM/EtAc/n-BuOH/H2O/Ac | MIC (2/NI/NI/0.25/2/NI/9.7 × 10−4) | [109] | |
| C. barbata (B) | EtOH | Re | [70] | |
| H. flagelliformis (R) | MeOH | NI | [73] | |
| C. myrica (B) | MeOH | |||
| S. boveanum (B) | H2O/MeOH/DCM | |||
| S. japonica (B) | Raw material/Raw material + catalyst/De-oiled/de-oiled + catalyst | NI/Re/NI/Re | [44] | |
| H. esperi (R)/C. prolifera (G) | MeOH | NI | [75] | |
| H. cuneiformis (B)/S. ilicifolium (B)/S. polycystum (B)/Sargassum sp. (B)/T. conoides (B)/T. ornata (B) | MeOH | Re | [76] | |
| Shigella sp. (reference strains: ATCC 9204 and 1457) | ||||
| H. cuneiformis (B)/S. ilicifolium (B)/S. incisifolium (B)/S. polycystum (B)/Sargassum sp. (B)/T. conoides (B)/T. decurrens (B)/T. ornata (B) | MeOH:DCM | Re | [76] | |
| S. muticum (B) | CHCl3/AcO/MeOH | Re | [98] | |
| C. rubrum (R) | DiEt/MeOH/EtOH/CHCl3 | Re/Re/I/Re | [88] | |
| S. vulgare (B) | Re/Re/NI/NI | |||
| S. fusiforme (B) | Re/NI/Re/Re | |||
| P. pavonia (B) | Re/Re/S/Re | |||
| T. conoides (B) | MeOH/AcO/PeEt/EtOH/EtAc/CHCl3/DiEt | Re | [101] | |
| P. gymnospora (B) | I/I/Re/Re/I/R e/I | |||
| S. tenerrimum (B) | I/Re/I/Re/I/R e/I | |||
| Pseudomonas aeruginosa (reference strains: ATCC 25619/27853/85327/9027; KCTC 1637; DSM 50071; MTCC 2453/424) | ||||
| L. brandenii (R) | MeOH | Re | [63] | |
| G. ornata (R) | H2O/EtOH/EtOH | Re | [64] | |
| G. changii (R) | MeOH | MIC (6.25) | Re | [119] |
| C. rupestris (G) | CHCl3:MeOH | Re | [120] | |
| C. trinodis (B) | (C2H5)2:EtOH:Hex | MIC (6.6) | [49] | |
| C. myrica (B)/C. trinodis (B)/P. gymnospora (B)/S. dentifolium (B)/S. hystrix (B)/A. fragilis (R)/Caulerpa racemose (G)/C. fragile (G) | MeOH/EtOH | Re | [47] | |
| P. australis (B) | MeOH/DCM/n-Hex | MIC (0.26/0.26/0.73) | [48] | |
| S.polycystum TK (B) | MIC (0.73/0.21/0.10) | |||
| S.polycystum CR (B) | MIC (0.21/0.21/0.10) | |||
| L. papillosa (R) | DCM/DCM:MeOH/MeOH/H2O | Re/I/Re/Re | [62] | |
| U. flexuosa (G)/P. antillarum (B)/P. boergeseni (B) | EtAc/MeOH | NI | [66] | |
| G. doryphora (R) | EtOH/MeOH/EtAc | Re | [68] | |
| Eisenia bicyclis (B) | MeOH/Hex/DCM/EtAc/BuOH | NI | [121] | |
| H. tuna (G)/D. dichotoma var intricate (B)/D. indica (B)/M. afaqhusainii (R)/S. lanceolatum (B) | EtOH/H2O | Re | [57] | |
| C. glomerata (G)/E. linza (G)/U. rigida (G)/C. barbata (B)/C. ciliatum (R)/C. officinalis (R) | EtOH | I/Re/Re/eRe/Re/Re | [50] | |
| D. membranacea (B) | H2O/CHCl3/EtAc | NI/NI/NI | [59] | |
| C. linum (G)/C. rupestris (G)/F. serratus (B)/F. vesiculosus (B)/H. tomentosus (B)/S. latissima(B)/B. hamifera (R)/C. corymbosum (R)/C. tenuicorn (R)/Ceramium virgatum (R)/D. baillouviana (R)/D. sanguinea (R)/D. contorta (R)/P. elongata (R)/P. nigra (R)/R. confervoides (R) | DCM | NI | [69] | |
| B. bifurcata (B) | MeOH/DCM | Re/Re | [53] | |
| T. conoides (B) | n-Hex/C6H12/MeOH/EtOH:H2O | NI/R e/Re/NI | [74] | |
| C. tamariscifolia (B)/P. pavonica (B)/R. confervoides (R)/U. lactuca (G) | MeOH | I/I/I/Re | [60] | |
| E. prolifera (G) | DiEt | MIC (1.25 × 10−3) | [58] | |
| S. portieriatum (B) | MeOH | MIC (7.5 × 10−4) | ||
| H. esperi (R) | MeOH | NI | [75] | |
| C. prolifera (G) | I | |||
| H. cuneiformis (B)/S. ilicifolium (B)/T. ornata (B) | MeOH | NI/Re/Re | [76] | |
| H. elongata (B) | H2O/MeOH | Re | [30] | |
| U. rigida (G) | EtOH:H2O/Hex/CHCl3 f/EtOAc f/ButOH f/H2O | Re/NI/Re/Re/NI/NI | [45] | |
| S. vulgare (B) | DIEt/MeOH/EtOH/CHCl3 | Re/NI/NI/NI | [88] | |
| S. fusiforme (B) | S/Re/Re/NI | |||
| P. pavonia (B) | I/NI/I/I | |||
| C. rubrum (R) | I/NI/I/NI | |||
| Seaweed | Solvent | MIC/IC50/MBC (mg/mL) | Agar Diffusion | Ref. |
|---|---|---|---|---|
| C. socialis (G) | AcO/MeOH | I/S | [118] | |
| S. latifolium (B) | I/S | |||
| S. platycarpum (B) | Re/I | |||
| C. elongatum (G)/L. obtusa (R)/C. multifida (B) | AcO | MIC (0.63 × 103) | [125] | |
| H. macroloba (G)/S. binderi (B)/Amphiroa sp. (B)/T. conoides (B) | AcO | Re | [99] | |
| U. lactuca (G)/C. fragile (G)/L. johnstonii (R)/Gymnogongrus martinensis (R)/D. flabellata (B)/Padina concrescens (B) | AcO:MeOH | Re/NI/I/NI/NI/NI | [127] | |
| U. flexuosa (G)/P. antillarum (B)/P. boergeseni (B) | MeOH/EtAc | MIC (3.75/1.87) | I/S | [66] |
| MIC (7.5/3.75) | I/S | |||
| MIC (15/3.75) | Re/I | |||
| U. rigida (G) | DCM | Re | [80] | |
| DCM:MeOH | ||||
| H. tuna (G)/D. dichotoma var intricata (B)/D. indica (B)/S. lanceolatum (B)/D. dichotoma var intricata (B)/M. afaqhusainii (R) | EtOH | Re | [57] | |
| C. glomerata (G)/E. linza (G)/U. rigida (G)/C. ciliatum (R)/C. barbata (B)/P. pavonica (B)/C. officinalis (B) | EtOH | MIC (>1.25/>2.5/>2.5/>10/>1.25/>1.25/>5) | I/Re/I/Re/I/I/Re | [50] |
| C. nodosa (G)/H. tuna (G)/C. barbata (G)/C. bursa (G) | MeOH | Re | [51] | |
| Oedogonium sp. (G) | MeOH/EtOH | NI | [72] | |
| Stigeoclonium sp. (G) | Re/I | |||
| Ulothrix sp. (G) | Re | |||
| Nitzschia sp. (G) | Re/I | |||
| E. prolifera (G) | EtAc | MIC (1.0 × 103) | [81] | |
| P. pavonica (B) | PeEt | MIC (1.25 × 103) | ||
| C. prolifera (G)/H. esperi (R) | MeOH | MIC (0.6/0.5) | I | [75] |
| U. reticulata (G) | H2O | S | [128] | |
| U. rigida (G) | Hex/CHCl3/EtOAc/ButOH/H2O/Hex/CHCl3/EtOAc/ButOH/H2O | NI/NI/Re/Re/Re/I/I/Re/NI/NI | [45] | |
| Ulva fasciata (G)/Chaetomorpha antennina (G)/Caula anthus okamurai (R)/Ahnfeltiopsis masudai (R)/P. hainanensis (R)/Sargassum hemiphyllum (R)/Sargassum vachellianum (R)/Pachydictyon coriaceum (B) | EtOH | Re/Re/Re/Re/Re/I/I/Re | [29] | |
| L. brandenii (R) | CHCl3/MeOH | I | [63] | |
| G. ornata (R) | MeOH | Re | [64] | |
| Gracilaria sp. (R) | PolySA/MeOH | NI | [65] | |
| L. papillosa (R) | EtOH/MOH/AcO | Re | [62] | |
| G. corticata (R)/G. edulis (R) | MeOH/DMSO | Re | [61] | |
| H. musciformis (R)/S. filamentosa (R)/S. lomentaria (B)/P. pavonica (B)/C. mediterranea (B) | MeOH | Re | [54] | |
| A. fragilis (R)/L. papillosa (R)/S. cinereum (R)/C. myrica (B)/H. cuneiformis (B)/T. turbinata (B) | DMSO | Re | [52] | |
| H. flagelliformis (R) | DCM/MeOH | Re | [73] | |
| C. myrica (B) | NI | |||
| S. boveanum (B) | NI | |||
| R. confervoides (R)/U. lactuca (G)/C. tamariscifolia (B)/P. pavonica (B) | MeOH | I | [60] | |
| L. complanata (R)/Grateloupia sp. (R)/G. corticata (R)/Halymenia sp. (R)/Spyridia sp. (R)/Metamastophora sp. (R)/Calloseris sp. (R)/N. fraxinifolia (R) | MeOH | Re | [77] | |
| P. gymnospora (B) | H2O | MIC (500 × 103) | [132] | |
| Sargassum oligocystum (B) | Hot H2O | MIC (3.18) | [129] | |
| E. bicyclis (B) | MeOH/Hex/DCM/EtAc/BuOH | MIC (1.02/256/512/128 × 103/512 × 103) | Re | [121] |
| C. trinodis (B) | DIEt:EtOH:Hex | MIC (1.031) | [49] | |
| S. vulgare (B) | EtAc | Re | [130] | |
| D. membranacea (B) | H2O/DCM/EtAc | Re | [59] | |
| E. cava (B) | EtOH/n-Hex/DCM/EtAc/n-BuOH/H2O | MIC (500 × 103/nd/nd/250 × 103/nd/nd) | [109] | |
| C. barbata (B) | EtOH | Re | [70] | |
| B. bifurcata (B) | MeOH/DCM | Re/NI | [53] | |
| D. dichotoma (B)/P. pavonia (B)/S. vulgare (B) | AcO | MIC (1.25/1.25/2.5) | [41] | |
| F. vesiculosus (B) | EtOH | IC50: (1.25) | [43] | |
| S. japonica (B) | CO2 | MIC (3) | [44] | |
| D. membranacea (B) | MeOH:DCM/EtOH/AcO | Re/S/S | [28] | |
| H. elongata (B) | Hex/EtOH/H2O | MBC (8.25/7.00/13.0) | [21] | |
| H. cuneiformis (B)/S. ilicifolium (B)/S. incisifolium (B)/Sargassum sp. (B)/T. conoides (B)/T. decurrens (B)/T. ornata (B) | MeOH | Re | [76] | |
| F. serratus (B)/F. vesiculosus (B) | H2O/MeOH/AcO/EtAc | I | [131] |
| Seaweed | Solvent | MIC/IC50/MBC (mg/mL) | Agar Diffusion | Ref |
|---|---|---|---|---|
| B. cereus (reference strains: ATCC 10876/11778/13061/14579; CMCC-B-63303) | ||||
| C. racemosa (G)/C. fragile (G)/A. fragilis (R)/C. myrica (B)/C. trinodis (B)/P. gymnospora (B)/S. dentifolium (B)/S. hystrix (B) | EtAc | Re | [47] | |
| C. glomerata (G)/E. linza (G)/U. rigida (G) | EtAc | MIC (>10) | Re | [50] |
| C. officinalis (R)/C. ciliatum (R)/C. barbata (B)/P. pavonica (B) | EtOH | MIC (>10) | Re | |
| P. capillacea (R)/D. membranacea (B) | Cold H2O/Hot H2O | Re/NA | [11] | |
| S. polycystum C, TK (B) | MeOH/DCM/n-Hex | MIC (0.13/0.21/0.37) | [48] | |
| S. polycystum C, CR (B) | MIC (0.21/0.21/0.07) | |||
| P. australis, CR (B) | MIC (0.21/0.21/0.07) | |||
| L. complanata (R)/Grateloupia sp. (R)/G. corticata (R)/Halymenia sp. (R)/Spyridia sp. (R)/Meta-mastophora sp. (R)/Calloseris sp. (R)/N. fraxinifolia (R) | MeOH | S/Re/Re/Re/Re/Re/Re/Re | [77] | |
| S. vulgare(B) | MeOH: CHCl3 | MIC (3.75) MBC (>15) | Re | [130] |
| Cladostephus hirsutus(B) | MIC (1.87) MBC (>15) | |||
| Rissoella verruculosa (R) | MIC (7.5) MBC (>15) | |||
| C. racemosa (G) | Hex/CHCl3/EtAc/EtOH/MeOH/H2O | MIC (0.3/0.6/0.3/2.5/1.2/-) MBC (0.3/0.6/0.3/2.5/-/-) | [57] | |
| Caulerpa sertularioides (G) | MIC (0.2/0.1/0.2/0.3/1.2/-) MBC (0.6/0.2/0.2/0.6/1.2/-) | |||
| K. alvarezii (R) | MIC (1.2/1.2/2.5/-/-) MBC (1.2/1.2/2.5/-/-) | |||
| K. alvarezi (R) | EtOH/H2O | Re | [71] | |
| S. ilicifolium (B)/S. incisifolium (B)/H. cuneiformis (B)/S. polycystum (B)/Sargassum sp. (B)/T. conoides (B)/T. ornata (B)/T. decurrens (B) | MeOH | Re | [76] | |
| C. anthus okamurai (R)/P. hainanensis (R)/Hypnea chordacea (R)/Hypnea japonica (R)/Hypnea boergesenii (R)/L. okamurai (R)/L. chinensis (R)/P. arborescens (B)/1 P. coriaceum (B)/Ulva conglobata (G)/U. fasciata (G)/C. antennina (G)/C. cylindricum (G) | AcO | Re/Re/Re/Re/Re/Re/Re/Re/I/Re/Re/I/I | [29] | |
| U. rigida (G) | MeOH/Hex f/CHCl3 f/EtAc f/BuOH f/H2O | Re/Re/I/Re/NI/NI | [45] | |
| Enterococus spp.: E. faecalis (ATCC 29737, 29219); E. faecium (ATCC 19434) | ||||
| C. rupestris (G) | CHCl3: MeOH | Re | [120] | |
| U. flexuosa (G) | MeOH/EtAc | MIC (15/3.75) | [57] | |
| P. antillarum (B) | MIC (15/7.5) | |||
| P. boergeseni (B) | MIC (15/7.5) | |||
| U. rigida(G) | DCM | Re/I | [80] | |
| DCM:MeOH | Re/I | |||
| C. nodosa (G)/H. tuna (G)/C. bursa (G)/C. barbata (B) | DCM:MeOH | N/D | [51] | |
| H. musciformis (G)/S. lomentaria (B)/S. filamentosa (R)/P. pavonica (B)/C. mediterranea (B) | MeOH | ND/Re/ND/Re/ND/ND | [54] | |
| U. rigida (G) | Hex f/CHCl3 f/EtAc f/BuOH f/H2O f | ND/I/Re/ND/ND | [45] | |
| Grateloupia doryphore (R) | MeOH/EtAc/EtOH | Re | [68] | |
| D. membranacea (B) | MeOH:DCM/EtOH f/AcO f/MeOH/DCM f/MeOH:DCM/EtOH f/AcO f/MeOH:DCM f | I/S/S/Re/Re/Re/Re/Re | [28] | |
| L. monocytogenes (reference strains: NCTC 11994; ATCC 19115) | ||||
| C. glomerata (G)/E. linza (G)/U. rigida (G)/C. officinalis (R)/C. ciliatum (R)/C. barbata (B)/P. pavonica (B) | EtOH | MBC (>1.25/>2.2/>10/>2.5/>2.5/>2.5/>1.25) | Re | [50] |
| U. rigida (G) | Hex f/CHCl3 f | S/Re | [45] | |
| D. membranacea (B) | H2O | I | [59] | |
| H. elongata (B) | H2O | Re | [30] | |
| 20% MeOH | Re | |||
| 40% MeOH | Re | |||
| 60% MeOH | Re | |||
| 80% MeOH | Re | |||
| MeOH | Re | |||
| C. glomerata (G)/U. rigida (G)/E. linza (G)/C. barbata (B)/P. pavonica (B)/C. ciliatum (R)/C. officinalis (R) | EtOH | I/Re/Re/Re/Re/Re/Re | [50] | |
| Streptococcus spp. (S. agalactiae; S. pneumoniae; S. suis; S. aureus; S. mutans; S. pyogenes) | ||||
| L. brandenii (R) | PeEt:DCM (6:4) f | Re | [154] | |
| C. rupestris (G) | DCM:MeOH | Re | [120] | |
| L. papillosa (R) | DCM/(DCM:MeOH)/MeOH/H2O | Re/Re/Re | [155] | |
| H. cuneiformis, (B)/T. ornata (B)/T. conoides (B)/S. polycystum. (B)/S. ilicifolium (B)/S. incisifolium (B)/T. decurrens (B)/Sargassum sp. (B) | MeOH | Re | [76] | |
| L. complanata (R) | MeOH/DCM f/(Pentene:DIEt) f/MeOH f | S/Re/Re/Re | [77] | |
| G. corticata (R)/Halymenia sp. (R)/Spyridia sp. (R)/Meta-mastophora sp. (R)/Calloseris sp. (R)/N. fraxinifolia (R) | MeOH | Re | [77] | |
| Ulva armoricana (G) | SPoly f | MIC (6.25) | [156] | |
| E. linza (G) | EtOH/MeOH/AcO | Re/Re/I | [157] | |
| T. conoides (B)/P. gymnospora (B)/S. tenerrimum (B) | MeOH/AcO/PeEt/EtOH/EtAc/CHCl3/DiEt | Re | [101] | |
| G. rugosa (R) | CHCl3/n-BuOH/H2O | Re/I/S | [97] | |
| L. hawaiiana (R) | S/NI/NI | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Silva, S.A.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Domingues, V.F.; Barroso, M.F.; Simal-Gandara, J.; et al. Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics 2020, 9, 712. https://doi.org/10.3390/antibiotics9100712
Silva A, Silva SA, Lourenço-Lopes C, Jimenez-Lopez C, Carpena M, Gullón P, Fraga-Corral M, Domingues VF, Barroso MF, Simal-Gandara J, et al. Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics. 2020; 9(10):712. https://doi.org/10.3390/antibiotics9100712
Chicago/Turabian StyleSilva, Aurora, Sofia A. Silva, C. Lourenço-Lopes, C. Jimenez-Lopez, M. Carpena, P. Gullón, M. Fraga-Corral, V. F. Domingues, M. Fátima Barroso, J. Simal-Gandara, and et al. 2020. "Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens" Antibiotics 9, no. 10: 712. https://doi.org/10.3390/antibiotics9100712
APA StyleSilva, A., Silva, S. A., Lourenço-Lopes, C., Jimenez-Lopez, C., Carpena, M., Gullón, P., Fraga-Corral, M., Domingues, V. F., Barroso, M. F., Simal-Gandara, J., & Prieto, M. A. (2020). Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics, 9(10), 712. https://doi.org/10.3390/antibiotics9100712













