Looking beyond Typical Treatments for Atypical Mycobacteria
Abstract
1. NTM Infections: Epidemiology and Clinical Presentations
1.1. Epidemiology
1.2. Relationship between Tuberculosis and NTM Infections
1.3. Clinical Presentations
2. NTM Biology and Interaction with the Host Cell
3. Current Treatments Available for NTM Infections
3.1. Limitations and Challenges
3.2. Base-Line Treatments for NTM
3.3. Second-Line Treatments for NTM
3.4. The Special Case of M. abscessus
3.5. New Antimycobacterial Compounds in Preclinical Studies
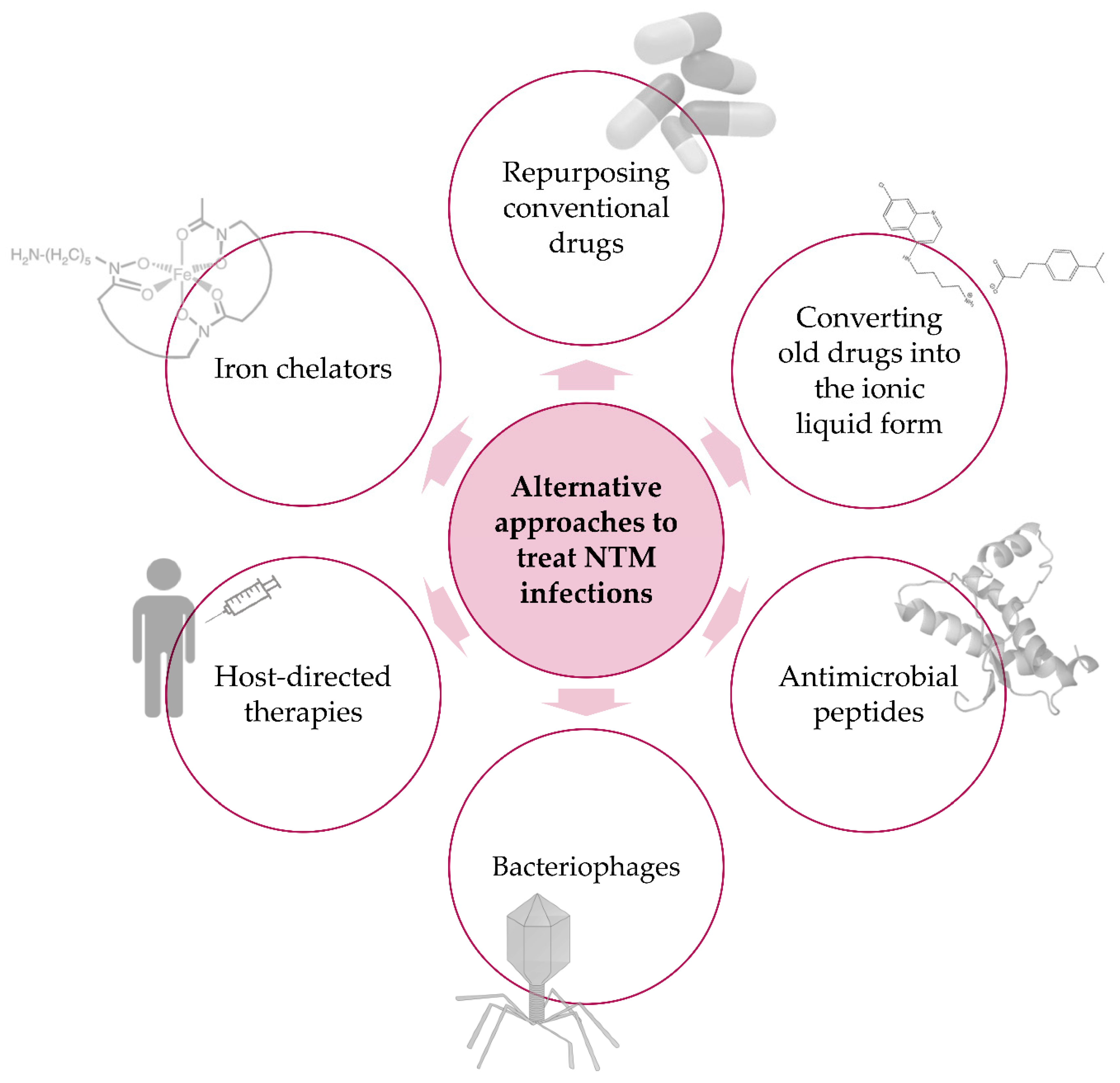
4. Alternative Approaches—Beyond Typical Treatments
4.1. Repurposing Old Drugs
4.2. Ionic Liquids
4.3. Antimicrobial Peptides
4.4. Bacteriophages
4.5. Iron Chelators
4.6. Host-Directed Therapies
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Anonymous. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am. J. Respir. Crit. Care Med. 1997, 156, S1–S25. [Google Scholar] [CrossRef]
- Wolinsky, E. Nontuberculous mycobacteria and associated diseases. Am. Rev. Respir. Dis. 1979, 119, 107–159. [Google Scholar] [CrossRef] [PubMed]
- Cowman, S.; van Ingen, J.; Griffith, D.E.; Loebinger, M.R. Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Daniel-Wayman, S.; Ricotta, E.; Prevots, D.R. Epidemiology of Nontuberculous Mycobacteriosis. Semin. Respir. Crit. Care Med. 2018, 39, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Drummond, W.K.; Kasperbauer, S.H. Nontuberculous Mycobacteria: Epidemiology and the Impact on Pulmonary and Cardiac Disease. Thorac. Surg. Clin. 2019, 29, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Falkinham, J.O., 3rd; Holland, S.M.; Prevots, D.R. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am. J. Respir. Crit. Care Med. 2012, 186, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886. [Google Scholar] [CrossRef]
- Nishiuchi, Y.; Iwamoto, T.; Maruyama, F. Infection Sources of a Common Non-tuberculous Mycobacterial Pathogen, Mycobacterium avium Complex. Front. Med. (Lausanne) 2017, 4, 27. [Google Scholar] [CrossRef]
- Feazel, L.M.; Baumgartner, L.K.; Peterson, K.L.; Frank, D.N.; Harris, J.K.; Pace, N.R. Opportunistic pathogens enriched in showerhead biofilms. Proc. Natl. Acad. Sci. USA 2009, 106, 16393–16399. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; Garcia-Coca, M. Mycobacterium Biofilms. Front. Microbiol. 2017, 8, 2651. [Google Scholar] [CrossRef] [PubMed]
- Brode, S.K.; Daley, C.L.; Marras, T.K. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: A systematic review. Int. J. Tuberc. Lung Dis. 2014, 18, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Shaw, P.A.; Strickland, D.; Jackson, L.A.; Raebel, M.A.; Blosky, M.A.; Montes de Oca, R.; Shea, Y.R.; Seitz, A.E.; Holland, S.M.; et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am. J. Respir. Crit. Care Med. 2010, 182, 970–976. [Google Scholar] [CrossRef]
- Horsburgh, C.R., Jr.; Hanson, D.L.; Jones, J.L.; Thompson, S.E., 3rd. Protection from Mycobacterium avium complex disease in human immunodeficiency virus-infected persons with a history of tuberculosis. J. Infect. Dis. 1996, 174, 1212–1217. [Google Scholar] [CrossRef]
- Gebert, M.J.; Delgado-Baquerizo, M.; Oliverio, A.M.; Webster, T.M.; Nichols, L.M.; Honda, J.R.; Chan, E.D.; Adjemian, J.; Dunn, R.R.; Fierer, N. Ecological Analyses of Mycobacteria in Showerhead Biofilms and Their Relevance to Human Health. MBio 2018, 9. [Google Scholar] [CrossRef]
- Nishiuchi, Y.; Maekura, R.; Kitada, S.; Tamaru, A.; Taguri, T.; Kira, Y.; Hiraga, T.; Hirotani, A.; Yoshimura, K.; Miki, M.; et al. The recovery of Mycobacterium avium-intracellulare complex (MAC) from the residential bathrooms of patients with pulmonary MAC. Clin. Infect. Dis. 2007, 45, 347–351. [Google Scholar] [CrossRef]
- Falkinham, J.O., 3rd; Iseman, M.D.; de Haas, P.; van Soolingen, D. Mycobacterium avium in a shower linked to pulmonary disease. J. Water Health 2008, 6, 209–213. [Google Scholar] [CrossRef]
- Bryant, J.M.; Grogono, D.M.; Rodriguez-Rincon, D.; Everall, I.; Brown, K.P.; Moreno, P.; Verma, D.; Hill, E.; Drijkoningen, J.; Gilligan, P.; et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 2016, 354, 751–757. [Google Scholar] [CrossRef]
- Lopez-Varela, E.; Garcia-Basteiro, A.L.; Santiago, B.; Wagner, D.; van Ingen, J.; Kampmann, B. Non-tuberculous mycobacteria in children: Muddying the waters of tuberculosis diagnosis. Lancet Respir. Med. 2015, 3, 244–256. [Google Scholar] [CrossRef]
- Daffe, M. The cell envelope of tubercle bacilli. Tuberculosis 2015, 95 (Suppl. 1), S155–S158. [Google Scholar] [CrossRef] [PubMed]
- Vilcheze, C.; Kremer, L. Acid-Fast Positive and Acid-Fast Negative Mycobacterium tuberculosis: The Koch Paradox. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Gomes, M.S.; Paul, S.; Moreira, A.L.; Appelberg, R.; Rabinovitch, M.; Kaplan, G. Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect. Immun. 1999, 67, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Frehel, C.; de Chastellier, C.; Lang, T.; Rastogi, N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect. Immun. 1986, 52, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Guirado, E.; Schlesinger, L.S.; Kaplan, G. Macrophages in tuberculosis: Friend or foe. Semin. Immunopathol. 2013, 35, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Fratti, R.A.; Chua, J.; Vergne, I.; Deretic, V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA 2003, 100, 5437–5442. [Google Scholar] [CrossRef] [PubMed]
- Appelberg, R. Pathogenesis of Mycobacterium avium infection: Typical responses to an atypical mycobacterium? Immunol. Res. 2006, 35, 179–190. [Google Scholar] [CrossRef]
- Gomes, M.S.; Appelberg, R. NRAMP1- or cytokine-induced bacteriostasis of Mycobacterium avium by mouse macrophages is independent of the respiratory burst. Microbiology 2002, 148, 3155–3160. [Google Scholar] [CrossRef]
- Appelberg, R. Macrophage nutriprive antimicrobial mechanisms. J. Leukoc. Biol. 2006, 79, 1117–1128. [Google Scholar] [CrossRef]
- Early, J.; Fischer, K.; Bermudez, L.E. Mycobacterium avium uses apoptotic macrophages as tools for spreading. Microb. Pathog. 2011, 50, 132–139. [Google Scholar] [CrossRef]
- Thegerstrom, J.; Jonsson, B.; Brudin, L.; Olsen, B.; Wold, A.E.; Ernerudh, J.; Friman, V. Mycobacterium avium subsp. avium and subsp. hominissuis give different cytokine responses after in vitro stimulation of human blood mononuclear cells. PLoS ONE 2012, 7, e34391. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.L.; Schultz, T.E.; Duke, T.J.; Blumenthal, A. Type I Interferons in the Pathogenesis of Tuberculosis: Molecular Drivers and Immunological Consequences. Front. Immunol. 2017, 8, 1633. [Google Scholar] [CrossRef]
- Abel, L.; Fellay, J.; Haas, D.W.; Schurr, E.; Srikrishna, G.; Urbanowski, M.; Chaturvedi, N.; Srinivasan, S.; Johnson, D.H.; Bishai, W.R. Genetics of human susceptibility to active and latent tuberculosis: Present knowledge and future perspectives. Lancet Infect. Dis. 2018, 18, e64–e75. [Google Scholar] [CrossRef]
- Loddenkemper, R.; Lipman, M.; Zumla, A. Clinical Aspects of Adult Tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 6, a017848. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E. Therapy of nontuberculous mycobacterial disease. Curr. Opin. Infect. Dis. 2007, 20, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F.; Leitch, A.; Loebinger, M.R.; Milburn, H.J.; Nightingale, M.; et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72, ii1–ii64. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef]
- Brode, S.K.; Chung, H.; Campitelli, M.A.; Kwong, J.C.; Marchand-Austin, A.; Winthrop, K.L.; Jamieson, F.B.; Marras, T.K. Prescribing Patterns for Treatment of Mycobacterium avium Complex and M. xenopi Pulmonary Disease in Ontario, Canada, 2001–2013. Emerg. Infect. Dis. 2019, 25, 1271–1280. [Google Scholar] [CrossRef]
- Adjemian, J.; Prevots, D.R.; Gallagher, J.; Heap, K.; Gupta, R.; Griffith, D. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann. Am. Thorac. Soc. 2014, 11, 9–16. [Google Scholar] [CrossRef]
- Diel, R.; Jacob, J.; Lampenius, N.; Loebinger, M.; Nienhaus, A.; Rabe, K.F.; Ringshausen, F.C. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur. Respir. J. 2017, 49, 1602109. [Google Scholar] [CrossRef]
- van Ingen, J.; Wagner, D.; Gallagher, J.; Morimoto, K.; Lange, C.; Haworth, C.S.; Floto, R.A.; Adjemian, J.; Prevots, D.R.; Griffith, D.E.; et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur. Respir. J. 2017, 49, 1601855. [Google Scholar] [CrossRef] [PubMed]
- Ricotta, E.E.; Olivier, K.N.; Lai, Y.L.; Prevots, D.R.; Adjemian, J. Hospital-based antibiotic use in patients with Mycobacterium avium complex. ERJ Open Res. 2018, 4, 00109-2018. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E. Treatment of Mycobacterium avium Complex (MAC). Semin. Respir. Crit. Care Med. 2018, 39, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Egelund, E.F.; Fennelly, K.P.; Peloquin, C.A. Medications and monitoring in nontuberculous mycobacteria infections. Clin. Chest Med. 2015, 36, 55–66. [Google Scholar] [CrossRef]
- TB Alliance. Clarithromycin. Tuberculosis 2008, 88, 92–95. [Google Scholar] [CrossRef]
- Philley, J.V.; Griffith, D.E. Treatment of slowly growing mycobacteria. Clin. Chest Med. 2015, 36, 79–90. [Google Scholar] [CrossRef]
- Lakshminarayana, S.B.; Huat, T.B.; Ho, P.C.; Manjunatha, U.H.; Dartois, V.; Dick, T.; Rao, S.P. Comprehensive physicochemical, pharmacokinetic and activity profiling of anti-TB agents. J. Antimicrob. Chemother. 2015, 70, 857–867. [Google Scholar] [CrossRef]
- Prideaux, B.; Via, L.E.; Zimmerman, M.D.; Eum, S.; Sarathy, J.; O’Brien, P.; Chen, C.; Kaya, F.; Weiner, D.M.; Chen, P.Y.; et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat. Med. 2015, 21, 1223–1227. [Google Scholar] [CrossRef]
- TB Alliance. Rifabutin. Tuberculosis 2008, 88, 145–147. [Google Scholar] [CrossRef]
- Ganapathy, U.S.; Dartois, V.; Dick, T. Repositioning rifamycins for Mycobacterium abscessus lung disease. Expert Opin. Drug Discov. 2019, 14, 867–878. [Google Scholar] [CrossRef]
- Aziz, D.B.; Low, J.L.; Wu, M.L.; Gengenbacher, M.; Teo, J.W.P.; Dartois, V.; Dick, T. Rifabutin Is Active against Mycobacterium abscessus Complex. Antimicrob. Agents Chemother. 2017, 61, e00155-17. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Iakhiaeva, E.; Griffith, D.E.; Woods, G.L.; Stout, J.E.; Wolfe, C.R.; Turenne, C.Y.; Wallace, R.J., Jr. In vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates. J. Clin. Microbiol. 2013, 51, 3389–3394. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Eagle, G.; Thomson, R.; Aksamit, T.R.; Hasegawa, N.; Morimoto, K.; Addrizzo-Harris, D.J.; O’Donnell, A.E.; Marras, T.K.; Flume, P.A.; et al. Amikacin Liposome Inhalation Suspension for Treatment-Refractory Lung Disease Caused by Mycobacterium avium Complex (CONVERT). A Prospective, Open-Label, Randomized Study. Am. J. Respir. Crit. Care Med. 2018, 198, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Philley, J.V.; Griffith, D.E. Medical Management of Pulmonary Nontuberculous Mycobacterial Disease. Thorac. Surg Clin. 2019, 29, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Dheda, K.; Chesov, D.; Mandalakas, A.M.; Udwadia, Z.; Horsburgh, C.R., Jr. Management of drug-resistant tuberculosis. Lancet 2019, 394, 953–966. [Google Scholar] [CrossRef]
- Smith, C.S.; Aerts, A.; Saunderson, P.; Kawuma, J.; Kita, E.; Virmond, M. Multidrug therapy for leprosy: A game changer on the path to elimination. Lancet Infect. Dis. 2017, 17, e293–e297. [Google Scholar] [CrossRef]
- Martiniano, S.L.; Wagner, B.D.; Levin, A.; Nick, J.A.; Sagel, S.D.; Daley, C.L. Safety and Effectiveness of Clofazimine for Primary and Refractory Nontuberculous Mycobacterial Infection. Chest 2017, 152, 800–809. [Google Scholar] [CrossRef]
- Yang, B.; Jhun, B.W.; Moon, S.M.; Lee, H.; Park, H.Y.; Jeon, K.; Kim, D.H.; Kim, S.Y.; Shin, S.J.; Daley, C.L.; et al. Clofazimine-Containing Regimen for the Treatment of Mycobacterium abscessus Lung Disease. Antimicrob. Agents Chemother. 2017, 61, e02052-16. [Google Scholar] [CrossRef]
- Field, S.K.; Cowie, R.L. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest 2003, 124, 1482–1486. [Google Scholar] [CrossRef]
- Jarand, J.; Davis, J.P.; Cowie, R.L.; Field, S.K.; Fisher, D.A. Long-term Follow-up of Mycobacterium avium Complex Lung Disease in Patients Treated With Regimens Including Clofazimine and/or Rifampin. Chest 2016, 149, 1285–1293. [Google Scholar] [CrossRef]
- van Ingen, J.; Totten, S.E.; Helstrom, N.K.; Heifets, L.B.; Boeree, M.J.; Daley, C.L. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob. Agents Chemother. 2012, 56, 6324–6327. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.H.; Wu, B.D.; Hu, S.T.; Lin, C.F.; Wu, K.M.; Chen, J.H. High efficacy of clofazimine and its synergistic effect with amikacin against rapidly growing mycobacteria. Int. J. Antimicrob. Agents 2010, 35, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Ruth, M.M.; Sangen, J.J.N.; Remmers, K.; Pennings, L.J.; Svensson, E.; Aarnoutse, R.E.; Zweijpfenning, S.M.H.; Hoefsloot, W.; Kuipers, S.; Magis-Escurra, C.; et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J. Antimicrob. Chemother. 2019, 74, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Wu, M.F.; Chen, H.C.; Huang, W.C. In vitro activity of aminoglycosides, clofazimine, d-cycloserine and dapsone against 83 Mycobacterium avium complex clinical isolates. J. Microbiol. Immunol. Infect. 2018, 51, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Leach, K.L.; Brickner, S.J.; Noe, M.C.; Miller, P.F. Linezolid, the first oxazolidinone antibacterial agent. Ann. N. Y. Acad. Sci. 2011, 1222, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; Ku, J.H.; Marras, T.K.; Griffith, D.E.; Daley, C.L.; Olivier, K.N.; Aksamit, T.R.; Varley, C.D.; Mackey, K.; Prevots, D.R. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur. Respir. J. 2015, 45, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jiang, Y.; Bao, P.; Li, Y.; Tang, L.; Zhou, Y.; Zhao, Y. Evaluation of the Efficacy of Novel Oxazolidinone Analogues against Nontuberculous Mycobacteria In Vitro. Jpn. J. Infect. Dis. 2015, 68, 520–522. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Wallace, R.J., Jr. In Vitro Susceptibility Testing of Tedizolid against Nontuberculous Mycobacteria. J. Clin. Microbiol. 2017, 55, 1747–1754. [Google Scholar] [CrossRef]
- Vera-Cabrera, L.; Brown-Elliott, B.A.; Wallace, R.J., Jr.; Ocampo-Candiani, J.; Welsh, O.; Choi, S.H.; Molina-Torres, C.A. In vitro activities of the novel oxazolidinones DA-7867 and DA-7157 against rapidly and slowly growing mycobacteria. Antimicrob. Agents Chemother. 2006, 50, 4027–4029. [Google Scholar] [CrossRef][Green Version]
- Kim, T.S.; Choe, J.H.; Kim, Y.J.; Yang, C.S.; Kwon, H.J.; Jeong, J.; Kim, G.; Park, D.E.; Jo, E.K.; Cho, Y.L.; et al. Activity of LCB01-0371, a Novel Oxazolidinone, against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2017, 61, e02752-16. [Google Scholar] [CrossRef]
- Deshpande, D.; Srivastava, S.; Pasipanodya, J.G.; Gumbo, T. Linezolid as treatment for pulmonary Mycobacterium avium disease. J. Antimicrob. Chemother. 2017, 72, i24–i29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wallace, R.J., Jr.; Brown-Elliott, B.A.; Ward, S.C.; Crist, C.J.; Mann, L.B.; Wilson, R.W. Activities of linezolid against rapidly growing mycobacteria. Antimicrob. Agents Chemother. 2001, 45, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, C.; Soyler, I.; Akinci, P. Activities of Linezolid against nontuberculous mycobacteria. New Microbiol. 2007, 30, 411–414. [Google Scholar]
- Brown-Elliott, B.A.; Wallace, R.J., Jr.; Blinkhorn, R.; Crist, C.J.; Mann, L.B. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin. Infect. Dis. 2001, 33, 1433–1434. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.D.; Porter, W.M. Mycobacterium chelonae infection successfully treated with oral clarithromycin and linezolid. Br. J. Dermatol. 2004, 151, 1101. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tsunoda, A.; Nishimoto, E.; Nishida, K.; Komatsubara, Y.; Onoe, R.; Saji, J.; Mineshita, M. Successful use of linezolid for refractory Mycobacterium abcessus infection: A case report. Respir. Med. Case Rep. 2018, 23, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Bostan, C.; Slim, E.; Choremis, J.; Boutin, T.; Brunette, I.; Mabon, M.; Talajic, J.C. Successful management of severe post-LASIK Mycobacterium abscessus keratitis with topical amikacin and linezolid, flap ablation, and topical corticosteroids. J. Cataract. Refract. Surg. 2019, 45, 1032–1035. [Google Scholar] [CrossRef]
- Yasar, K.K.; Pehlivanoglu, F.; Sengoz, G.; Cabioglu, N. Successfully treated Mycobacterium abscessus mastitis: A rare cause of breast masses. Indian J. Med. Microbiol. 2011, 29, 425–427. [Google Scholar] [CrossRef]
- Diacon, A.H.; Pym, A.; Grobusch, M.P.; de los Rios, J.M.; Gotuzzo, E.; Vasilyeva, I.; Leimane, V.; Andries, K.; Bakare, N.; De Marez, T.; et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N. Engl. J. Med. 2014, 371, 723–732. [Google Scholar] [CrossRef]
- Diacon, A.H.; Pym, A.; Grobusch, M.; Patientia, R.; Rustomjee, R.; Page-Shipp, L.; Pistorius, C.; Krause, R.; Bogoshi, M.; Churchyard, G.; et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 2009, 360, 2397–2405. [Google Scholar] [CrossRef]
- TB Alliance. Tmc-207. Tuberculosis 2008, 88, 168–169. [Google Scholar] [CrossRef]
- Martin, A.; Godino, I.T.; Aguilar-Ayala, D.A.; Mathys, V.; Lounis, N.; Villalobos, H.R. In vitro activity of bedaquiline against slow-growing nontuberculous mycobacteria. J. Med. Microbiol. 2019, 68, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jhun, B.W.; Moon, S.M.; Kim, S.Y.; Jeon, K.; Kwon, O.J.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. In Vitro Activity of Bedaquiline and Delamanid against Nontuberculous Mycobacteria, Including Macrolide-Resistant Clinical Isolates. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Ayala, D.A.; Cnockaert, M.; Andre, E.; Andries, K.; Gonzalez, Y.M.J.A.; Vandamme, P.; Palomino, J.C.; Martin, A. In vitro activity of bedaquiline against rapidly growing nontuberculous mycobacteria. J. Med. Microbiol. 2017, 66, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ye, M.; Guo, Q.; Zhang, Z.; Yang, S.; Ma, W.; Yu, F.; Chu, H. Determination of MIC Distribution and Mechanisms of Decreased Susceptibility to Bedaquiline among Clinical Isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zheng, H.; Tan, Y.; Song, Y.; Zhao, Y. In Vitro Activity of Bedaquiline against Nontuberculous Mycobacteria in China. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Dupont, C.; Viljoen, A.; Thomas, S.; Roquet-Baneres, F.; Herrmann, J.L.; Pethe, K.; Kremer, L. Bedaquiline Inhibits the ATP Synthase in Mycobacterium abscessus and Is Effective in Infected Zebrafish. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Wallace, R.J., Jr. In Vitro Susceptibility Testing of Bedaquiline against Mycobacterium abscessus Complex. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Philley, J.V.; Griffith, D.E.; Thakkar, F.; Wallace, R.J., Jr. In Vitro Susceptibility Testing of Bedaquiline against Mycobacterium avium Complex. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Yu, X.; Gao, X.; Li, C.; Luo, J.; Wen, S.; Zhang, T.; Ma, Y.; Dong, L.; Wang, F.; Huang, H. In Vitro Activities of Bedaquiline and Delamanid against Nontuberculous Mycobacteria Isolated in Beijing, China. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Lerat, I.; Cambau, E.; Roth Dit Bettoni, R.; Gaillard, J.L.; Jarlier, V.; Truffot, C.; Veziris, N. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J. Infect. Dis. 2014, 209, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Lounis, N.; Gevers, T.; Van den Berg, J.; Vranckx, L.; Andries, K. ATP synthase inhibition of Mycobacterium avium is not bactericidal. Antimicrob. Agents Chemother. 2009, 53, 4927–4929. [Google Scholar] [CrossRef] [PubMed]
- Obregon-Henao, A.; Arnett, K.A.; Henao-Tamayo, M.; Massoudi, L.; Creissen, E.; Andries, K.; Lenaerts, A.J.; Ordway, D.J. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob. Agents Chemother. 2015, 59, 6904–6912. [Google Scholar] [CrossRef] [PubMed]
- Philley, J.V.; Wallace, R.J., Jr.; Benwill, J.L.; Taskar, V.; Brown-Elliott, B.A.; Thakkar, F.; Aksamit, T.R.; Griffith, D.E. Preliminary Results of Bedaquiline as Salvage Therapy for Patients With Nontuberculous Mycobacterial Lung Disease. Chest 2015, 148, 499–506. [Google Scholar] [CrossRef]
- Alexander, D.C.; Vasireddy, R.; Vasireddy, S.; Philley, J.V.; Brown-Elliott, B.A.; Perry, B.J.; Griffith, D.E.; Benwill, J.L.; Cameron, A.D.; Wallace, R.J., Jr. Emergence of mmpT5 Variants during Bedaquiline Treatment of Mycobacterium intracellulare Lung Disease. J. Clin. Microbiol. 2017, 55, 574–584. [Google Scholar] [CrossRef]
- Lindman, M.; Dick, T. Bedaquiline Eliminates Bactericidal Activity of beta-Lactams against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Svensson, E.M.; Murray, S.; Karlsson, M.O.; Dooley, K.E. Rifampicin and rifapentine significantly reduce concentrations of bedaquiline, a new anti-TB drug. J. Antimicrob. Chemother. 2015, 70, 1106–1114. [Google Scholar] [CrossRef]
- Liu, Y.; Matsumoto, M.; Ishida, H.; Ohguro, K.; Yoshitake, M.; Gupta, R.; Geiter, L.; Hafkin, J. Delamanid: From discovery to its use for pulmonary multidrug-resistant tuberculosis (MDR-TB). Tuberculosis 2018, 111, 20–30. [Google Scholar] [CrossRef]
- Krieger, D.; Schonfeld, N.; Vesenbeckh, S.; Bettermann, G.; Bauer, T.T.; Russmann, H.; Mauch, H. Is delamanid a potential agent in the treatment of diseases caused by Mycobacterium avium-intracellulare? Eur. Respir. J. 2016, 48, 1803–1804. [Google Scholar] [CrossRef]
- Pandey, R.; Chen, L.; Manca, C.; Jenkins, S.; Glaser, L.; Vinnard, C.; Stone, G.; Lee, J.; Mathema, B.; Nuermberger, E.L.; et al. Dual beta-Lactam Combinations Highly Active against Mycobacterium abscessus Complex In Vitro. MBio 2019, 10, e02895-18. [Google Scholar] [CrossRef]
- Story-Roller, E.; Maggioncalda, E.C.; Lamichhane, G. Select beta-Lactam Combinations Exhibit Synergy against Mycobacterium abscessus In Vitro. Antimicrob. Agents Chemother. 2019, 63, e02613-18. [Google Scholar] [CrossRef] [PubMed]
- Story-Roller, E.; Maggioncalda, E.C.; Lamichhane, G. Synergistic Efficacy of beta-Lactam Combinations against Mycobacterium abscessus Pulmonary Infection in Mice. Antimicrob. Agents Chemother. 2019, 63, e00614-19. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Ammerman, N.C.; Lee, J.; Martins, O.; Kreiswirth, B.N.; Lamichhane, G.; Parrish, N.M.; Nuermberger, E.L. In Vitro Activity of the New beta-Lactamase Inhibitors Relebactam and Vaborbactam in Combination with beta-Lactams against Mycobacterium abscessus Complex Clinical Isolates. Antimicrob. Agents Chemother. 2019, 63, e02623-18. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Gupta, C.; Fisher, S.; Story-Roller, E.; Galanis, C.; Parrish, N.; Lamichhane, G. Combinations of avibactam and carbapenems exhibit enhanced potencies against drug-resistant Mycobacterium abscessus. Future Microbiol. 2017, 12, 473–480. [Google Scholar] [CrossRef]
- Lefebvre, A.L.; Le Moigne, V.; Bernut, A.; Veckerle, C.; Compain, F.; Herrmann, J.L.; Kremer, L.; Arthur, M.; Mainardi, J.L. Inhibition of the beta-Lactamase BlaMab by Avibactam Improves the In Vitro and In Vivo Efficacy of Imipenem against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2017, 61, e02440-16. [Google Scholar] [CrossRef]
- Dubee, V.; Bernut, A.; Cortes, M.; Lesne, T.; Dorchene, D.; Lefebvre, A.L.; Hugonnet, J.E.; Gutmann, L.; Mainardi, J.L.; Herrmann, J.L.; et al. beta-Lactamase inhibition by avibactam in Mycobacterium abscessus. J. Antimicrob. Chemother. 2015, 70, 1051–1058. [Google Scholar] [CrossRef]
- Dubee, V.; Soroka, D.; Cortes, M.; Lefebvre, A.L.; Gutmann, L.; Hugonnet, J.E.; Arthur, M.; Mainardi, J.L. Impact of beta-lactamase inhibition on the activity of ceftaroline against Mycobacterium tuberculosis and Mycobacterium abscessus. Antimicrob. Agents Chemother. 2015, 59, 2938–2941. [Google Scholar] [CrossRef]
- Lefebvre, A.L.; Dubee, V.; Cortes, M.; Dorchene, D.; Arthur, M.; Mainardi, J.L. Bactericidal and intracellular activity of beta-lactams against Mycobacterium abscessus. J. Antimicrob. Chemother. 2016, 71, 1556–1563. [Google Scholar] [CrossRef]
- Bentur, L.; Gur, M.; Ashkenazi, M.; Livnat-Levanon, G.; Mizrahi, M.; Tal, A.; Ghaffari, A.; Geffen, Y.; Aviram, M.; Efrati, O. Pilot study to test inhaled nitric oxide in cystic fibrosis patients with refractory Mycobacterium abscessus lung infection. J. Cyst. Fibros. 2019. [Google Scholar] [CrossRef]
- Yaacoby-Bianu, K.; Gur, M.; Toukan, Y.; Nir, V.; Hakim, F.; Geffen, Y.; Bentur, L. Compassionate Nitric Oxide Adjuvant Treatment of Persistent Mycobacterium Infection in Cystic Fibrosis Patients. Pediatr. Infect. Dis. J. 2018, 37, 336–338. [Google Scholar] [CrossRef]
- Chau, T.; Blade, K.; Da Silva, J.; Ghaffari, A.; Zelazny, A.; Olivier, K. High Efficacy of High-dose Nitric Oxide and its Synergistic Effect with Antibiotics against Mycobacterium Abscessus. Eur. Respirat. J. 2019, 54, OA4950. [Google Scholar] [CrossRef]
- Dupont, C.; Viljoen, A.; Dubar, F.; Blaise, M.; Bernut, A.; Pawlik, A.; Bouchier, C.; Brosch, R.; Guerardel, Y.; Lelievre, J.; et al. A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol. Microbiol. 2016, 101, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Onajole, O.K.; Stec, J.; Dupont, C.; Viljoen, A.; Richard, M.; Chaira, T.; Lun, S.; Bishai, W.; Raj, V.S.; et al. Targeting Mycolic Acid Transport by Indole-2-carboxamides for the Treatment of Mycobacterium abscessus Infections. J. Med. Chem. 2017, 60, 5876–5888. [Google Scholar] [CrossRef] [PubMed]
- Franz, N.D.; Belardinelli, J.M.; Kaminski, M.A.; Dunn, L.C.; Calado Nogueira de Moura, V.; Blaha, M.A.; Truong, D.D.; Li, W.; Jackson, M.; North, E.J. Design, synthesis and evaluation of indole-2-carboxamides with pan anti-mycobacterial activity. Bioorg. Med. Chem. 2017, 25, 3746–3755. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Rubio, A.; Wallace, R.J., Jr. In Vitro Susceptibility Testing of a Novel Benzimidazole, SPR719, against Nontuberculous Mycobacteria. Antimicrob. Agents Chemother. 2018, 62, e01503-18. [Google Scholar] [CrossRef]
- Locher, C.P.; Jones, S.M.; Hanzelka, B.L.; Perola, E.; Shoen, C.M.; Cynamon, M.H.; Ngwane, A.H.; Wiid, I.J.; van Helden, P.D.; Betoudji, F.; et al. A novel inhibitor of gyrase B is a potent drug candidate for treatment of tuberculosis and nontuberculosis mycobacterial infections. Antimicrob. Agents Chemother. 2015, 59, 1455–1465. [Google Scholar] [CrossRef]
- Cynamon, M.; Jureller, J.; Desai, B.; Ramachandran, K.; Sklaney, M.; Grossman, T.H. In vitro activity of TP-271 against Mycobacterium abscessus, Mycobacterium fortuitum, and Nocardia species. Antimicrob. Agents Chemother. 2012, 56, 3986–3988. [Google Scholar] [CrossRef]
- Madani, A.; Ridenour, J.N.; Martin, B.P.; Paudel, R.R.; Abdul Basir, A.; Le Moigne, V.; Herrmann, J.L.; Audebert, S.; Camoin, L.; Kremer, L.; et al. Cyclipostins and Cyclophostin Analogues as Multitarget Inhibitors That Impair Growth of Mycobacterium abscessus. ACS Infect. Dis. 2019, 5, 1597–1608. [Google Scholar] [CrossRef]
- Nguyen, P.C.; Madani, A.; Santucci, P.; Martin, B.P.; Paudel, R.R.; Delattre, S.; Herrmann, J.L.; Spilling, C.D.; Kremer, L.; Canaan, S.; et al. Cyclophostin and Cyclipostins analogues, new promising molecules to treat mycobacterial-related diseases. Int. J. Antimicrob. Agents 2018, 51, 651–654. [Google Scholar] [CrossRef]
- Baranyai, Z.; Kratky, M.; Vinsova, J.; Szabo, N.; Senoner, Z.; Horvati, K.; Stolarikova, J.; David, S.; Bosze, S. Combating highly resistant emerging pathogen Mycobacterium abscessus and Mycobacterium tuberculosis with novel salicylanilide esters and carbamates. Eur. J. Med. Chem. 2015, 101, 692–704. [Google Scholar] [CrossRef]
- Kratky, M.; Bosze, S.; Baranyai, Z.; Szabo, I.; Stolarikova, J.; Paraskevopoulos, G.; Vinsova, J. Synthesis and in vitro biological evaluation of 2-(phenylcarbamoyl)phenyl 4-substituted benzoates. Bioorg. Med. Chem. 2015, 23, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, T.; Bogatcheva, E.; Krishnan, M.Y.; Collins, M.T.; Einck, L.; Nacy, C.A.; Reddy, V.M. In vitro antimicrobial activities of capuramycin analogues against non-tuberculous mycobacteria. J. Antimicrob. Chemother. 2010, 65, 2590–2597. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, Y.; Cai, W.; Green, K.D.; Goswami, A.; Garneau-Tsodikova, S.; Nonaka, K.; Baba, S.; Funabashi, M.; Yang, Z.; et al. A biocatalytic approach to capuramycin analogues by exploiting a substrate permissive N-transacylase CapW. Org. Biomol. Chem. 2016, 14, 3956–3962. [Google Scholar] [CrossRef] [PubMed]
- Molina-Torres, C.A.; Ocampo-Candiani, J.; Rendon, A.; Pucci, M.J.; Vera-Cabrera, L. In vitro activity of a new isothiazoloquinolone, ACH-702, against Mycobacterium tuberculosis and other mycobacteria. Antimicrob. Agents Chemother. 2010, 54, 2188–2190. [Google Scholar] [CrossRef]
- Moraski, G.C.; Cheng, Y.; Cho, S.; Cramer, J.W.; Godfrey, A.; Masquelin, T.; Franzblau, S.G.; Miller, M.J.; Schorey, J. Imidazo[1,2-a]Pyridine-3-Carboxamides Are Active Antimicrobial Agents against Mycobacterium avium Infection In Vivo. Antimicrob. Agents Chemother. 2016, 60, 5018–5022. [Google Scholar] [CrossRef]
- Millar, B.C.; Moore, J.E. Antimycobacterial strategies to evade antimicrobial resistance in the nontuberculous mycobacteria. Int. J. Mycobacteriol. 2019, 8, 7–21. [Google Scholar] [CrossRef]
- Chopra, S.; Matsuyama, K.; Hutson, C.; Madrid, P. Identification of antimicrobial activity among FDA-approved drugs for combating Mycobacterium abscessus and Mycobacterium chelonae. J. Antimicrob. Chemother. 2011, 66, 1533–1536. [Google Scholar] [CrossRef]
- Marini, E.; Di Giulio, M.; Ginestra, G.; Magi, G.; Di Lodovico, S.; Marino, A.; Facinelli, B.; Cellini, L.; Nostro, A. Efficacy of carvacrol against resistant rapidly growing mycobacteria in the planktonic and biofilm growth mode. PLoS ONE 2019, 14, e0219038. [Google Scholar] [CrossRef]
- Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of Antimicrobial Action of Cinnamon and Oregano Oils, Cinnamaldehyde, Carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods 2017, 6, 72. [Google Scholar] [CrossRef]
- Bax, H.I.; de Vogel, C.P.; Mouton, J.W.; de Steenwinkel, J.E.M. Omadacycline as a promising new agent for the treatment of infections with Mycobacterium abscessus. J. Antimicrob. Chemother. 2019, 74, 2930–2933. [Google Scholar] [CrossRef]
- Minhas, R.; Sharma, S.; Kundu, S. Utilizing the Promise of Omadacycline in a Resistant, Non-tubercular Mycobacterial Pulmonary Infection. Cureus 2019, 11, e5112. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Ammerman, N.C.; Martins, O.; Parrish, N.M.; Nuermberger, E.L. In Vitro Activity of New Tetracycline Analogs Omadacycline and Eravacycline against Drug-Resistant Clinical Isolates of Mycobacterium abscessus. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Shoen, C.; Benaroch, D.; Sklaney, M.; Cynamon, M. In Vitro Activities of Omadacycline against Rapidly Growing Mycobacteria. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, L.E.; Kolonoski, P.; Wu, M.; Aralar, P.A.; Inderlied, C.B.; Young, L.S. Mefloquine Is Active In Vitro and In Vivo against Mycobacterium avium Complex. Antimicrob. Agents Chemother. 1999, 43, 1870–1874. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Inderlied, C.B.; Kolonoski, P.; Chee, C.B.; Aralar, P.; Petrofsky, M.; Parman, T.; Green, C.E.; Lewin, A.H.; Ellis, W.Y.; et al. Identification of (+)-erythro-mefloquine as an active enantiomer with greater efficacy than mefloquine against Mycobacterium avium infection in mice. Antimicrob. Agents Chemother. 2012, 56, 4202–4206. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Kolonoski, P.; Petrofsky, M.; Wu, M.; Inderlied, C.B.; Young, L.S. Mefloquine, Moxifloxacin, and Ethambutol Are a Triple-Drug Alternative to Macrolide-Containing Regimens for Treatment of Mycobacterium avium Disease. J. Infect. Dis. 2003, 187, 1977–1980. [Google Scholar] [CrossRef][Green Version]
- Deshpande, D.; Srivastava, S.; Musuka, S.; Gumbo, T. Thioridazine as Chemotherapy for Mycobacterium avium Complex Diseases. Antimicrob. Agents Chemother. 2016, 60, 4652–4658. [Google Scholar] [CrossRef]
- Srivastava, S.; Deshpande, D.; Sherman, C.M.; Gumbo, T. A ‘shock and awe’ thioridazine and moxifloxacin combination-based regimen for pulmonary Mycobacterium avium-intracellulare complex disease. J. Antimicrob. Chemother. 2017, 72, i43–i47. [Google Scholar] [CrossRef]
- Boelaert, J.R.; Appelberg, R.; Gomes, M.S.; Blasi, E.; Mazzolla, R.; Grosset, J.; Lounis, N.; Soteriadou, K.; Thiakaki, M.; Taramelli, D.; et al. Experimental Results on Chloroquine and AIDS-Relataed Opportunistic Infections. J. Acquir. Immunodefic. Syndr. 2001, 300–301. [Google Scholar] [CrossRef]
- Pavic, K.; Perkovic, I.; Pospisilova, S.; Machado, M.; Fontinha, D.; Prudencio, M.; Jampilek, J.; Coffey, A.; Endersen, L.; Rimac, H.; et al. Primaquine hybrids as promising antimycobacterial and antimalarial agents. Eur. J. Med. Chem. 2018, 143, 769–779. [Google Scholar] [CrossRef]
- Teixeira, C.; Vale, N.; Perez, B.; Gomes, A.; Gomes, J.R.; Gomes, P. “Recycling” classical drugs for malaria. Chem. Rev. 2014, 114, 11164–11220. [Google Scholar] [CrossRef] [PubMed]
- Coatney, G.R. Pitfalls in a discovery: The chronicle of chloroquine. Am. J. Trop. Med. Hyg. 1963, 12, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Moreira, R.; Gomes, P. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 2009, 44, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Lougheed, K.E.; Taylor, D.L.; Osborne, S.A.; Bryans, J.S.; Buxton, R.S. New anti-tuberculosis agents amongst known drugs. Tuberculosis 2009, 89, 364–370. [Google Scholar] [CrossRef]
- Ferraz, R.; Noronha, J.; Murtinheira, F.; Nogueira, F.; Machado, M.; Prudêncio, M.; Parapini, S.; D’Alessandro, S.; Teixeira, C.; Gomes, A.; et al. Primaquine-based ionic liquids as a novel class of antimalarial hits. RSC Adv. 2016, 6, 56134–56138. [Google Scholar] [CrossRef]
- Ferraz, R.; Pinheiro, M.; Gomes, A.; Teixeira, C.; Prudencio, C.; Reis, S.; Gomes, P. Effects of novel triple-stage antimalarial ionic liquids on lipid membrane models. Bioorg. Med. Chem. Lett. 2017, 27, 4190–4193. [Google Scholar] [CrossRef]
- Ferraz, R.; Teixeira, C.; Gomes, P.; Prudêncio, C. Chapter 16. Bioactivity of Ionic Liquids. In Ionic Liquid Devices; The Royal Society of Chemistry: London, UK, 2018; pp. 404–422. [Google Scholar]
- Ferraz, R.; Teixeira, V.; Rodrigues, D.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Antibacterial activity of Ionic Liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef]
- Gomes, A.; Ferraz, R.; Ficker, L.; Collins, M.S.; Prudencio, C.; Cushion, M.T.; Teixeira, C.; Gomes, P. Chloroquine Analogues as Leads against Pneumocystis Lung Pathogens. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Bento, C.M. Evaluation of the Effects of Selected Ionic Liquids against Mycobacterium avium. Master’s Thesis, University of Porto, Porto, Portugal, 2019. [Google Scholar]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Silva, T.; Gomes, M.S. Immuno-Stimulatory Peptides as a Potential Adjunct Therapy against Intra-Macrophagic Pathogens. Molecules 2017, 22, 1297. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Saikia, U.N.; Sharma, S.; Verma, I. Activity of human beta defensin-1 and its motif against active and dormant Mycobacterium tuberculosis. Appl. Microbiol. Biotechnol. 2017, 101, 7239–7248. [Google Scholar] [CrossRef] [PubMed]
- Gutsmann, T. Interaction between antimicrobial peptides and mycobacteria. Biochim. Biophys. Acta 2016, 1858, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- AlMatar, M.; Makky, E.A.; Yakici, G.; Var, I.; Kayar, B.; Koksal, F. Antimicrobial peptides as an alternative to anti-tuberculosis drugs. Pharmacol. Res. 2018, 128, 288–305. [Google Scholar] [CrossRef]
- Yuk, J.M.; Shin, D.M.; Lee, H.M.; Yang, C.S.; Jin, H.S.; Kim, K.K.; Lee, Z.W.; Lee, S.H.; Kim, J.M.; Jo, E.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Castaneda-Delgado, J.E.; Rivas Santiago, C.E.; Waldbrook, M.; Gonzalez-Curiel, I.; Leon-Contreras, J.C.; Enciso-Moreno, J.A.; del Villar, V.; Mendez-Ramos, J.; Hancock, R.E.; et al. Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis infections in animal models. PLoS ONE 2013, 8, e59119. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, I.; Khuller, G.K. Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis. Antimicrob. Agents Chemother. 2001, 45, 639–640. [Google Scholar] [CrossRef]
- Kalita, A.; Verma, I.; Khuller, G.K. Role of human neutrophil peptide-1 as a possible adjunct to antituberculosis chemotherapy. J. Infect. Dis. 2004, 190, 1476–1480. [Google Scholar] [CrossRef]
- Yoshida, N.; Tani, Y.; Ogata, K. Cryomycin, a new peptide antibiotic produced only at low temperature. J. Antibiot. 1972, 25, 653–659. [Google Scholar] [CrossRef][Green Version]
- Gao, W.; Kim, J.Y.; Anderson, J.R.; Akopian, T.; Hong, S.; Jin, Y.Y.; Kandror, O.; Kim, J.W.; Lee, I.A.; Lee, S.Y.; et al. The cyclic peptide ecumicin targeting ClpC1 is active against Mycobacterium tuberculosis in vivo. Antimicrob. Agents Chemother. 2015, 59, 880–889. [Google Scholar] [CrossRef]
- Gavrish, E.; Sit, C.S.; Cao, S.; Kandror, O.; Spoering, A.; Peoples, A.; Ling, L.; Fetterman, A.; Hughes, D.; Bissell, A.; et al. Lassomycin, a ribosomally synthesized cyclic peptide, kills mycobacterium tuberculosis by targeting the ATP-dependent protease ClpC1P1P2. Chem. Biol. 2014, 21, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Draper, L.A.; O’Connor, P.M.; Coffey, A.; Hill, C.; Ross, R.P.; Cotter, P.D.; O’Mahony, J. Comparison of the activities of the lantibiotics nisin and lacticin 3147 against clinically significant mycobacteria. Int. J. Antimicrob. Agents 2010, 36, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Field, D.; O’Connor, P.M.; Cotter, P.D.; Coffey, A.; Hill, C.; Ross, R.P.; O’Mahony, J. Gene encoded antimicrobial peptides, a template for the design of novel anti-mycobacterial drugs. Bioeng. Bugs 2010, 1, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.I.; Saudi, A.M.; Albrecht, R.; Talaat, A.M. The inhibitory effect of nisin on Mycobacterium avium ssp. paratuberculosis and its effect on mycobacterial cell wall. J. Dairy Sci. 2019, 102, 4935–4944. [Google Scholar] [CrossRef]
- Cirone, K.M.; Lahiri, P.; Holani, R.; Tan, Y.L.; Arrazuria, R.; De Buck, J.; Barkema, H.W.; Cobo, E.R. Synthetic cathelicidin LL-37 reduces Mycobacterium avium subsp. paratuberculosis internalization and pro-inflammatory cytokines in macrophages. Cell Tissue Res. 2019. [Google Scholar] [CrossRef]
- Mohanty, S.; Jena, P.; Mehta, R.; Pati, R.; Banerjee, B.; Patil, S.; Sonawane, A. Cationic antimicrobial peptides and biogenic silver nanoparticles kill mycobacteria without eliciting DNA damage and cytotoxicity in mouse macrophages. Antimicrob. Agents Chemother. 2013, 57, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Linzer, B.A.; Zuberi, R.I.; Ganz, T.; Lehrer, R.I.; Catanzaro, A. Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium-Mycobacterium intracellulare. Infect. Immun. 1992, 60, 4720–4725. [Google Scholar] [CrossRef]
- Silva, T.; Magalhaes, B.; Maia, S.; Gomes, P.; Nazmi, K.; Bolscher, J.G.; Rodrigues, P.N.; Bastos, M.; Gomes, M.S. Killing of Mycobacterium avium by lactoferricin peptides: Improved activity of arginine- and D-amino-acid-containing molecules. Antimicrob. Agents Chemother. 2014, 58, 3461–3467. [Google Scholar] [CrossRef]
- Silva, T.; Moreira, A.C.; Nazmi, K.; Moniz, T.; Vale, N.; Rangel, M.; Gomes, P.; Bolscher, J.G.M.; Rodrigues, P.N.; Bastos, M.; et al. Lactoferricin Peptides Increase Macrophages’ Capacity To Kill Mycobacterium avium. mSphere 2017, 2, e00301-17. [Google Scholar] [CrossRef]
- Adhya, M.; Jeung, H.D.; Kang, H.S.; Choi, K.S.; Lee, D.S.; Cho, M. Cloning and localization of MCdef, a defensin from Manila clams (Ruditapes philippinarum). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 161, 25–31. [Google Scholar] [CrossRef]
- Trentini, M.M.; das Neves, R.C.; Santos, B.P.; DaSilva, R.A.; de Souza, A.C.; Mortari, M.R.; Schwartz, E.F.; Kipnis, A.; Junqueira-Kipnis, A.P. Non-disulfide-Bridge Peptide 5.5 from the Scorpion Hadrurus gertschi Inhibits the Growth of Mycobacterium abscessus subsp. massiliense. Front. Microbiol. 2017, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- das Neves, R.C.; Trentini, M.M.; de Castro e Silva, J.; Simon, K.S.; Bocca, A.L.; Silva, L.P.; Mortari, M.R.; Kipnis, A.; Junqueira-Kipnis, A.P. Antimycobacterial Activity of a New Peptide Polydim-I Isolated from Neotropical Social Wasp Polybia dimorpha. PLoS ONE 2016, 11, e0149729. [Google Scholar] [CrossRef] [PubMed]
- Marques-Neto, L.M.; Trentini, M.M.; das Neves, R.C.; Resende, D.P.; Procopio, V.O.; da Costa, A.C.; Kipnis, A.; Mortari, M.R.; Schwartz, E.F.; Junqueira-Kipnis, A.P. Antimicrobial and Chemotactic Activity of Scorpion-Derived Peptide, ToAP2, against Mycobacterium massiliensis. Toxins 2018, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Neto, L.M.; Neves, R.C.; Goncalves, J.C.; Trentini, M.M.; Mucury-Filho, R.; Smidt, K.S.; Fensterseifer, I.C.; Silva, O.N.; Lima, L.D.; et al. Evaluation of the antimicrobial activity of the mastoparan Polybia-MPII isolated from venom of the social wasp Pseudopolybia vespiceps testacea (Vespidae, Hymenoptera). Int. J. Antimicrob. Agents 2017, 49, 167–175. [Google Scholar] [CrossRef]
- D’Herelle, F. Sur un microbe invisible antagoniste des bacilles dysentériques. C. R. Acad. Sci. 1917, 165, 373–375. [Google Scholar]
- Azimi, T.; Mosadegh, M.; Nasiri, M.J.; Sabour, S.; Karimaei, S.; Nasser, A. Phage therapy as a renewed therapeutic approach to mycobacterial infections: A comprehensive review. Infect. Drug Resist. 2019, 12, 2943–2959. [Google Scholar] [CrossRef]
- Dams, D.; Briers, Y. Enzybiotics: Enzyme-Based Antibacterials as Therapeutics. Adv. Exp. Med. Biol. 2019, 1148, 233–253. [Google Scholar] [CrossRef]
- Nieth, A.; Verseux, C.; Barnert, S.; Suss, R.; Romer, W. A first step toward liposome-mediated intracellular bacteriophage therapy. Expert Opin. Drug Deliv. 2015, 12, 1411–1424. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, M.; Fan, X.; Yan, J.; Li, W.; Xie, J. Mycobacteriophage SWU1 gp39 can potentiate multiple antibiotics against Mycobacterium via altering the cell wall permeability. Sci. Rep. 2016, 6, 28701. [Google Scholar] [CrossRef]
- Broxmeyer, L.; Sosnowska, D.; Miltner, E.; Chacon, O.; Wagner, D.; McGarvey, J.; Barletta, R.G.; Bermudez, L.E. Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: A model for phage therapy of intracellular bacterial pathogens. J. Infect. Dis. 2002, 186, 1155–1160. [Google Scholar] [CrossRef]
- Danelishvili, L.; Young, L.S.; Bermudez, L.E. In vivo efficacy of phage therapy for Mycobacterium avium infection as delivered by a nonvirulent mycobacterium. Microb. Drug Resist. 2006, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.; Sun, Q.; Sacchettini, J.; Hatfull, G.F. Mycobacteriophage Lysin B is a novel mycolylarabinogalactan esterase. Mol. Microbiol. 2009, 73, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Catalao, M.J.; Milho, C.; Gil, F.; Moniz-Pereira, J.; Pimentel, M. A second endolysin gene is fully embedded in-frame with the lysA gene of mycobacteriophage Ms6. PLoS ONE 2011, 6, e20515. [Google Scholar] [CrossRef] [PubMed]
- Grover, N.; Paskaleva, E.E.; Mehta, K.K.; Dordick, J.S.; Kane, R.S. Growth inhibition of Mycobacterium smegmatis by mycobacteriophage-derived enzymes. Enzyme Microb. Technol. 2014, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.J.; Liu, C.C.; Jiang, S.J.; Soo, P.C.; Tu, M.H.; Lee, J.J.; Chen, Y.H.; Chang, K.C. Antimycobacterial Activities of Endolysins Derived From a Mycobacteriophage, BTCU-1. Molecules 2015, 20, 19277–19290. [Google Scholar] [CrossRef]
- Silva-Gomes, S.; Vale-Costa, S.; Appelberg, R.; Gomes, M.S. Iron in intracellular infection: To provide or to deprive? Front. Cell Infect. Microbiol. 2013, 3, 96. [Google Scholar] [CrossRef]
- Sritharan, M. Iron Homeostasis in Mycobacterium tuberculosis: Mechanistic Insights into Siderophore-Mediated Iron Uptake. J. Bacteriol. 2016, 198, 2399–2409. [Google Scholar] [CrossRef]
- Jones, C.M.; Niederweis, M. Mycobacterium tuberculosis can utilize heme as an iron source. J. Bacteriol. 2011, 193, 1767–1770. [Google Scholar] [CrossRef][Green Version]
- Tullius, M.V.; Harmston, C.A.; Owens, C.P.; Chim, N.; Morse, R.P.; McMath, L.M.; Iniguez, A.; Kimmey, J.M.; Sawaya, M.R.; Whitelegge, J.P.; et al. Discovery and characterization of a unique mycobacterial heme acquisition system. Proc. Natl. Acad. Sci. USA 2011, 108, 5051–5056. [Google Scholar] [CrossRef] [PubMed]
- Nambu, S.; Matsui, T.; Goulding, C.W.; Takahashi, S.; Ikeda-Saito, M. A new way to degrade heme: The Mycobacterium tuberculosis enzyme MhuD catalyzes heme degradation without generating CO. J. Biol. Chem. 2013, 288, 10101–10109. [Google Scholar] [CrossRef]
- Kelley, V.A.; Schorey, J.S. Mycobacterium’s arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol. Biol. Cell 2003, 14, 3366–3377. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Hatfull, G.F. The role of iron in Mycobacterium smegmatis biofilm formation: The exochelin siderophore is essential in limiting iron conditions for biofilm formation but not for planktonic growth. Mol. Microbiol. 2007, 66, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Thomas, J.; Li, Y.; Vilcheze, C.; Derbyshire, K.M.; Jacobs, W.R., Jr.; Ojha, A.K. Defining a temporal order of genetic requirements for development of mycobacterial biofilms. Mol. Microbiol. 2017, 105, 794–809. [Google Scholar] [CrossRef] [PubMed]
- De Voss, J.J.; Rutter, K.; Schroeder, B.G.; Su, H.; Zhu, Y.; Barry, C.E., 3rd. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 2000, 97, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.; Puri, R.V.; Chauhan, P.; Kar, R.; Rohilla, A.; Khera, A.; Tyagi, A.K. Disruption of mycobactin biosynthesis leads to attenuation of Mycobacterium tuberculosis for growth and virulence. J. Infect. Dis. 2013, 208, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Moreira, A.C.; Mesquita, G.; Gomes, M.S. Modulation of Iron Metabolism in Response to Infection: Twists for All Tastes. Pharmaceuticals 2018, 11, 84. [Google Scholar] [CrossRef]
- Gomes, M.S.; Dom, G.; Pedrosa, J.; Boelaert, J.R.; Appelberg, R. Effects of iron deprivation on Mycobacterium avium growth. Tuber Lung Dis. 1999, 79, 321–328. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Nunes, A.; Gomes, A.R.; de Castro, B.; Hider, R.C.; Rangel, M.; Appelberg, R.; Gomes, M.S. Identification of a new hexadentate iron chelator capable of restricting the intramacrophagic growth of Mycobacterium avium. Microbes Infect. 2010, 12, 287–294. [Google Scholar] [CrossRef]
- Moniz, T.; Leite, A.; Silva, T.; Gameiro, P.; Gomes, M.S.; de Castro, B.; Rangel, M. The influence of functional groups on the permeation and distribution of antimycobacterial rhodamine chelators. J. Inorg. Biochem. 2017, 175, 138–147. [Google Scholar] [CrossRef]
- Moniz, T.; Nunes, A.; Silva, A.M.; Queiros, C.; Ivanova, G.; Gomes, M.S.; Rangel, M. Rhodamine labeling of 3-hydroxy-4-pyridinone iron chelators is an important contribution to target Mycobacterium avium infection. J. Inorg. Biochem. 2013, 121, 156–166. [Google Scholar] [CrossRef]
- Moniz, T.; Silva, D.; Silva, T.; Gomes, M.S.; Rangel, M. Antimycobacterial activity of rhodamine 3,4-HPO iron chelators against Mycobacterium avium: Analysis of the contribution of functional groups and of chelator’s combination with ethambutol. MedChemComm 2015, 6, 2194–2203. [Google Scholar] [CrossRef]
- Tatano, Y.; Kanehiro, Y.; Sano, C.; Shimizu, T.; Tomioka, H. ATP exhibits antimicrobial action by inhibiting bacterial utilization of ferric ions. Sci. Rep. 2015, 5, 8610. [Google Scholar] [CrossRef] [PubMed]
- Dragset, M.S.; Poce, G.; Alfonso, S.; Padilla-Benavides, T.; Ioerger, T.R.; Kaneko, T.; Sacchettini, J.C.; Biava, M.; Parish, T.; Arguello, J.M.; et al. A novel antimycobacterial compound acts as an intracellular iron chelator. Antimicrob. Agents Chemother. 2015, 59, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Torfs, E.; Piller, T.; Cos, P.; Cappoen, D. Opportunities for Overcoming Mycobacterium tuberculosis Drug Resistance: Emerging Mycobacterial Targets and Host-Directed Therapy. Int. J. Mol. Sci. 2019, 20, 2868. [Google Scholar] [CrossRef]
- Silva, R.A.; Pais, T.F.; Appelberg, R. Evaluation of IL-12 in Immunotherapy and Vaccine Design in Experimental Mycobacterium avium Infections. J. Immunol. 1998, 5578–5585. [Google Scholar]
- Kim, S.H.; Cho, D.; Kim, T.S. Induction of in vivo resistance to Mycobacterium avium infection by intramuscular injection with DNA encoding interleukin-18. Immunology 2001, 234–241. [Google Scholar] [CrossRef]
- Skerry, C.; Harper, J.; Klunk, M.; Bishai, W.R.; Jain, S.K. Adjunctive TNF inhibition with standard treatment enhances bacterial clearance in a murine model of necrotic TB granulomas. PLoS ONE 2012, 7, e39680. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Chang, E.; Yamashita, S.; Iademarco, M.F.; LoBue, P.A. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg. Infect. Dis. 2009, 15, 1556–1561. [Google Scholar] [CrossRef]
- Yoo, J.W.; Jo, K.W.; Kang, B.H.; Kim, M.Y.; Yoo, B.; Lee, C.K.; Kim, Y.G.; Yang, S.K.; Byeon, J.S.; Kim, K.J.; et al. Mycobacterial diseases developed during anti-tumour necrosis factor-alpha therapy. Eur. Respir. J. 2014, 44, 1289–1295. [Google Scholar] [CrossRef]
- Dorhoi, A.; Kaufmann, S.H. Tumor necrosis factor alpha in mycobacterial infection. Semin. Immunol. 2014, 26, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.D.; Zhu, G.; Tsai, M.C.; Mohan, V.P.; Marino, S.; Kirschner, D.E.; Huang, L.; Flynn, J.; Chan, J. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect. Immun. 2008, 76, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.C.; Wang, J.Y.; Wu, M.F.; Wu, C.T.; Lai, H.C.; Lee, L.N.; Chiang, B.L.; Yu, C.J. Attenuation of lymphocyte immune responses during Mycobacterium avium complex-induced lung disease due to increasing expression of programmed death-1 on lymphocytes. Sci. Rep. 2017, 7, 42004. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef]
- Zullo, A.J.; Jurcic Smith, K.L.; Lee, S. Mammalian target of Rapamycin inhibition and mycobacterial survival are uncoupled in murine macrophages. BMC Biochem. 2014, 15, 4. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Zullo, A.J.; Lee, S. Mycobacterial induction of autophagy varies by species and occurs independently of mammalian target of rapamycin inhibition. J. Biol. Chem. 2012, 287, 12668–12678. [Google Scholar] [CrossRef]
| Compound | Description | NTM | Ref. |
|---|---|---|---|
| Nitric oxide | Inhaled nitric oxide to treat MABC lung disease with ongoing clinical trials. Shows synergistic effect with antimycobacterial antibiotics, such as clofazimine. | M. abscessus | [109,110,111] |
| PIPD1 | Piperidinol-based molecule that targets mycolic acid transport. | M. abscessus | [112] |
| Indolecarboxamide analogs | Structure-activity relationship studies of a series of indolecarboxamide analogs that target mycolic acid transport. | M. abscessus; M. chelonae; M. massiliense; M. bolletii; MAC; M. xenopi | [113,114] |
| Benzimidazole SPR719 | Active form of the prodrug SPR720, is an aminobenzimidazole that inhibits the ATPase activity of gyrase in Mtb. | MAC; MABC; M. chelonae; M. immunogenum; M. fortuitum; M. mucogenicum; M. kansasii; M. marinum; M. simiae | [115,116] |
| TP-271 | Novel fluorocycline antimicrobial related to tetracycline; active in vitro against NTM isolates. | M. abscessus; M. fortuitum | [117] |
| CyCs | Cyclipostins and cyclophostin analogs with selective in vitro and intramacrophagic activity against mycobacteria; mechanism of action related to enzyme-inhibition involved in lipid metabolism and/or cell wall biosynthesis. | M. abscessus; M. marinum; M. smegmatis | [118,119] |
| Salicylanilide esters, carbamates and benzoates | De novo synthesized molecules with in vitro potency against M. abscessus; ability to inhibit various bacterial enzymes and to function as proton shuttles, destroying the cellular proton gradient killing the bacteria. | M. abscessus; M. avium; M. kansasii | [120,121] |
| Capuramycin analogs | Nucleoside antibiotics that target peptidoglycan synthesis, with in vitro activity against several species of NTM. | MAC; M. paratuberculosis; M. kansasii; M. abscessus; M. smegmatis; M. ulcerans | [122,123] |
| ACH-702 | Isothiazoloquinolones, analogs related to quinolones, which target bacterial replication; in vitro activity against NTM. | MAC; M. fortuitum | [124] |
| IAPs | Imidazo [1,2-a]pyridine-3-carboxamides; potential in vitro and in vivo activity against MAC. | MAC | [125] |
| Compound | Description | NTM | Ref. |
|---|---|---|---|
| Carvacrol | Major constituent of many essential oils of the Labiatae family; Generally recognized as safe (GRAS) and approved for use in food; Antioxidant, anti-inflammatory, antitumor, analgesic, antihepatotoxic, and insecticidal activities; Activity in vitro against planktonic and biofilm cells of several RGM. | M. abscessus; M. fortuitum; M. chelonae; M. mucogenicum; M. smegmatis. Biofilm inhibiting activity | [128,129] |
| Omadacycline | Tetracycline, used for skin infections and community-acquired pneumonia caused by Gram-positive bacteria; In vitro activity against M. abscessus. | M. abscessus; M. chelonae; M. fortuitum | [130,131,132,133] |
| Mefloquine and enantiomers | Derivative of 4-quinolinemethanol; An antimicrobial drug used against chloroquine-resistant Plasmodium falciparum; Active in vitro and in vivo against MAC; Synergistic effect with antimycobacterial drugs in vivo. | MAC | [134,135,136] |
| Thioridazine | Phenothiazine derivative, an antipsychotic drug with activity against Mtb, by inhibition of the electron transport chain; In vitro activity in a hollow-fiber system model for pulmonary MAC disease (HFS-MAC). | MAC | [137,138] |
| Chloroquine | Antimalarial with activity in vitro and in vivo against M. avium. Also active in vitro against HIV-1. | MAC | [139] |
| Primaquine | Urea derivatives of this antimalarial showed high activity in vitro against M. avium. | MAC | [140] |
| AMP | Origin | NTM Species | Activity | Ref. |
|---|---|---|---|---|
| Ecumicin | Extracts from actinomycetes | M. abscessus; M. chelonae; M. marinum; M. kansasii; M. avium | Axenic | [162] |
| Lassomycin | Extracts from actinomycetes | M. avium | Axenic | [163] |
| Nisin | Lactococcus lactis | M. paratuberculosis | Axenic | [166] |
| Nisin A, S, T, and V | Lactococcus lactis | M. kansasii; M. avium | Axenic | [165] |
| Lacticin 3147 | Lactococcus lactis | M. kansasii; M. avium | Axenic | [164] |
| LL-37 | Human Cathelicidin | M. avium | Macrophages | [167] |
| LLKKK-18 (plus nanoparticles) | Cathelicidin LL-37 | M. marinum | Axenic; macrophages | [168] |
| NK-2 (plus nanoparticles) | NK cells and cytotoxic T cells | M. marinum | Axenic; macrophages | [168] |
| HNP-1, 2 and 3 | Human neutrophils | M. avium | Axenic | [169] |
| hLFcin1-11 and variants | Human lactoferricin | M. avium | Axenic | [170] |
| LFcin17-30 and variants | Bovine lactoferricin | M. avium | Axenic; macrophages | [170,171] |
| Mcdef | Manila clams (Ruditapes philippinarum) | M. fortuitum | Axenic | [172] |
| NDBP-5.5 | Scorpion (Hadrurus gertschi) | M. abscessus | Anexic; macrophages; in vivo | [173] |
| ToAP2 | Scorpion (Tityus obscurus) | M. massiliense | Axenic; macrophages; in vivo | [175] |
| Polydim-I | Wasp (Polybia dimorpha) | M. abscessus | Anexic; macrophages; in vivo | [174] |
| Polybia-MPII | Mastoparans from wasp (Pseudopolybia vespiceps) | M. abscessus sp. massiliense | Axenic; macrophages | [176] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento, C.M.; Gomes, M.S.; Silva, T. Looking beyond Typical Treatments for Atypical Mycobacteria. Antibiotics 2020, 9, 18. https://doi.org/10.3390/antibiotics9010018
Bento CM, Gomes MS, Silva T. Looking beyond Typical Treatments for Atypical Mycobacteria. Antibiotics. 2020; 9(1):18. https://doi.org/10.3390/antibiotics9010018
Chicago/Turabian StyleBento, Clara M., Maria Salomé Gomes, and Tânia Silva. 2020. "Looking beyond Typical Treatments for Atypical Mycobacteria" Antibiotics 9, no. 1: 18. https://doi.org/10.3390/antibiotics9010018
APA StyleBento, C. M., Gomes, M. S., & Silva, T. (2020). Looking beyond Typical Treatments for Atypical Mycobacteria. Antibiotics, 9(1), 18. https://doi.org/10.3390/antibiotics9010018






