In the Right Light: Photodynamic Inactivation of Microorganisms Using a LED-Based Illumination Device Tailored for the Antimicrobial Application
Abstract
1. Introduction
2. Results
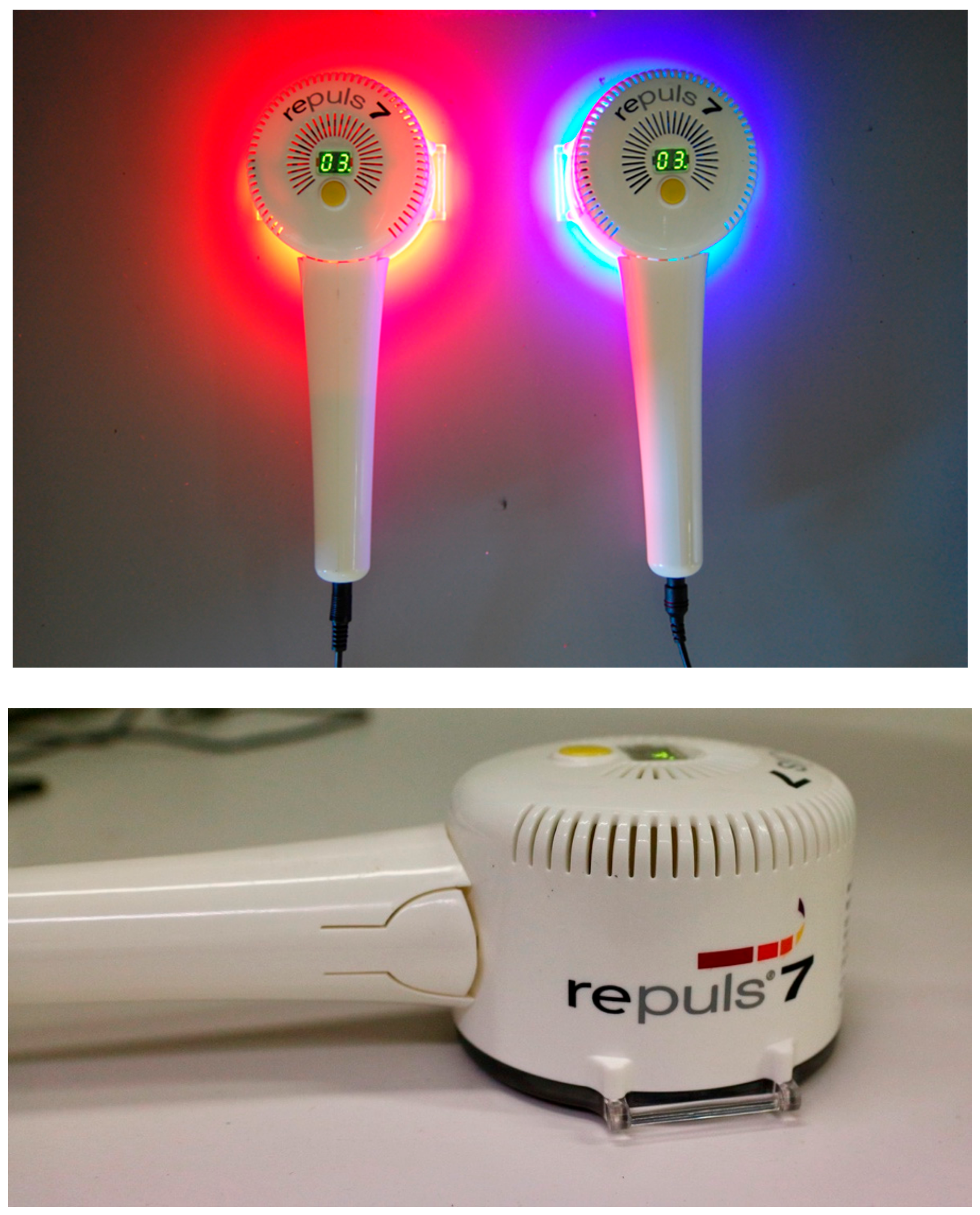
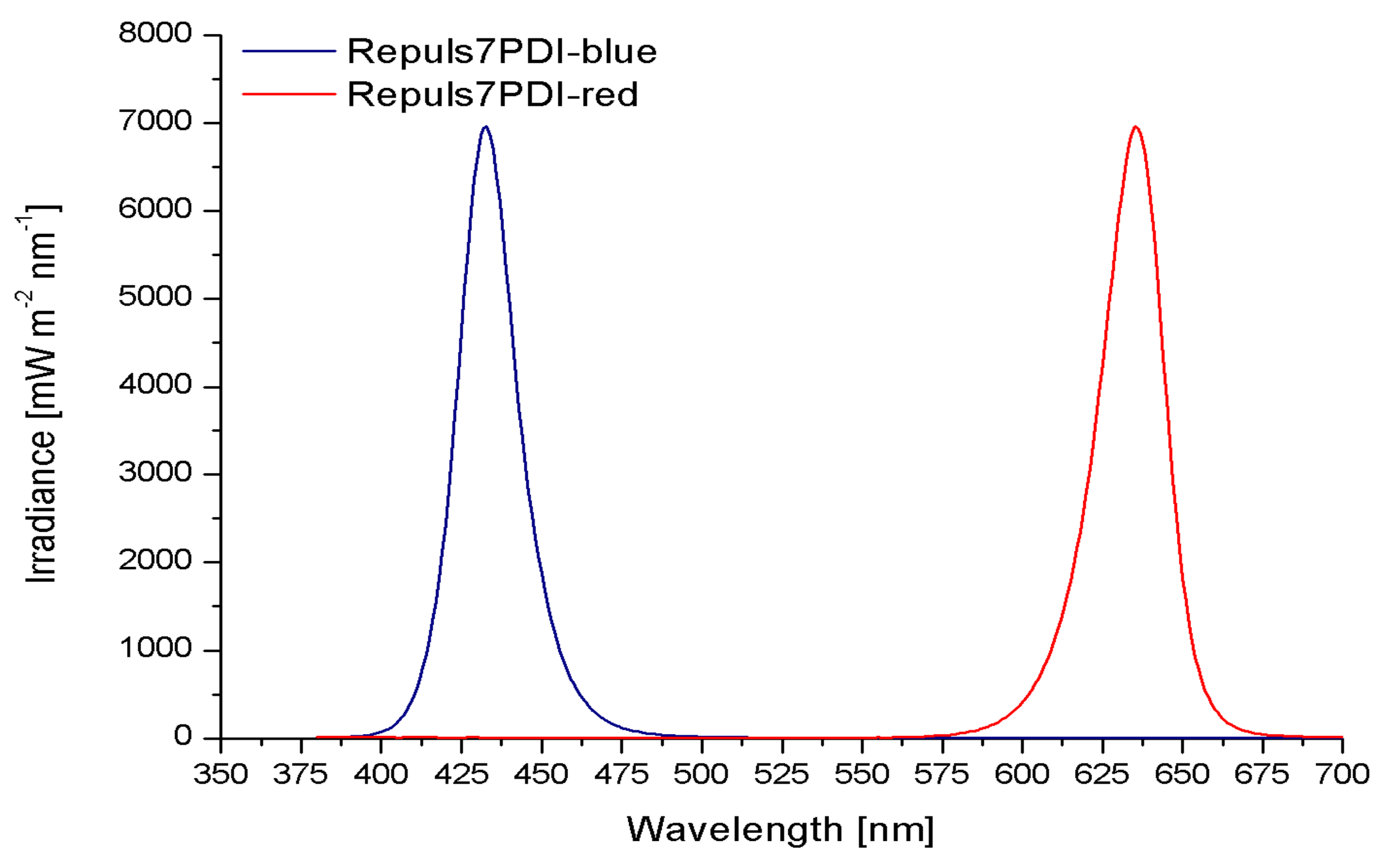
2.1. Irradiance Spectrum of the Repuls7PDI-Red and -Blue
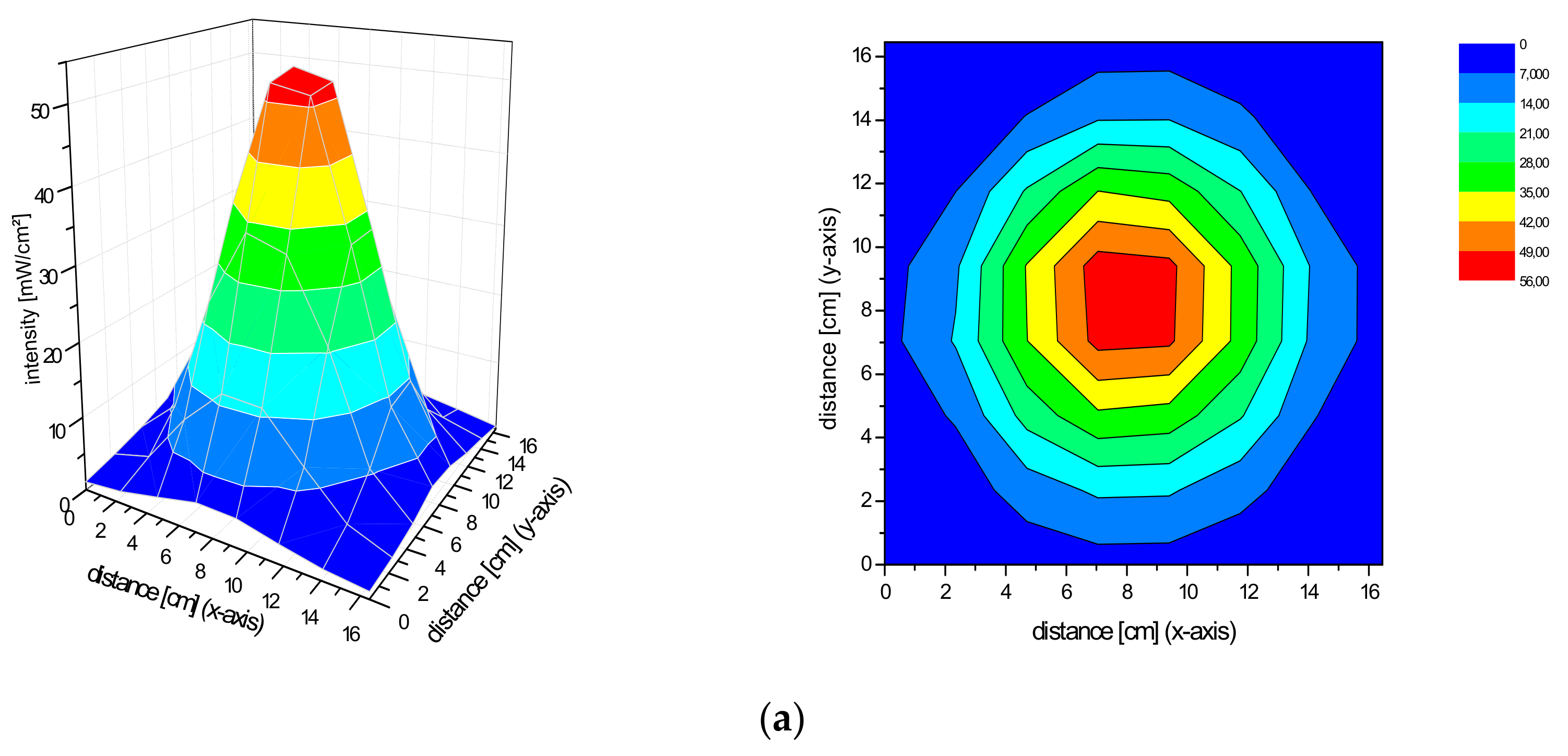
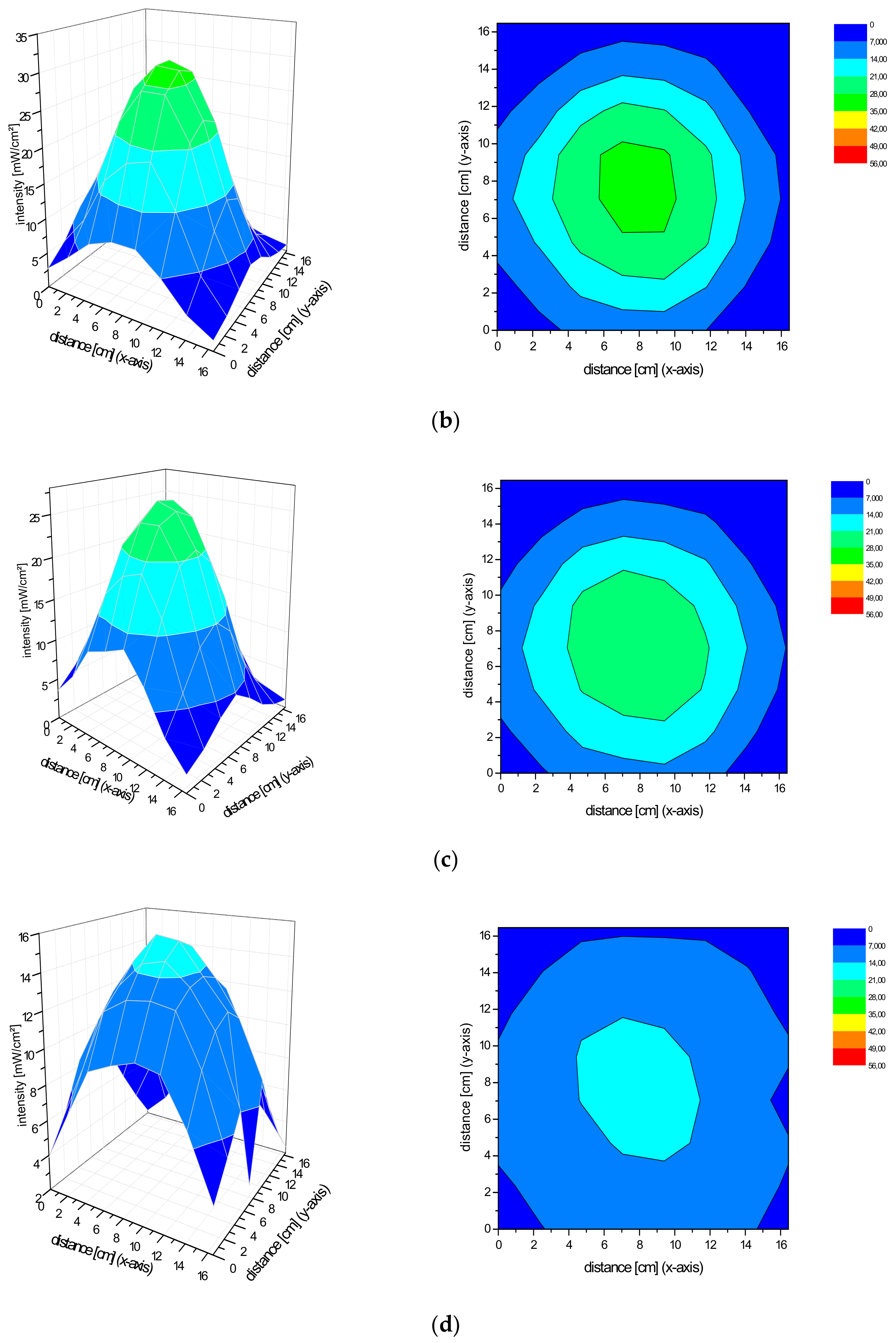
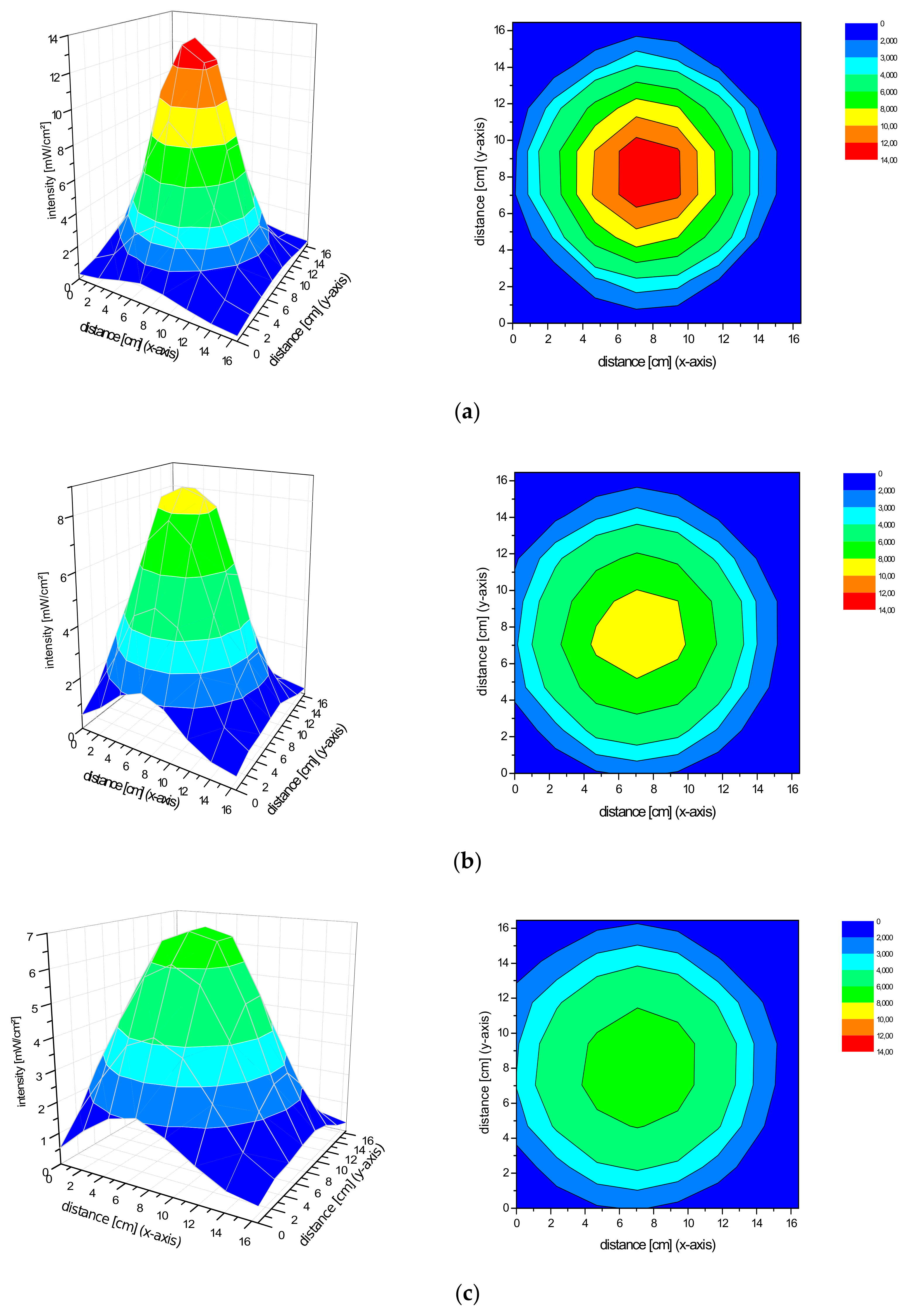
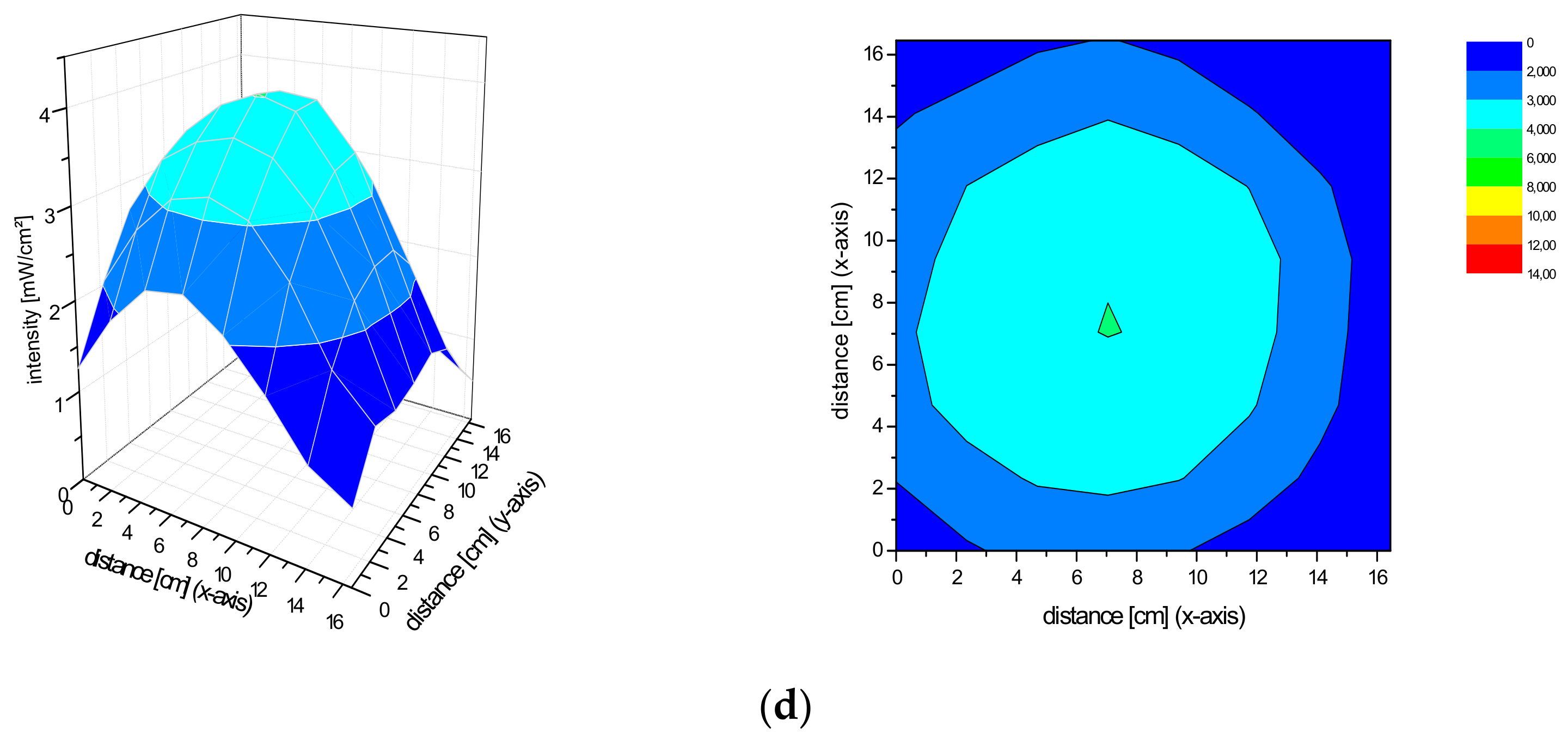
2.2. Spatial Distribution of the Irradiance of the Repuls7PDI-Red and Blue Light Source
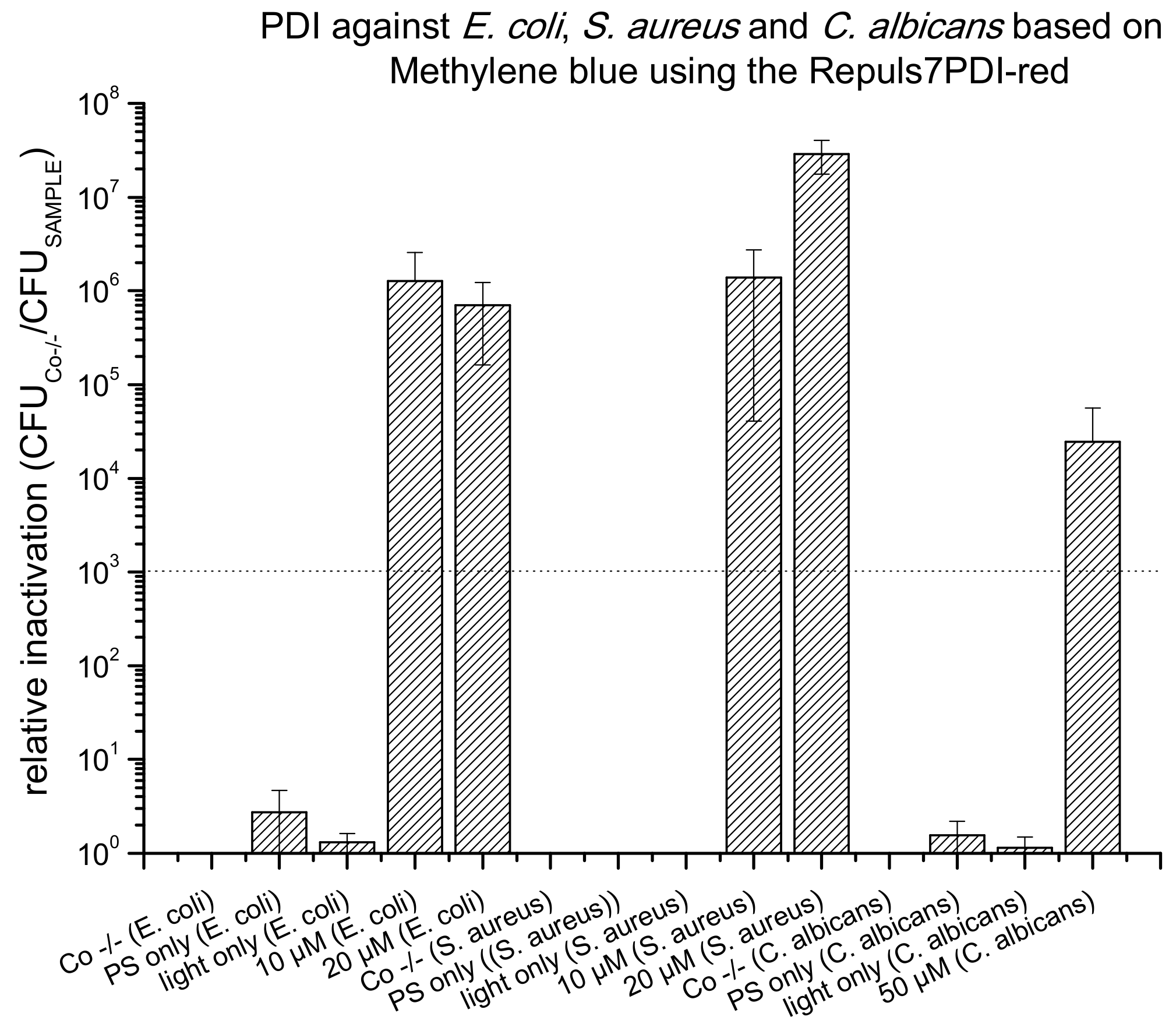
2.3. PDI Based on Methylene Blue against E. coli, S. aureus and C. albicans
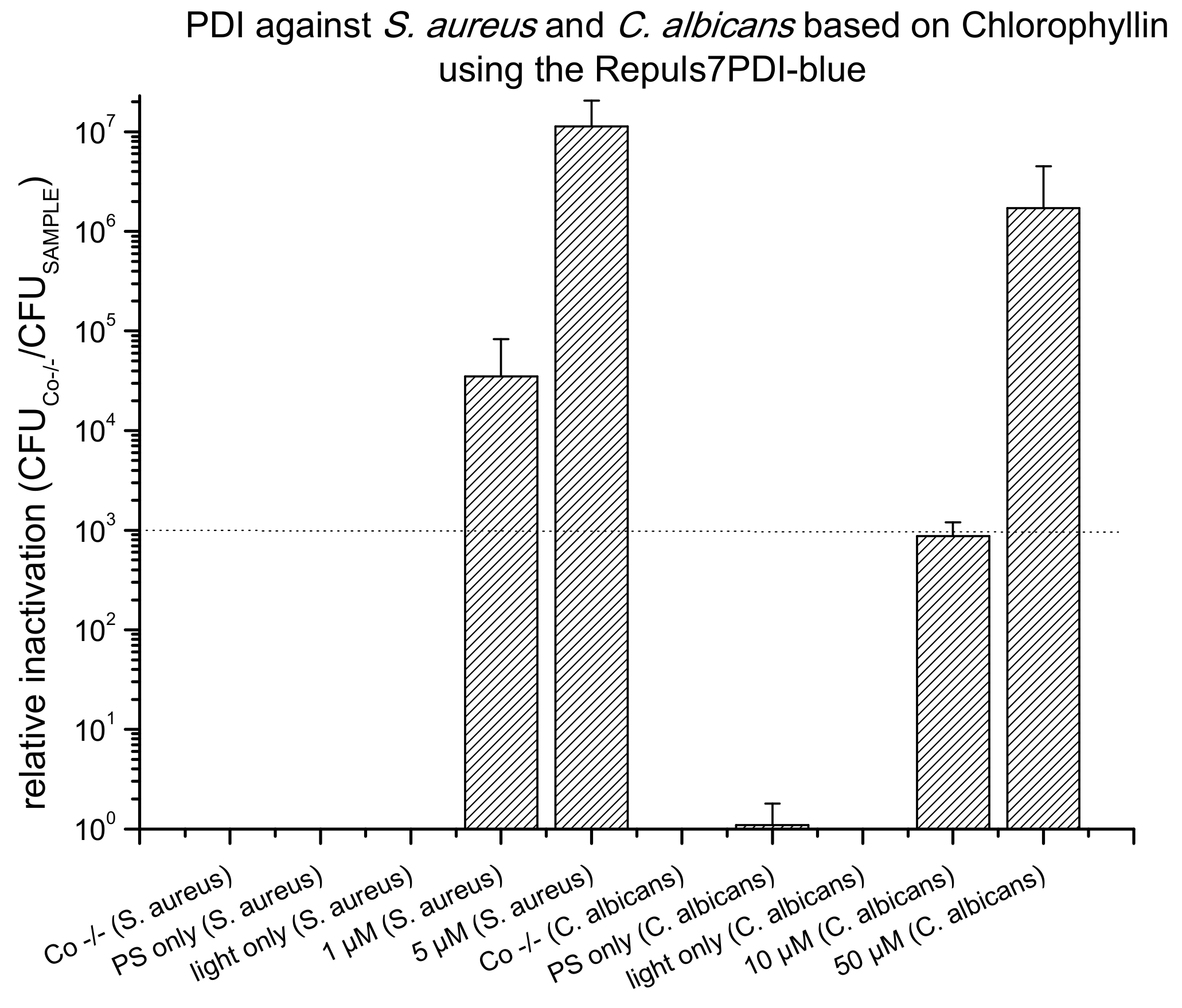
2.4. PDI Based on Chlorophyllin against E. coli, S. aureus and C. albicans
3. Discussion
4. Materials and Methods
4.1. Measurement of the Irradiance Spectrum and Field Homogeneity of the REPULS7PDI-Red and -Blue
4.2. Preparation of Stock Solutions
4.3. Bacterial Culture
4.4. PDI Against Gram(+) S. aureus, Gram(−) E. coli and the Yeast C. albicans
4.5. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Antimicrobial Resistance Home Page. 2019. Available online: http://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 16 June 2019).
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Glueck, M.; Hamminger, C.; Fefer, M.; Liu, J.; Plaetzer, K. Save the crop: Photodynamic Inactivation of plant pathogens I: Bacteria. Photochem. Photobiol. Sci. 2019, 18, 1700–1708. [Google Scholar] [CrossRef]
- Krüger, M.; Richter, P.; Strauch, S.M.; Nasir, A.; Burkovski, A.; Antunes, C.A.; Meißgeier, T.; Schlücker, E.; Schwab, S.; Lebert, M. What an Escherichia coli Mutant Can Teach Us About the Antibacterial Effect of Chlorophyllin. Microorganisms 2019, 7, 59. [Google Scholar] [CrossRef]
- Pieslinger, A.; Plaetzer, K.; Oberdanner, C.B.; Berlanda, J.; Mair, H.; Krammer, B.; Kiesslich, T. Characterization of a simple and homogeneous irradiation device based on light-emitting diodes: A possible low-cost supplement to conventional light sources for photodynamic treatment. Med. Laser Appl. 2006, 21, 277–283. [Google Scholar] [CrossRef]
- Crosbie, J.; Winser, K.; Collins, P. Mapping the light field of the Waldmann PDT 1200 lamp: Potential for wide-field low light irradiance aminolevulinic acid photodynamic therapy. Photochem. Photobiol. 2002, 76, 204–207. [Google Scholar] [CrossRef]
- King, A. Antibiotic Resistance Will Kill 300 Million People by 2050. 2014. Available online: https://www.scientificamerican.com/article/antibiotic-resistance-will-kill-300-million-people-by-2050/ (accessed on 25 April 2018).
- Harrison, J.W.; Svec, T.A. The beginning of the end of the antibiotic era? Part II. Proposed solutions to antibiotic abuse. Quintessence Int. 1998, 29, 223–229. [Google Scholar]
- Yilmaz, A.; Ozkiraz, S.; Akcan, A.B.; Canpolat, M. Low-cost Home-use Light-emitting-diode Phototherapy as an alternative to Conventional Methods. J. Trop. Pediatr. 2015, 61, 113–118. [Google Scholar] [CrossRef]
- Peloi, L.S.; Soares, R.R.; Biondo, C.E.; Souza, V.R.; Hioka, N.; Kimura, E. Photodynamic effect of light-emitting diode light on cell growth inhibition induced by methylene blue. J. Biosci. 2008, 33, 231–237. [Google Scholar] [CrossRef]
- Repuls ist Nach den Neuesten Anforderungen der MDD (Medizinprodukterichtlinie) Zugelassen. Available online: https://www.repuls.at/ (accessed on 25 September 2019).
- Stockett, M.H.; Musbat, L.; Kjær, C.; Houmøller, J.; Toker, Y.; Rubio, A.; Milne, B.F.; Nielsen, S.B. The Soret absorption band of isolated chlorophyll a and b tagged with quaternary ammonium ions. Phys. Chem. Chem. Phys. 2015, 17, 25793–25798. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials-are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Tortik, N.; Steinbacher, P.; Maisch, T.; Spaeth, A.; Plaetzer, K. A comparative study on the antibacterial photodynamic efficiency of a curcumin derivative and a formulation on a porcine skin model. Photochem. Photobiol. Sci. 2016, 15, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Khachemoune, A. An update on topical photodynamic therapy for clinical dermatologists. J. Dermatol. Treat. 2019, 30, 732–744. [Google Scholar] [CrossRef]
- Waldmann PDT 1200 Instruction for Use Manual; Update Status A; Waldmann Medical Division: Wheeling, IL, USA, 1997.
- Winter, R.; Dungel, P.; Reischies, F.M.J.; Rohringer, S.; Slezak, P.; Smolle, C.; Spendel, S.; Kamolz, L.P.; Ghaffari-Tabrizi-Wizsy, N.; Schicho, K. Photobiomodulation (PBM) promotes angiogenesis in-vitro and in chick embryo chorioallantoic membrane model. Sci. Rep. 2018, 8, 17080. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Serrano, R.; Zimmermann, L.M.; Matos, L.L.; Baptista, M.S.; Pinhal, M.A.S.; Atallah, Á.N. Is surgical debridement necessary in the diabetic foot treated with photodynamic therapy? Diabet. Foot Ankle 2017, 8. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. In Recent Patents on Anti-Infective Drug Discovery; Bentham Science Publishers: Sharjah, UAE, 2013; Volume 8, pp. 108–120. [Google Scholar]
- Teli, M.D.; Sheikh, J. Antibacterial and acid and cationic dyeable bamboo cellulose (rayon) fabric on grafting. Carbohydr. Polym. 2012, 88, 1281–1287. [Google Scholar] [CrossRef]
- Gollmer, A.; Felgenträger, A.; Bäumler, W.; Maisch, T.; Späth, A. A novel set of symmetric methylene blue derivatives exhibits effective bacteria photokilling—A structure-response study. Photochem. Photobiol. Sci. 2015, 14, 335–351. [Google Scholar] [CrossRef]
- Freire, F.; Ferraresi, C.; Jorge, A.O.C.; Hamblin, M.R. Photodynamic therapy of oral Candida infection in a mouse model. J. Photochem. Photobiol. B Biol. 2016, 159, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zudyte, B.; Luksiene, Z. Toward better microbial safety of wheat sprouts: Chlorophyllin-based photosensitization of seeds. Photochem. Photobiol. Sci. 2019, 18, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Paskeviciute, E.; Zudyte, B.; Luksiene, Z. Innovative Nonthermal Technologies: Chlorophyllin and Visible Light Significantly Reduce Microbial Load on Basil. Food Technol. Biotechnol. 2019, 57, 126–132. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Hamblin, M.R.; Kishen, A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem. Photobiol. Sci. 2009, 8, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Seely, G.R.; Jensen, R.G. Effect of solvent on the spectrum of chlorophyll. Spectrochim. Acta 1965, 21, 1835–1845. [Google Scholar] [CrossRef]
- Glueck, M.; Schamberger, B.; Eckl, P.; Plaetzer, K. New horizons in microbiological food safety: Photodynamic Decontamination based on a curcumin derivative. Photochem. Photobiol. Sci. 2017, 16, 1784–1791. [Google Scholar] [CrossRef]

| Manufacturer | Model | Wavelength(s) Emitted | Weblink |
|---|---|---|---|
| OmniLux | OminLux blueTM | 415 ± 10 nm | https://omniluxled.com/products/omnilux-blue/ |
| HUBEI YJT Technology | Aesthetic Phototherapy Lamp | Red light: 630 nm ± 10 nm Blue light: 465 nm ± 10 nm | https://www.medicalexpo.com/prod/hubei-yjt-technology/product-126671-924289.html |
| Poly | Clear | 415 nm | https://www.mypolyled.com/clear/ |
| Poly | Rejuv | 433 nm | https://www.mypolyled.com/rejuv/ |
| BIOPTRON | Bioptron Medall | 630 nm | https://bioptron.at/index.php/bioptron-produkte.html |
| Molteni Therapeutics | VULNOLIGHT® | 630 nm | http://www.moltenitherapeutics.it/products/vulnolight®.aspx |
| Biofrontera | BF-RHODOLED® | 635 nm | https://www.biofrontera.com/de/produkte.html |
| Theralase® | TLC-1000 | 660 nm | https://theralase.com/tlc-1000/ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasenleitner, M.; Plaetzer, K. In the Right Light: Photodynamic Inactivation of Microorganisms Using a LED-Based Illumination Device Tailored for the Antimicrobial Application. Antibiotics 2020, 9, 13. https://doi.org/10.3390/antibiotics9010013
Hasenleitner M, Plaetzer K. In the Right Light: Photodynamic Inactivation of Microorganisms Using a LED-Based Illumination Device Tailored for the Antimicrobial Application. Antibiotics. 2020; 9(1):13. https://doi.org/10.3390/antibiotics9010013
Chicago/Turabian StyleHasenleitner, Martina, and Kristjan Plaetzer. 2020. "In the Right Light: Photodynamic Inactivation of Microorganisms Using a LED-Based Illumination Device Tailored for the Antimicrobial Application" Antibiotics 9, no. 1: 13. https://doi.org/10.3390/antibiotics9010013
APA StyleHasenleitner, M., & Plaetzer, K. (2020). In the Right Light: Photodynamic Inactivation of Microorganisms Using a LED-Based Illumination Device Tailored for the Antimicrobial Application. Antibiotics, 9(1), 13. https://doi.org/10.3390/antibiotics9010013






