Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens
Abstract
1. Introduction
2. Traditional Medicinal Plants
Phytocomponent Fractions and Antimicrobial Methods
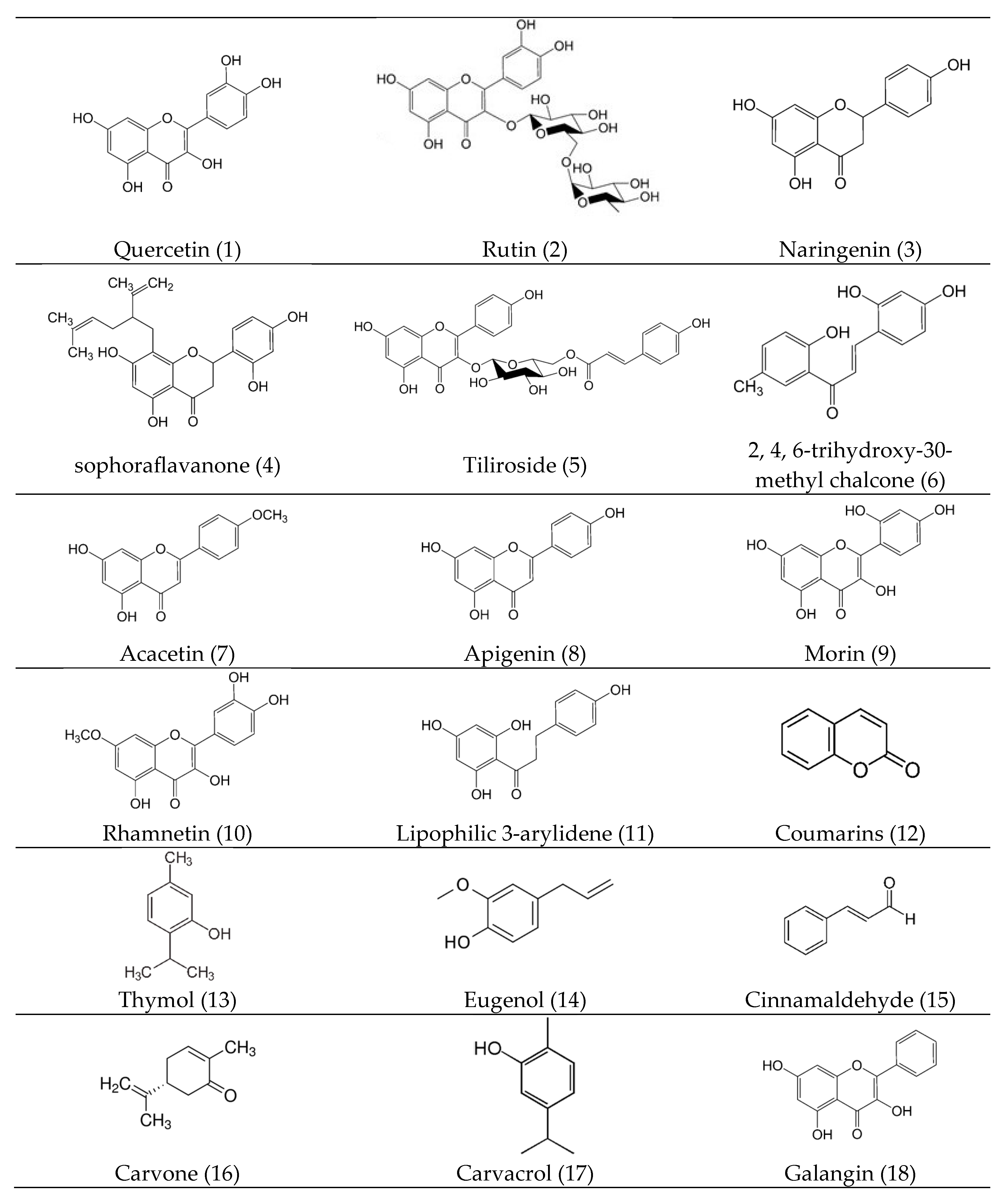
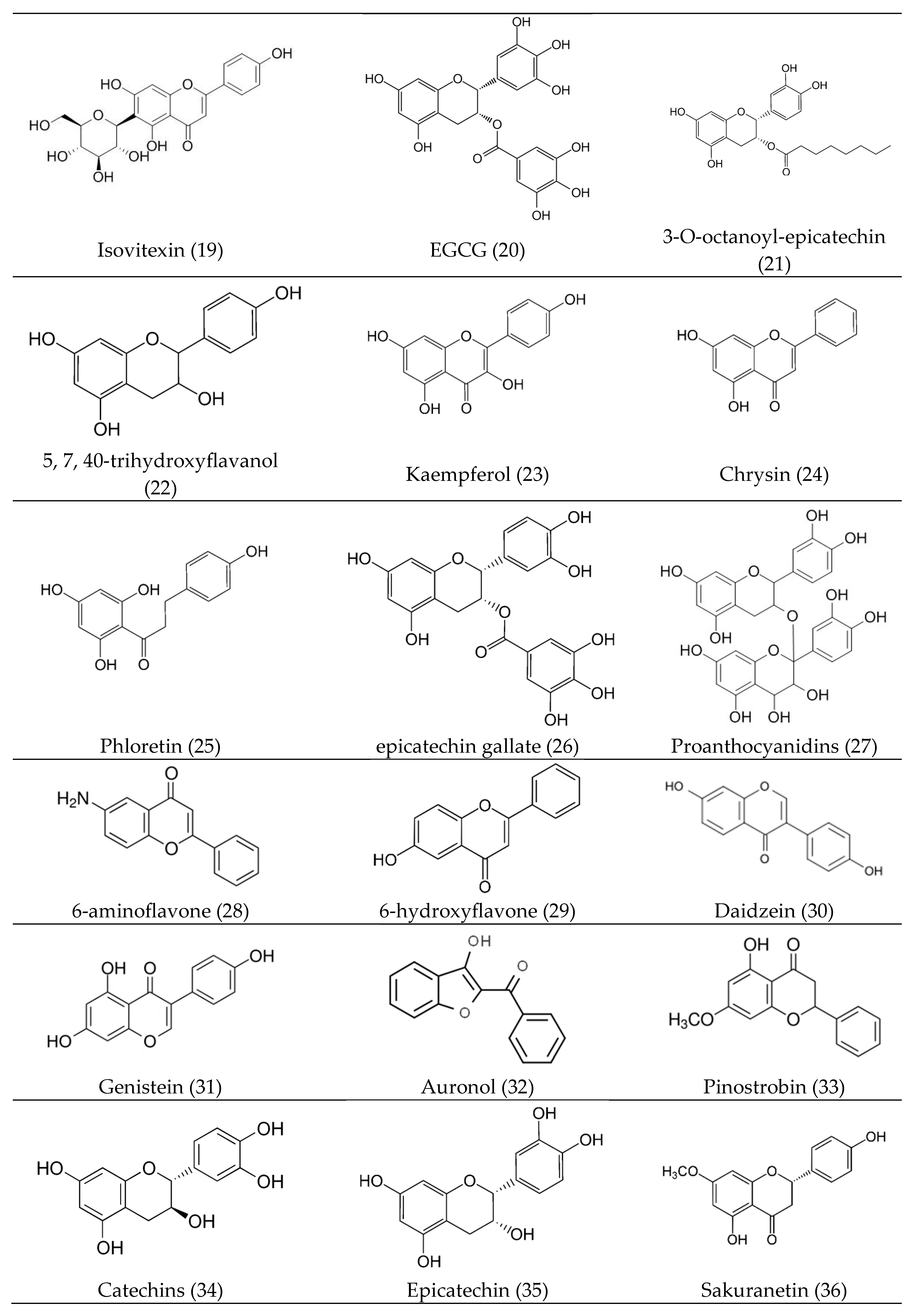
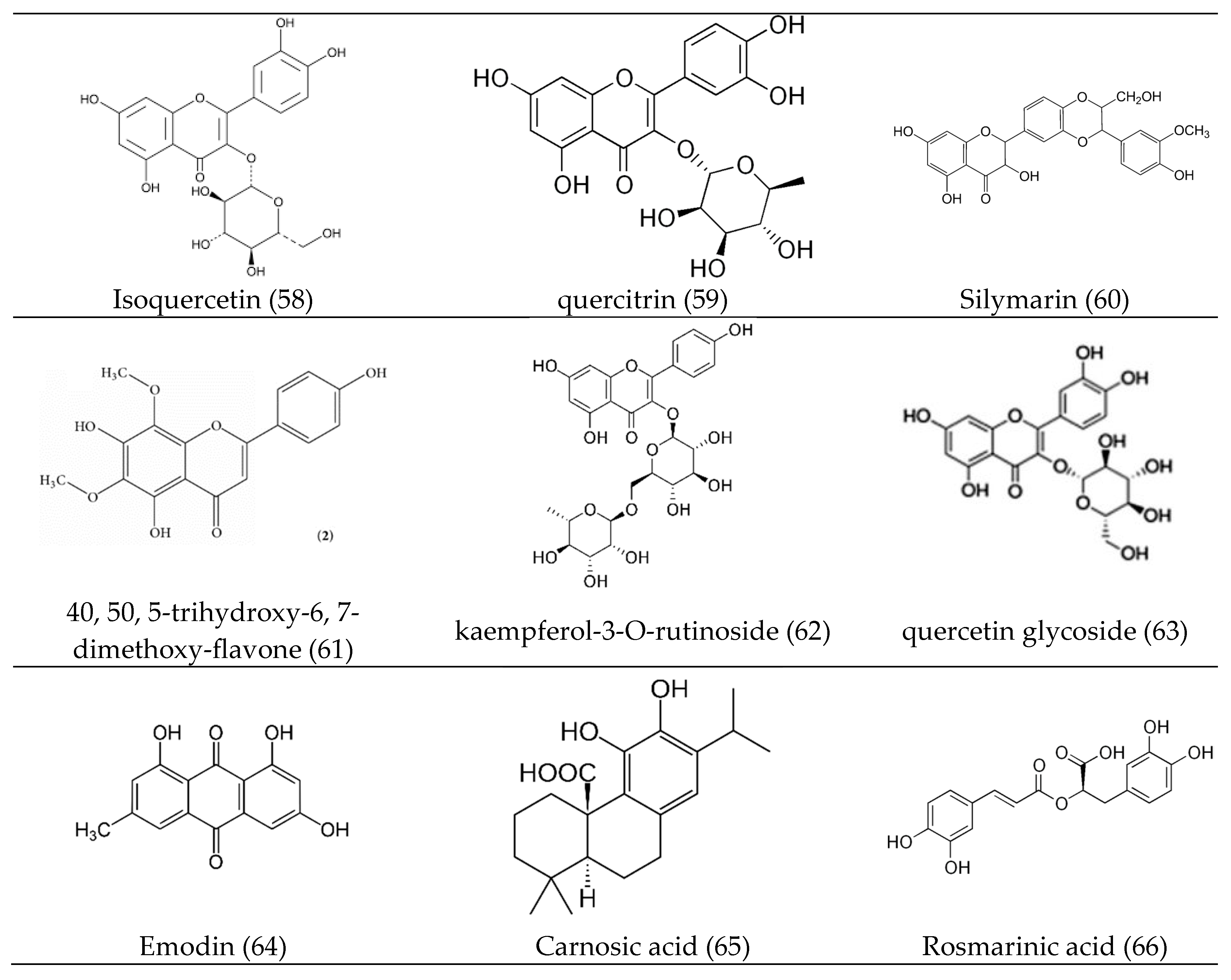
3. Bioactive Compounds (Bioactive Phytocomponents)
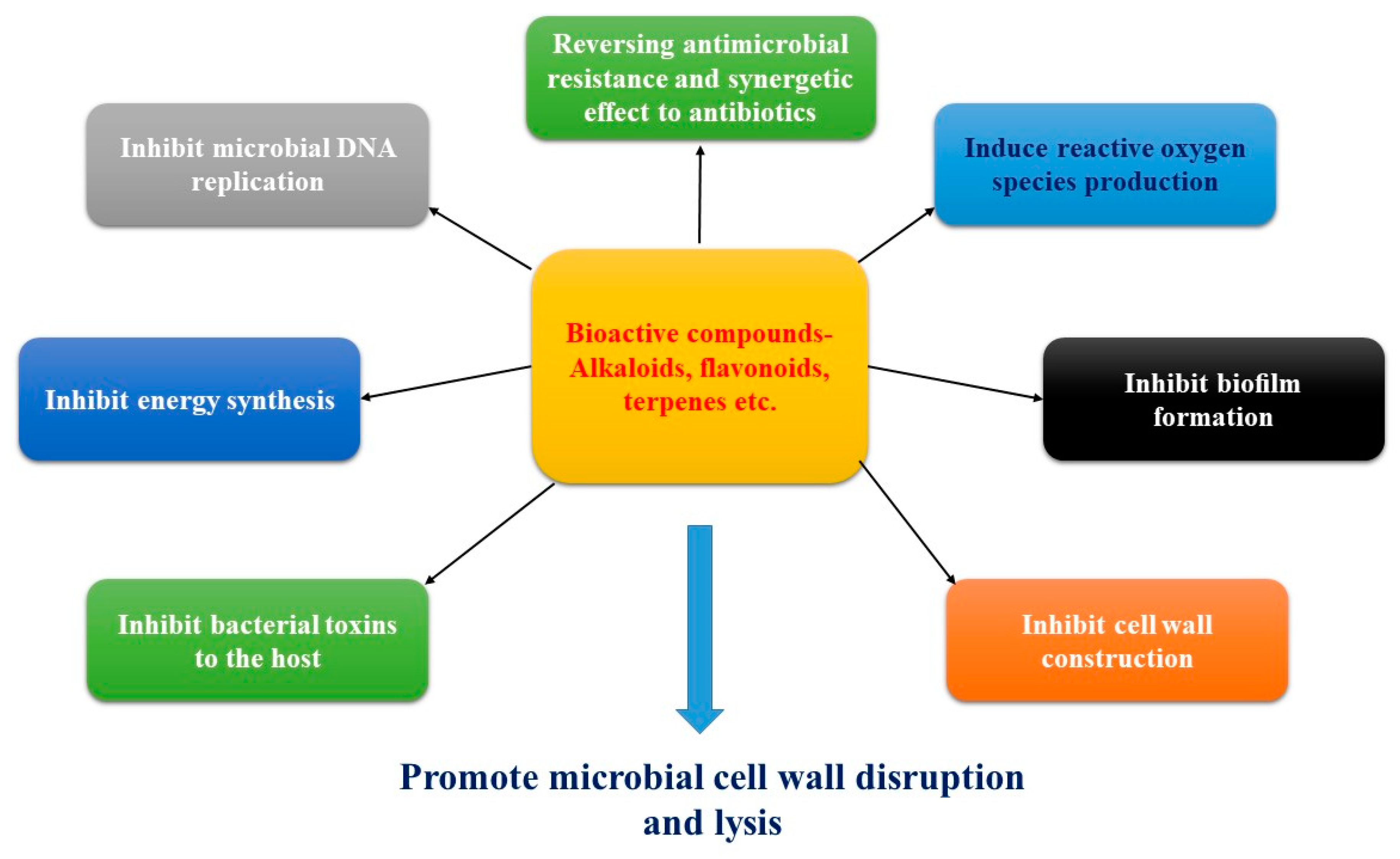
4. Mechanism of Actions of Antibacterial Bioactive Compounds
4.1. Promote Cell Wall Disruption and Lysis
4.2. Inhibition of Biofilm Formation
4.3. Inhibition of Cell Wall Construction
4.4. Inhibition of Prokaryotic DNA Replication
4.5. Inhibition of Energy Production
4.6. Inhibition of Bacterial Toxins
4.7. Mechanism of Resistance to Antibacterial Agents
4.8. Antimicrobial Action with Generation of Reactive Oxygen Species
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A. bohemicus | Acinetobacter bohemicus |
| A. flavus | Aspergillus flavus |
| A. fumigatus | Aspergillus fumigatus |
| A. niger | Aspergillus niger |
| A. solani | Alternaria solani |
| B. agri | Brevibacillus agri |
| B. brevis | Brevibacillus brevis |
| B. cereus | Bacillus cereus |
| B. megaterium | Bacillus megaterium |
| B. pumilus | Bacillus pumilus |
| B. subtilis | Bacillus subtilis |
| C. albicans | Candida albicans |
| C. Dipthieriae | Corynebacterium Dipthieriae |
| C. dubliniensis | Candida dubliniensis |
| C. glabrata | Candida glabrata |
| C. graminicola | Colletotrichum graminicola |
| C. jejuni | Campylobacter jejuni |
| C. krusei | Candida krusei |
| C. lunat | Candida lunat |
| C. lunatus | Cochliobolus lunatus |
| C. macrocarpum | Cladosporium macrocarpum |
| C. neoformans | Cryptococcus neoformans |
| C. parapsilosis | Candida parapsilosis |
| C. sphaerospermum | Cladosporium sphaerospermum |
| C. tropicalis | Candida tropicalis |
| C. maydis | Cercospora zeae-maydis |
| D. turcica | Drechslera turcica |
| E. aerogenes | Enterobacter aerogenes |
| E. cloacae | Enterobacter cloacae |
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| E. ficariae | Entyloma ficariae |
| E. floccosum | Epidermophyton floccosum |
| F. nucleatum | Fusobacterium nucleatum |
| F. oxysporum | Fusarium oxysporum |
| F. verticillioides | Fusarium verticillioides |
| H. carbonum | Helminthosporium carbonum |
| H. pylori | Helicobacter pylori |
| K. aerogenes | Klebsiella aerogenes |
| K. kristinae | Kocuria kristinae |
| K. pneumonia | Klebsiella pneumonia |
| L. acidophilus | Lactobacillus acidophilus |
| L. casei | Lactobacillus casei |
| L. innocua | Listeria innocua |
| L. monocytogenes | Listeria monocytogenes |
| L. sporogenes | Lactobacillus sporogenes |
| M. canis | Microsporum canis |
| M. luteus | Micrococcus luteus |
| M. morganii | Morganella morganii |
| M. ruber | Monascus ruber |
| M. smegmatis | Mycobacterium smegmatis |
| M. tuberculosis | Mycobacterium tuberculosis |
| M. verticillata | Mortierella verticillata |
| P. acnes | Propionibacterium acnes |
| P. aeruginosa | Pseudomonas aeruginosa |
| P. brasiliensis | Paracoccidioides brasiliensis |
| P. fluorescens | Pseudomonas fluorescens |
| P. gingivalis | Porphrymonas gingivalis |
| P. herbarum | Pleospora herbarum |
| P. innundatus | Protomyces innundatus |
| P. intermedia | Prevotella intermedia |
| P. lilacinum | Purpureocillium lilacinum |
| P. mirabilis | Proteus mirabilis |
| P. sojae | Phytophthora sojae |
| P. vulgaris | Proteus vulgaris |
| R. rubrum | Rhodospirillum rubrum |
| R. solanacearum | Ralstonia solanacearum |
| R. solani | Rhizoctonia solani |
| R. stolonifera | Rhizopus stolonifera |
| S. agalactiae | Streptococcus agalactiae |
| S. anginosus | Streptococcus anginosus |
| S. aureus | Staphylococcus aureus |
| S. auricularis | Staphylococcus auricularis |
| S. boydii | Shigella boydii |
| S. dysenteriae | shigella dysenteriae |
| S. epidermidis | Staphylococcus epidermidis |
| S. fecalis | Streptococcus fecalis |
| S. flexneri | Shigella flexneri |
| S. gordonii | Streptococcus gordonii |
| S. haemolyticus | Staphylococcus haemolyticus |
| S. heidelberg | Salmonella heidelberg |
| S. hominis | Staphylococcus hominis |
| S. japonicas | Schizosaccharomyces japonicas |
| S. kneipii | Spizellomyces kneipii |
| S. lutea | Sarcina lutea |
| S. marcescens | Serratia marcescens |
| S. mutans | Streptococcus mutans |
| S. para typhi | Salmonella para typhi |
| S. pneumoniae | Streptococcus pneumoniae |
| S. pseudodichotomus | Spizellomyces pseudodichotomus |
| S. pyogenes | Streptococcus pyogenes |
| S. sanguis | Streptococcus sanguis |
| S. saprophyticus | Staphylococcus saprophyticus |
| S. shiga | Shigella shiga |
| S. typhi | Salmonella typhi |
| T. deformans | Taphrina deformans |
| T. mentagraphytes | Trichophyton mentagraphytes |
| T. rubrum | Trichophyton rubrum |
| T. tonsurans | Trichophyton tonsurans |
| T. urans | Trichophytontonsurans |
| V. cholerae | Vibrio cholerae |
| V. fischeri | Vibrio fischeri |
| X. axonopodis Pv. malvacearum | Xanthomonas axonopodis pv. Malvacearum |
| X. vesicatoria | Xanthomonas vesicatoria |
| Y. enterocolitica | Yersinia enterocolitica |
References
- Mozirandi, W.; Tagwireyi, D.; Mukanganyama, S. Evaluation of antimicrobial activity of chondrillasterol isolated from Vernonia adoensis (Asteraceae). BMC Complement. Altern. Med. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Mickymaray, S. One-step synthesis of silver nanoparticles using Saudi Arabian desert seasonal plant Sisymbrium irio and antibacterial activity against multidrug-resistant bacterial strains. Biomolecules 2019, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, M.; Manuel, V.N.; Raja, V.; Thambidurai, P.; Mickymaray, S.; Nooruddin, T. Antimicrobial activity of the ethanolic and aqueous extracts of Salacia chinensis Linn. against human pathogens. Asian Pac. J. Trop. Dis. 2012, 2, S416–S420. [Google Scholar] [CrossRef]
- Kannaiyan, M.; Meseret Abebe, G.; Kanimozhi, C.; Thambidurai, P.; Ashokapuram Selvam, S.; Vinodhini, R.; Suresh, M. Prevalence of extended-spectrum beta-lactamase producing enterobacteriaceae members isolated from clinically suspected patients. Asian J. Pharm. Clin. Res. 2018, 11, 364. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Aboody, M.; AlFonaisan, M.; Turaiki, W.; Mickymaray, S.; Mariappan, P.; Alsagaby, S.; Sandle, T. Determination of Minimum inhibitory concentrations of Common Biocides to Multidrug-Resistant Gram-negative bacteria. Appl. Med. Res. 2016, 2, 56. [Google Scholar] [CrossRef]
- Mickymaray, S.; Alturaiki, W. Antifungal efficacy of marine macroalgae against fungal isolates from bronchial asthmatic cases. Molecules 2018, 23, 3032. [Google Scholar] [CrossRef]
- Fotso, G.W.; Mogue Kamdem, L.; Dube, M.; Fobofou, S.A.; Ndjie Ebene, A.; Arnold, N.; Tchaleu Ngadjui, B. Antimicrobial secondary metabolites from the stem barks and leaves of Monotes kerstingii Gilg (Dipterocarpaceae). Fitoterapia 2019, 137, 104239. [Google Scholar] [CrossRef]
- Houlihan, A.J.; Conlin, P.; Chee-Sanford, J.C. Water-soluble exudates from seeds of Kochia scoparia exhibit antifungal activity against Colletotrichum graminicola. PLoS ONE 2019, 14, e0218104. [Google Scholar] [CrossRef]
- Mickymaray, S.; Al Aboody, M.S.; Rath, P.K.; Annamalai, P.; Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 185–191. [Google Scholar] [CrossRef]
- Casciaro, B.; Calcaterra, A.; Cappiello, F.; Mori, M.; Loffredo, M.R.; Ghirga, F.; Mangoni, M.L.; Botta, B.; Quaglio, D. Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections. Toxins 2019, 11, 511. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical Characteristics and Antimicrobial Activity of Australian Grown Garlic (Allium Sativum L.) Cultivars. Foods 2019, 8, 358. [Google Scholar] [CrossRef]
- Toiu, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.-M.; Oniga, I. Solidago graminifolia L. Salisb. (Asteraceae) as a Valuable Source of Bioactive Polyphenols: HPLC Profile, In Vitro Antioxidant and Antimicrobial Potential. Molecules 2019, 24, 2666. [Google Scholar] [CrossRef]
- Değirmenci, H.; Erkurt, H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. J. Infect. Public Health 2019. [Google Scholar] [CrossRef]
- Gómez-Rivera, A.; González-Cortazar, M.; Herrera-Ruíz, M.; Zamilpa, A.; Rodríguez-López, V. Sessein and isosessein with anti-inflammatory, antibacterial and antioxidant activity isolated from Salvia sessei Benth. J. Ethnopharmacol. 2018, 217, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Sukalingam, K.; Ganesan, K.; Ponnusamy, K. Evaluation of antidiabetic activity of polyherbal formulations on type 2 diabetic patients: A single blinded randomized study. Int. J. Integ. Medl. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Sukalingam, K.; Ganesan, K.; Xu, B. Trianthema portulacastrum L. (giant pigweed): Phytochemistry and pharmacological properties. Phytochem. Rev. 2017, 16, 461–478. [Google Scholar] [CrossRef]
- Karalija, E.; Parić, A.; Dahija, S.; Bešta-Gajević, R.; Ćavar Zeljković, S. Phenolic compounds and bioactive properties of Verbascum glabratum subsp. bosnense (K. Malý) Murb., an endemic plant species. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Dewapriya, P.; Khalil, Z.G.; Prasad, P.; Salim, A.A.; Cruz-Morales, P.; Marcellin, E.; Capon, R.J. Talaropeptides A-D: Structure and Biosynthesis of Extensively N-methylated Linear Peptides From an Australian Marine Tunicate-Derived Talaromyces sp. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P.; Shaw, M.; Mukhopadhyay, M.K. Bottle Gourd (Lagenaria Siceraria). In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2018; Volume II, pp. 909–920. [Google Scholar] [CrossRef]
- Prasannabalaji, N.; Muralitharan, G.; Sivanandan, R.N.; Kumaran, S.; Pugazhvendan, S.R. Antibacterial activities of some Indian traditional plant extracts. Asian Pac. J. Trop. Dis. 2012, 2, S291–S295. [Google Scholar] [CrossRef]
- Mabona, U.; Viljoen, A.; Shikanga, E.; Marston, A.; Van Vuuren, S. Antimicrobial activity of southern African medicinal plants with dermatological relevance: From an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J. Ethnopharmacol. 2013, 148, 45–55. [Google Scholar] [CrossRef]
- Benevides Bahiense, J.; Marques, F.M.; Figueira, M.M.; Vargas, T.S.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Potential anti-inflammatory, antioxidant and antimicrobial activities ofSambucus australis. Pharm. Biol. 2017, 55, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Akhalwaya, S.; van Vuuren, S.; Patel, M. An in vitro investigation of indigenous South African medicinal plants used to treat oral infections. J. Ethnopharmacol. 2018, 210, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Selvaraj, A.; Ng, Z.Y.; Palanisamy, M.; Mickmaray, S.; Cheong, P.C.H.; Lim, R.L.H. Isolation of actinomycetes with antibacterial activity against multi-drug resistant bacteria. Malays. J. Microbiol. 2018. [Google Scholar] [CrossRef]
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef]
- Muhaisen, H.M.H.; Ab–Mous, M.M.; Ddeeb, F.A.; Rtemi, A.A.; Taba, O.M.; Parveen, M. Antimicrobial agents from selected medicinal plants in Libya. Chin. J. Integr. Med. 2015, 22, 177–184. [Google Scholar] [CrossRef]
- Mubarack, H.; Doss, A.; Vijayasanthi, M.; Venkataswamy, R. Antimicrobial drug susceptibility of Staphylococcus aureus from subclinical bovine mastitis in Coimbatore, Tamilnadu, South India. Vet. World 2012, 5, 352. [Google Scholar] [CrossRef]
- Okwu, M.U.; Olley, M.; Akpoka, A.O.; Izevbuwa, O.E. Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: A brief review. AIMS Microbiol. 2019, 5, 117–137. [Google Scholar] [CrossRef]
- Obeidat, M.; Shatnawi, M.; Al-alawi, M.; Al-Zu‘bi, E.; Al-Dmoor, H.; Al-Qudah, M.; El-Qudah, J.; Otri, I. Antimicrobial Activity of Crude Extracts of Some Plant Leaves. Res. J. Microbiol. 2012, 7, 59–67. [Google Scholar] [CrossRef]
- Aristolochia indica Linn. In SpringerReference; Springer: Berlin, Germany, 2016.
- Vinodhini, R.; Moorthy, K.; Suresh, M. Incidence and virulence traits of Candida dubliniensis isolated from clinically suspected patients. Asian J. Pharm. Clin. Res. 2016, 9, 77. [Google Scholar] [CrossRef]
- Zuo, G.-Y.; Zhang, X.-J.; Yang, C.-X.; Han, J.; Wang, G.-C.; Bian, Z.-Q. Evaluation of Traditional Chinese Medicinal Plants for Anti-MRSA Activity with Reference to the Treatment Record of Infectious Diseases. Molecules 2012, 17, 2955–2967. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Kumar, B.; Srivastava, M.; Arya, K.R.; Sharma, K.R. Distribution and Discrimination Study of Bioactive Compounds from Berberis species using HPLC-ESI-QTOF-MS/MS with Principle Component Analysis. Nat. Prod. Commun. 2016, 11, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Kariu, T.; Nakao, R.; Ikeda, T.; Nakashima, K.; Potempa, J.; Imamura, T. Inhibition of gingipains andPorphyromonas gingivalisgrowth and biofilm formation by prenyl flavonoids. J. Periodontal Res. 2016, 52, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Spathodea campanulata Beauv. In SpringerReference; Springer: Berlin, Germany, 2016.
- dos Santos, E.; Pereira, M.; da Silva, C.; Souza-Neta, L.; Geris, R.; Martins, D.; Santana, A.; Barbosa, L.; Silva, H.; Freitas, G.; et al. Antibacterial Activity of the Alkaloid-Enriched Extract from Prosopis juliflora Pods and Its Influence on in Vitro Ruminal Digestion. Int. J. Mol. Sci. 2013, 14, 8496–8516. [Google Scholar] [CrossRef]
- Kumar, G.; Maheswaran, R.; Sharmila Banu, G. Antihyperlipideamic effect of Solanum trilobatum L. leaves extract on streptozotocin induced diabetic rats. Asian J. Biomed. Pharm. Sci. 2013, 3, 51–57. [Google Scholar]
- Semalty, M.; Semalty, A.; Badola, A.; Joshi, G.; Rawat, M.S.M. Semecarpus anacardium Linn.: A review. Pharm. Rev. 2010, 4, 88. [Google Scholar] [CrossRef]
- Rawat, S.; Jugran, A.K.; Bahukhandi, A.; Bahuguna, A.; Bhatt, I.D.; Rawal, R.S.; Dhar, U. Anti-oxidant and anti-microbial properties of some ethno-therapeutically important medicinal plants of Indian Himalayan Region. 3 Biotech. 2016, 6, 154. [Google Scholar] [CrossRef]
- Gujjeti, R.P.; Namthabad, S.; Mamidala, E. HIV-1 reverse transcriptase inhibitory activity of Aerva lanata plant extracts. BMC Infect. Dis. 2014, 14. [Google Scholar] [CrossRef][Green Version]
- Mohotti, S.; Rajendran, S.; Muhammad, T.; Strömstedt, A.A.; Adhikari, A.; Burman, R.; de Silva, E.D.; Göransson, U.; Hettiarachchi, C.M.; Gunasekera, S. Screening for bioactive secondary metabolites in Sri Lankan medicinal plants by microfractionation and targeted isolation of antimicrobial flavonoids from Derris scandens. J. Ethnopharmacol. 2020, 246, 112158. [Google Scholar] [CrossRef]
- Ghasemi, P.; Jahanbazi, P.; Enteshari, S.; Malekpoor, F.; Hamedi, B. Antimicrobial activity of some Iranian medicinal plants. Arch. Biol. Sci. 2010, 62, 633–641. [Google Scholar] [CrossRef]
- Mickymaray, S.; Al Aboody, M.S. In Vitro Antioxidant and Bactericidal Efficacy of 15 Common Spices: Novel Therapeutics for Urinary Tract Infections? Medicina 2019, 55, 289. [Google Scholar] [CrossRef]
- Banothu, V.; Neelagiri, C.; Adepally, U.; Lingam, J.; Bommareddy, K. Phytochemical screening and evaluation of in vitro antioxidant and antimicrobial activities of the indigenous medicinal plant Albizia odoratissima. Pharm. Biol. 2017, 55, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.C.; Padhy, R.N. In vitro antibacterial potency of Butea monosperma Lam. against 12 clinically isolated multidrug resistant bacteria. Asian Pac. J. Trop. Dis. 2013, 3, 217–226. [Google Scholar] [CrossRef]
- Wikaningtyas, P.; Sukandar, E.Y. The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens. Asian Pac. J. Trop. Biomed. 2016, 6, 16–19. [Google Scholar] [CrossRef]
- Mwinga, J.L.; Asong, J.A.; Amoo, S.O.; Nkadimeng, S.M.; McGaw, L.J.; Aremu, A.O.; Otang-Mbeng, W. In vitro antimicrobial effects of Hypoxis hemerocallidea against six pathogens with dermatological relevance and its phytochemical characterization and cytotoxicity evaluation. J. Ethnopharmacol. 2019, 242, 112048. [Google Scholar] [CrossRef]
- Armas, J.; Quiroz, J.; Roman, R.; Sanchez, J.; Pacheco, M.; Valdivia, L.; Rivera, E.; Asmat, R.; Anampa, A. Antibacterial Activities of Essential Oils from Three Medicinal Plants in Combination with EDTA against Methicillin-resistant Staphylococcus aureus. Br. Microbiol. Res. J. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Ferhat, M.; Erol, E.; Beladjila, K.A.; Çetintaş, Y.; Duru, M.E.; Öztürk, M.; Kabouche, A.; Kabouche, Z. Antioxidant, anticholinesterase and antibacterial activities of Stachys guyoniana and Mentha aquatica. Pharm. Biol. 2016, 55, 324–329. [Google Scholar] [CrossRef]
- Guan, C.P.; Luo, H.X.; Fang, H.E.; Zhou, X.Z. Global Transcriptome Changes of Biofilm-Forming Staphylococcus epidermidis Responding to Total Alkaloids of Sophorea alopecuroides. Pol. J. Microbiol. 2018, 67, 223–226. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Braun, M.; Wetterauer, P.; Wetterauer, B.; Wink, M. Antioxidant, Cytotoxic, and Antimicrobial Activities of Glycyrrhiza glabra L., Paeonia lactiflora Pall., and Eriobotrya japonica (Thunb.) Lindl. Extracts. Medicines 2019, 6, 43. [Google Scholar] [CrossRef]
- Arefin, M.K.; Rahman, M.M.; Uddin, M.Z.; Hassan, M.A. Angiosperm flora of Satchari National Park, Habiganj, Bangladesh. Bangladesh J. Plant. Taxon. 1970, 18, 117–140. [Google Scholar] [CrossRef]
- Koona, S.; Budida, S. Antibacterial Potential of the Extracts of the Leaves of Azadirachta indica Linn. Not. Sci. Biol. 2011, 3, 65–69. [Google Scholar] [CrossRef]
- Durairaj, B.; Dorai, A. Antiplatelet activity of white and pink Nelumbo nucifera Gaertn flowers. Braz. J. Pharm. Sci. 2010, 46, 579–583. [Google Scholar] [CrossRef]
- Bhattacharjee, I.; Chatterjee, S.K.; Chandra, G. Isolation and identification of antibacterial components in seed extracts of Argemone mexicana L. (Papaveraceae). Asian Pac. J. Trop. Med. 2010, 3, 547–551. [Google Scholar] [CrossRef]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Eur. J. Pharmcol. 2018, 829, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.K.; Bhattacharjee, I.; Chandra, G. Isolation and identification of bioactive antibacterial components in leaf extracts of Vangueria spinosa (Rubiaceae). Asian Pac. J. Trop. Med. 2011, 4, 35–40. [Google Scholar] [CrossRef]
- Gaziano, R.; Campione, E.; Iacovelli, F.; Marino, D.; Pica, F.; Di Francesco, P.; Aquaro, S.; Menichini, F.; Falconi, M.; Bianchi, L. Antifungal activity of Cardiospermum halicacabum L. (Sapindaceae) against Trichophyton rubrum occurs through molecular interaction with fungal Hsp90. Drug Des. Dev. 2018, 12, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Nefzi, A.; Ben Abdallah, R.A. Antifungal activity of aqueous and organic extracts from Withania somnifera L. against Fusarium oxysporum f. sp. Radicis lycopersici. J. Microb. Biochem. Technol. 2016, 8. [Google Scholar] [CrossRef]
- Chahal, S.S.; Matthews, H.R.; Bradbury, E.M. Acetylation of histone H4 and its role in chromatin structure and function. Nature 1980, 287, 76–79. [Google Scholar] [CrossRef]
- Venkataswamy, R.; Doss, A.; Sukumar, M.; Mubarack, H.M. Preliminary phytochemical screening and antimicrobial studies of Lantana indica roxb. Ind. J. Pharm. Sci. 2010, 72, 229. [Google Scholar] [CrossRef]
- Pandian, M.R.; Banu, G.S.; Kumar, G. A study of the antimicrobial activity of Alangium salviifolium. Indian J. Pharm. 2006, 38, 203. [Google Scholar] [CrossRef]
- Arulmozhi, P.; Vijayakumar, S.; Kumar, T. Phytochemical analysis and antimicrobial activity of some medicinal plants against selected pathogenic microorganisms. Microb. Pathog. 2018, 123, 219–226. [Google Scholar] [CrossRef]
- Kahaliw, W.; Aseffa, A.; Abebe, M.; Teferi, M.; Engidawork, E. Evaluation of the antimycobacterial activity of crude extracts and solvent fractions of selected Ethiopian medicinal plants. BMC Complement. Altern. Med. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Ginovyan, M.; Petrosyan, M.; Trchounian, A. Antimicrobial activity of some plant materials used in Armenian traditional medicine. BMC Complement. Altern. Med. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Asgarpanah, J.; Hashemi, S.J.; Hashemi, E.; Askari, K. In vitro antifungal activity of some traditional Persian medicinal plants on pathogenic fungi. Chin. J. Integ. Med. 2015, 23, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Flores-Vallejo, R.D.C.; Cardoso-Taketa, A.; Villarreal, M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017, 208, 264–329. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Mahmoud, E.A.; Al-Mishari, A.A.; Alsaid, M.S. Antimicrobial activities of some Saudi Arabian herbal plants. Afr. J. Trad. Complement. Altern. Med. 2017, 14, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Cioch, M.; Satora, P.; Skotniczny, M.; Semik-Szczurak, D.; Tarko, T. Characterisation of Antimicrobial Properties of Extracts of Selected Medicinal Plants. Pol. J. Microbiol. 2017, 66, 463–472. [Google Scholar] [CrossRef]
- Voukeng, I.K.; Beng, V.P.; Kuete, V. Antibacterial activity of six medicinal Cameroonian plants against Gram-positive and Gram-negative multidrug resistant phenotypes. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef]
- Anyanwu, M.U.; Okoye, R.C. Antimicrobial activity of Nigerian medicinal plants. J. Intercult Ethnopharmacol. 2017, 6, 240–259. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2017, 19, 13. [Google Scholar] [CrossRef]
- Sukalingam, K.; Ganesan, K.; Xu, B. Protective Effect of Aqueous Extract from the Leaves of Justicia tranquebariesis against Thioacetamide-Induced Oxidative Stress and Hepatic Fibrosis in Rats. Antioxidants 2018, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Polyphenol-Rich Lentils and Their Health Promoting Effects. Int. J.Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Gani, S.B.; Ganesan Murugesan, A. Influence of Helicteres isora L. bark extracts on glycemic control and renoprotective activity in streptozotocin-induced diabetic rats. Int. J. Pharm. Sci. Nanotechnol. 2008, 1, 275–280. [Google Scholar]
- Kumar, G.; Murugesan, A.G. Hypolipidaemic activity of Helicteres isora L. bark extracts in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2008, 116, 161–166. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.S.; Murugesan, A.G.; Pandian, M.R. Hypoglycaemic effect of Helicteres isora bark extract in rats. J. Ethnopharmacol. 2006, 107, 304–307. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Murugesan, A.G.; Rajasekara Pandian, M. Effect ofHelicteres isora. Bark Extracts on Brain Antioxidant Status and Lipid Peroxidation in Streptozotocin Diabetic Rats. Pharm. Biol. 2007, 45, 753–759. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Ganesan Murugesan, A. Effect of Helicteres isora bark extracts on heart antioxidant status and lipid peroxidation in streptozotocin diabetic rats. J. Appl. Biomed. 2008, 6, 89–95. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-Obesity Effects of Medicinal and Edible Mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef]
- Ganesan, K.; Gani, S.B.; Ganesan Murugesan, A. Antidiabetic activity of Helicteres isora L. bark extracts on streptozotocin-induced diabetic rats. Int. J. Pharm. Sci. Nanotechnol. 2009, 1, 379–382. [Google Scholar]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Madikizela, B.; Aderogba, M.A.; Van Staden, J. Isolation and characterization of antimicrobial constituents of Searsia chirindensis L. (Anacardiaceae) leaf extracts. J. Ethnopharmacol. 2013, 150, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Alolga, R.N.; Chávez León, M.A.S.C.; Osei-Adjei, G.; Onoja, V. GC-MS-based metabolomics, antibacterial and anti-inflammatory investigations to characterize the quality of essential oil obtained from dried Xylopia aethiopica fruits from Ghana and Nigeria. J. Pharm. Pharmacol. 2019, 71, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.L.A.; Johann, S.; Hughes, F.M.; Rosa, C.A.; Rosa, L.H. The diversity and antimicrobial activity of endophytic fungi associated with medicinal plant Baccharis trimera (Asteraceae) from the Brazilian savannah. Can. J. Microbiol. 2014, 60, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Lourenção Brighenti, F.; Salvador, M.J.; Vidal Lacerda Gontijo, A.; Botazzo Delbem, A.C.; Botazzo Delbem, Á.C.; Soares, C.P.; Carvalho de Oliveira, M.A.; Miorelli Girondi, C.; Koga-Ito, C.Y. Plant extracts: Initial screening, identification of bioactive compounds and effect against Candida albicansbiofilms. Fut. Microbiol. 2017, 12, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Efferth, T.; Salgueiro, L. Artemisia herba-alba essential oil from Buseirah (South Jordan): Chemical characterization and assessment of safe antifungal and anti-inflammatory doses. J. Ethnopharmacol. 2015, 174, 153–160. [Google Scholar] [CrossRef]
- Gazoni, V.F.; Balogun, S.O.; Arunachalam, K.; Oliveira, D.M.; Filho, V.C.; Lima, S.R.; Colodel, E.M.; Soares, I.M.; Ascêncio, S.D.; Martins, D.T.d.O. Assessment of toxicity and differential antimicrobial activity of methanol extract of rhizome of Simaba ferruginea A. St.-Hil. and its isolate canthin-6-one. J. Ethnopharmacol. 2018, 223, 122–134. [Google Scholar] [CrossRef]
- Perianayagam, J.B.; Sharma, S.K.; Pillai, K.K.; Pandurangan, A.; Kesavan, D. Evaluation of antimicrobial activity of ethanol extract and compounds isolated from Trichodesma indicum (Linn.) R. Br. root. J. Ethnopharmacol. 2012, 142, 283–286. [Google Scholar] [CrossRef]
- Dandashire, B.; Magashi, A.; Abdulkadir, B.; Abbas, M.; Goni, M.; Yakubu, A. Toxicological studies and bioactivity-guided identification of antimicrobially active compounds from crude aqueous stem bark extract of Boswellia dalzielii. J. Advan. Vet. Anim. Res. 2019, 6, 183. [Google Scholar] [CrossRef]
- Olmedo-Juárez, A.; Briones-Robles, T.I.; Zaragoza-Bastida, A.; Zamilpa, A.; Ojeda-Ramírez, D.; Mendoza de Gives, P.; Olivares-Pérez, J.; Rivero-Perez, N. Antibacterial activity of compounds isolated from Caesalpinia coriaria (Jacq) Willd against important bacteria in public health. Microb. Pathog. 2019, 136, 103660. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, B.; Aderogba, M.A.; Finnie, J.F.; Van Staden, J. Isolation and characterization of antimicrobial compounds from Terminalia phanerophlebia Engl. & Diels leaf extracts. J. Ethnopharmacol. 2014, 156, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, G.R.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, Á.C.B.; Khouri, S.; Gontijo, A.V.L.; Pascoal, A.C.R.F.; Salvador, M.J.; Koga-Ito, C.Y. Antifungal activity of extracts and isolated compounds fromBuchenavia tomentosaonCandida albicansand non-albicans. Fut. Microbiol. 2015, 10, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Pramila, D.M. Phytochemical analysis and antimicrobial potential of methanolic leaf extract of peppermint (Mentha piperita: Lamiaceae). J. Med. Plants Res. 2012, 6. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; FlaminiGuido; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and Ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19. [Google Scholar] [CrossRef]
- Mustaffa, F.; Indurkar, J.; Ismail, S.; Shah, M.; Mansor, S.M. An Antimicrobial Compound Isolated from Cinnamomum Iners Leaves with Activity against Methicillin-Resistant Staphylococcus Aureus. Molecules 2011, 16, 3037–3047. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. The Tea Catechin Epigallocatechin Gallate Suppresses Cariogenic Virulence Factors ofStreptococcus mutans. Antimicrob. Agents Chemother. 2010, 55, 1229–1236. [Google Scholar] [CrossRef]
- Kuete, V.; Ango, P.Y.; Fotso, G.W.; Kapche, G.D.W.F.; Dzoyem, J.P.; Wouking, A.G.; Ngadjui, B.T.; Abegaz, B.M. Antimicrobial activities of the methanol extract and compounds from Artocarpus communis (Moraceae). BMC Complement. Altern. Med. 2011, 11. [Google Scholar] [CrossRef]
- Bouzabata, A.; Bazzali, O.; Cabral, C.; Gonçalves, M.J.; Cruz, M.T.; Bighelli, A.; Cavaleiro, C.; Casanova, J.; Salgueiro, L.; Tomi, F. New compounds, chemical composition, antifungal activity and cytotoxicity of the essential oil from Myrtus nivellei Batt. & Trab., an endemic species of Central Sahara. J. Ethnopharmacol. 2013, 149, 613–620. [Google Scholar] [CrossRef]
- González-Alamilla, E.N.; Gonzalez-Cortazar, M.; Valladares-Carranza, B.; Rivas-Jacobo, M.A.; Herrera-Corredor, C.A.; Ojeda-Ramírez, D.; Zaragoza-Bastida, A.; Rivero-Perez, N. Chemical Constituents of Salix babylonica L. and Their Antibacterial Activity Against Gram-Positive and Gram-Negative Animal Bacteria. Molecules 2019, 24, 2992. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Arch. Oral Biol. 2012, 57, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int. J. Antimicrob. Agents 2004, 24, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Hamilton, V.E.S.; Chapman, D.G.; Taylor, P.W.; Lamb, A.J. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef]
- El-Adawi, H. Inhibitory effect of grape seed extract (GSE) on cariogenic bacteria. J. Med. Plants Res. 2012, 6. [Google Scholar] [CrossRef]
- Awolola, G.V.; Koorbanally, N.A.; Chenia, H.; Shode, F.O.; Baijnath, H. Antibacterial and Anti-Biofilm Activity of Flavonoids and Triterpenes Isolated from The Extracts of Ficus Sansibarica Warb. Subsp. Sansibarica (Moraceae) Extracts. Afr. J. Trad. Complement. Altern. Med. 2014, 11, 124. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata ). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Iinuma, M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine 2000, 7, 161–165. [Google Scholar] [CrossRef]
- Sanver, D.; Murray, B.S.; Sadeghpour, A.; Rappolt, M.; Nelson, A.L. Experimental Modeling of Flavonoid–Biomembrane Interactions. Langmuir 2016, 32, 13234–13243. [Google Scholar] [CrossRef]
- Stepanović, S.; Antić, N.; Dakić, I.; Švabić-Vlahović, M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ollila, F.; Halling, K.; Vuorela, P.; Vuorela, H.; Slotte, J.P. Characterization of Flavonoid–Biomembrane Interactions. Arch. Biochem. Biophys. 2002, 399, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Mishra, A.; Kehri, H.K.; Sharma, B.; Pandey, A.K. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The Antibacterial Activity of Coriolus versicolor Methanol Extract and Its Effect on Ultrastructural Changes of Staphylococcus aureus and Salmonella Enteritidis. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, A.; Rozalski, M.; Karolczak, W.; Wieckowska-Szakiel, M.; Sadowska, B.; Rozalska, B. Synthetic 3-Arylidenefl avanones as Inhibitors of the Initial Stages of Biofilm Formation by Staphylococcus aureus and Enterococcus faecalis. Z. Für Naturforschung C 2011, 66, 0104. [Google Scholar] [CrossRef]
- Ganesan, K.; Jayachandran, M.; Xu, B. A critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutr. 2017, 58, 1165–1229. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A Review on Pharmacological Properties of Coumarins. Mini-Rev. Med. Chem. 2018, 18. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Sahakyan, N.; Babayan, A.; Chichoyan, N.; Petrosyan, M.; Trchounian, A. Essential Oil and Ethanol Extract of Oregano (Origanum vulgare L.) from Armenian Flora as a Natural Source of Terpenes, Flavonoids and other Phytochemicals with Antiradical, Antioxidant, Metal Chelating, Tyrosinase Inhibitory and Antibacterial Activity. Curr. Pharm. Des. 2019, 25, 1809–1816. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Silva, N.C.C.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.G.N.V.; krishna, B.V.; Swamy, P.L.; Rao, T.S.; Rao, G.S. Antibacterial synergy between quercetin and polyphenolic acids against bacterial pathogens of fish. Asian Pac. J. Trop. Dis. 2014, 4, S326–S329. [Google Scholar] [CrossRef]
- Thiago, J.D.S.B.; Andréa, F.F.; Ana, C.D.P.R.I.; Norma, A. Cytotoxic, antibacterial and antibiofilm activities of aqueous extracts of leaves and flavonoids occurring in Kalanchoe pinnata (Lam.) Pers. J. Med. Plants Res. 2016, 10, 763–770. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors inPseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.-P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids SuppressPseudomonas aeruginosaVirulence through Allosteric Inhibition of Quorum-sensing Receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2017, 9, 522–554. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane Interactions: A Protective Role of Flavonoids at the Membrane Surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- Vasconcelos, M.A.; Arruda, F.V.S.; de Alencar, D.B.; Saker-Sampaio, S.; Albuquerque, M.R.J.R.; dos Santos, H.S.; Bandeira, P.N.; Pessoa, O.D.L.; Cavada, B.S.; Henriques, M.; et al. Antibacterial and Antioxidant Activities of Derriobtusone A Isolated from Lonchocarpus obtusus. Biomed. Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Christena, L.R.; Subramaniam, S.; Vidhyalakshmi, M.; Mahadevan, V.; Sivasubramanian, A.; Nagarajan, S. Dual role of pinostrobin-a flavonoid nutraceutical as an efflux pump inhibitor and antibiofilm agent to mitigate food borne pathogens. RSC Adv. 2015, 5, 61881–61887. [Google Scholar] [CrossRef]
- Lee, P.; Tan, K.S. Effects of Epigallocatechin gallate against Enterococcus faecalis biofilm and virulence. Arch. Oral Biol. 2015, 60, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the β-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef]
- Jeong, K.-W.; Lee, J.-Y.; Kang, D.-I.; Lee, J.-U.; Shin, S.Y.; Kim, Y. Screening of Flavonoids as Candidate Antibiotics againstEnterococcus faecalis. J. Nat. Prod. 2009, 72, 719–724. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Evaluation of Epigallocatechin Gallate and Related Plant Polyphenols as Inhibitors of the FabG and FabI Reductases of Bacterial Type II Fatty-acid Synthase. J. Biol. Chem. 2004, 279, 30994–31001. [Google Scholar] [CrossRef]
- Elmasri, W.A.; Zhu, R.; Peng, W.; Al-Hariri, M.; Kobeissy, F.; Tran, P.; Hamood, A.N.; Hegazy, M.F.; Paré, P.W.; Mechref, Y. Multitargeted Flavonoid Inhibition of the Pathogenic Bacterium Staphylococcus aureus: A Proteomic Characterization. J. Proteome Res. 2017, 16, 2579–2586. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, S.-Y.; Ye, Y.-B.; Zhao, W.-H.; Sun, X.-G.; Wang, Z.-Q.; Li, R.; Sun, Y.-H.; Tian, W.-X.; Zhang, Y.-X. The antibacterial efficacy of an aceraceous plant [Shantung maple (Acer truncatum Bunge)] may be related to inhibition of bacterial β-oxoacyl-acyl carrier protein reductase (FabG). Biotechnol. Appl. Biochem. 2008, 51, 73. [Google Scholar] [CrossRef]
- Brown, A.K.; Papaemmanouil, A.; Bhowruth, V.; Bhatt, A.; Dover, L.G.; Besra, G.S. Flavonoid inhibitors as novel antimycobacterial agents targeting Rv0636, a putative dehydratase enzyme involved in Mycobacterium tuberculosis fatty acid synthase II. Microbiology 2007, 153, 3314–3322. [Google Scholar] [CrossRef]
- Fujita, M.; Shiota, S.; Kuroda, T.; Hatano, T.; Yoshida, T.; Mizushima, T.; Tsuchiya, T. Remarkable Synergies between Baicalein and Tetracycline, and Baicalein and β-Lactams against Methicillin-ResistantStaphylococcus aureus. Microbiol. Immunol. 2005, 49, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Eumkeb, G.; Siriwong, S.; Phitaktim, S.; Rojtinnakorn, N.; Sakdarat, S. Synergistic activity and mode of action of flavonoids isolated from smaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli. J. Appl. Microbiol. 2011, 112, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Sandle, T.; Al-Aboody, M.S.; AlFonaisan, M.K.; Alturaiki, W.; Mickymaray, S.; Premanathan, M.; Alsagaby, S.A. Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii - A first report from the Kingdom of Saudi Arabia. J. Infect. Public Health 2018, 11, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, S.; Wójciak-Kosior, M.; Dziągwa-Becker, M.; Gleńsk, M.; Sowa, I.; Fijałkowski, K.; Rurańska-Smutnicka, D.; Matkowski, A.; Junka, A. The Activity of Isoquinoline Alkaloids and Extracts from Chelidonium majus against Pathogenic Bacteria and Candida sp. Toxins 2019, 11, 406. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophy. Res. Comm. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Verdrengh, M.; Collins, L.V.; Bergin, P.; Tarkowski, A. Phytoestrogen genistein as an anti-staphylococcal agent. Microb. Infect. 2004, 6, 86–92. [Google Scholar] [CrossRef]
- Ulanowska, K.; Tkaczyk, A.; Konopa, G.; Węgrzyn, G. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 2005, 184, 271–278. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. d-Alanine:d-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef]
- Xu, H. Flavones inhibit the hexameric replicative helicase RepA. Nucleic Acids Res. 2001, 29, 5058–5066. [Google Scholar] [CrossRef]
- Shadrick, W.R.; Ndjomou, J.; Kolli, R.; Mukherjee, S.; Hanson, A.M.; Frick, D.N. Discovering New Medicines Targeting Helicases. J. Biomol. Screen. 2013, 18, 761–781. [Google Scholar] [CrossRef]
- Bhosle, A.; Chandra, N. Structural analysis of dihydrofolate reductases enables rationalization of antifolate binding affinities and suggests repurposing possibilities. FEBS J. 2016, 283, 1139–1167. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Martinez, M.D.; Navarro-Peran, E.; Cabezas-Herrera, J.; Ruiz-Gomez, J.; Garcia-Canovas, F.; Rodriguez-Lopez, J.N. Antifolate Activity of Epigallocatechin Gallate against Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2005, 49, 2914–2920. [Google Scholar] [CrossRef] [PubMed]
- Raju, A.; Degani, M.S.; Khambete, M.P.; Ray, M.K.; Rajan, M.G.R. Antifolate Activity of Plant Polyphenols againstMycobacterium tuberculosis. Phytother. Res. 2015, 29, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structure determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.W.; Walker, J.E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl. Acad. Sci. USA 2007, 104, 13632–13637. [Google Scholar] [CrossRef]
- Chinnam, N.; Dadi, P.K.; Sabri, S.A.; Ahmad, M.; Kabir, M.A.; Ahmad, Z. Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol. 2010, 46, 478–486. [Google Scholar] [CrossRef]
- Shah, S.; Stapleton, P.D.; Taylor, P.W. The polyphenol (−)-epicatechin gallate disrupts the secretion of virulence-related proteins by Staphylococcus aureus. Lett. Appl. Microbiol. 2007, 46, 181–185. [Google Scholar] [CrossRef]
- Lee, J.-H.; Regmi, S.C.; Kim, J.-A.; Cho, M.H.; Yun, H.; Lee, C.-S.; Lee, J. Apple Flavonoid Phloretin Inhibits Escherichia coli O157:H7 Biofilm Formation and Ameliorates Colon Inflammation in Rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef]
- Choi, O.; Yahiro, K.; Morinaga, N.; Miyazaki, M.; Noda, M. Inhibitory effects of various plant polyphenols on the toxicity of Staphylococcal α-toxin. Microb. Pathog. 2007, 42, 215–224. [Google Scholar] [CrossRef]
- Ruddock, P.S.; Charland, M.; Ramirez, S.; López, A.; Neil Towers, G.H.; Arnason, J.T.; Liao, M.; Dillon, J.-A.R. Antimicrobial Activity of Flavonoids From Piper lanceaefolium and Other Colombian Medicinal Plants Against Antibiotic Susceptible and Resistant Strains of Neisseria gonorrhoeae. Sex. Transm. Dis. 2011, 38, 82–88. [Google Scholar] [CrossRef]
- Rasul, A.; Millimouno, F.M.; Ali Eltayb, W.; Ali, M.; Li, J.; Li, X. Pinocembrin: A Novel Natural Compound with Versatile Pharmacological and Biological Activities. Biomed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. Bmc Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Hertel, W.; Peschel, G.; Ozegowski, J.-H.; Müller, P.-J. Inhibitory Effects of Triterpenes and Flavonoids on the Enzymatic Activity of Hyaluronic Acid-Splitting Enzymes. Arch. Pharm. 2006, 339, 313–318. [Google Scholar] [CrossRef]
- Bush, K.; Fisher, J.F. Epidemiological Expansion, Structural Studies, and Clinical Challenges of New β-Lactamases from Gram-Negative Bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef]
- Bush, K. The ABCD’s of β-lactamase nomenclature. J. Infect. Chemother. 2013, 19, 549–559. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. In Virulence Mechanisms of Bacterial Pathogens, 5th ed.; American Society of Microbiology: Washington, DC, USA, 2016; pp. 481–511. [Google Scholar] [CrossRef]
- Yan, X.; Gu, S.; Shi, Y.; Cui, X.; Wen, S.; Ge, J. The effect of emodin on Staphylococcus aureus strains in planktonic form and biofilm formation in vitro. Arch. Microbiol. 2017, 199, 1267–1275. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, S.; Yao, F.; Hou, B.; Huang, Y.; Hua, D.; Zheng, Y.; Qian, Y. Baicalein Suppresses the SOS Response System of Staphylococcus Aureus Induced by Ciprofloxacin. Cell. Physiol. Biochem. 2011, 28, 1045–1050. [Google Scholar] [CrossRef]
- Klančnik, A.; Možina, S.S.; Zhang, Q. Anti-Campylobacter Activities and Resistance Mechanisms of Natural Phenolic Compounds in Campylobacter. PLoS ONE 2012, 7, e51800. [Google Scholar] [CrossRef]
- Fathima, A.; Rao, J.R. Selective toxicity of Catechin—A natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Taylor, P.W.; Nagaoka, Y.; Uesato, S.; Hara, Y.; Lamb, A.J. Investigation of the antibacterial activity of 3-O-octanoyl-(-)-epicatechin. J. Appl. Microbiol. 2008, 105, 1461–1469. [Google Scholar] [CrossRef]

| Botanical Name | Family | Plant Used | Extracts | MIC * | Gram Positive | Gram Negative | Fungi | References |
|---|---|---|---|---|---|---|---|---|
| Barleria prionitis L. | Acanthaceae | Leaves | Pet. Ether | 3.33–33.3 mg/mL | B. subtilis, M. luteus, B. cereus, S. mutans, S. aureus, L. sporogenes | S. typhi, V. Cholera, M. luteus, Citrobacter | - | [19] |
| Chloroform | 5–50 mg/mL | B. subtilis, L. sporogenes | S. typhi, V. cholerae, Citrobacter, Providencia | - | ||||
| Methanol | 10–100 mg/mL | B. subtilis, L. sporogenes | V. cholerae, S. typhi, | - | ||||
| Ethanol | 50–600 μg/mL | - | S. typhi | - | ||||
| Bark | Acetone | 25, 50, 100 mg/mL | Bacillus spp., S. mutans, S. aureus, | Pseudomonas spp., | S. cerevisiae, C. albicans | |||
| Ethanol | 25, 50, 100 mg/mL | |||||||
| Methanol | 25, 50, 100 mg/mL | |||||||
| Adhatoda vasica L. | Acanthaceae | Leaves | Aqueous | 4% v/v | M. tuberculosis, | E. coli, S. typhi | - | [20] |
| Methanol | 625 µg/mL | S. aureus | E. coli, S. typhi | - | ||||
| Pellaea calomelanos L. | Adiantaceae | Leaves, Rhizomes | Aqueous, | 250 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Sambucus australis Cham. & Schltdl. | Adoxaceae | Leaves and Bark | Hexane | 50 μg/mL | S. aureus, S. agalactiae | E. coli, S. typhimurium and K. pneumoniae | C. albicans | [22] |
| Ethanol | ||||||||
| Carpobrotus edulis L N.E.Br. | Aizoaceae | Leaves | Aqueous | 100 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrataC. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Achyranthes aspera L. | Amaranthaceae | Root, Leaves, Stem | Ethanol | 1 mg/mL | S. aureus, B. subtilis, | E. coli, P. vulgaris, K. pneumoniae | - | [16] |
| Alternanthera Sessile L. | Amaranthaceae | Leaves | Ethanol | 75 μg/mL | S. pyogenes | S. typhi | - | [24,25] |
| Amaranthus caudatus L. | Amaranthaceae | Leaves | Ethyl Acetate | 162.2–665 mg/mL | S. aureus, Bacillus spp. | E. coli, S. typhi, P. mirabilis | - | [26] |
| Chloroform | 1.25 mg/mL | |||||||
| Methanol | 3–5 mg/mL | |||||||
| Amaranthus hybridus L. | Amaranthaceae | Leaves | Ethyl Acetate | 200–755 mg/mL | - | E. coli, S. typhi, k. pneumoniae, P. aeruginosa | - | [26] |
| Chloroform | 1.25 mg/mL | |||||||
| Methanol | 3–5 mg/mL | |||||||
| Amaranthus spinosus L. | Amaranthaceae | Leaves | Ethyl Acetate | 129 mg/mL | - | S. typhi | - | [26] |
| Chloroform | 1.25 mg/mL | |||||||
| Methanol | 3–5 mg/mL | |||||||
| Boophane disticha L.f. | Amaryllidaceae | Leaves | Aqueous, | 20–100 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Scadoxus puniceus (L.) Friis &Nordal. | Amaryllidaceae | Rhizomes, Roots | Aqueous | 50 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Harpephyllum caffrum Bernh. exKrauss | Anacardiaceae | Bark, Leaves | Aqueous | 125–500 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Lannea discolor Engl. | Anacardiaceae | Leaves | Aqueous | 50–200 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Polyalthia cerascides L. | Annonaceae | Stem Bark | Dichloromethane | 100 μg/mL | C. Dipthieriae | - | - | [27] |
| Berula erecta Huds., Coville | Apiaceae | Rhizome, Leaves, Stem | Aqueous | 2–16 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Acokanthera oppositifolia L. Codd. | Apocynaceae | Leaves, Stem | Aqueous | 25–200 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Plumeria ruba L. | Apocynaceae | Leaves | Aqueous | 50–200 μg/mL | S. epidermidis | E. coli | - | [16] |
| Dichloromethane/Methanol | 100 μg/mL | |||||||
| Acokanthera oppositifolia (Laim.) Codd., | Apocynaceae | Leaves | Aqueous | 10–50 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Rauvolfia caffra Sond. | Apocynaceae | Leaves | Aqueous | 25, 50 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Calotropis gigantea L. | Apocynaceae | Latex | Ethanol | 1–8 mg/mL | - | - | C. albicans, T. mentagrophytes, T. rubrum | [16] |
| Plumeria alba L. | Apocynaceae | Root | Methanol | 10–40 μg/mL | - | E. coli | [16] | |
| Ilex mitis Radlk. | Aquifoliaceae | Bark, Leaves | Aqueous | 1–8 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Anchomanes difformis Engl. | Araceae | Roots | Methanol | 20–100 mg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Zantedeschia aethiopica Spreng | Araceae | Leaves | Aqueous | 50 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 15–150 μg/mL | |||||||
| Arum dioscoridis L. | Araceae | Leaves | Aqueous | 125–500 μg/mL | S. aureus, S. pneumoniae | E. coli, S. typhi, P. aeruginosa | - | [29] |
| Aristolochia Indica L. | Aristolochiaceae | Leaves | Ethanol | 1–8 mg/mL | - | - | A. niger A. flavus A. fumigatus | [3,4,30,31] |
| Vernonia blumeoides Hook. f. | Asteraceae | Aerial Part | Ethanol | 100 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Artemisia afra Jacq. ex Willd. | Asteraceae | Leaves, Stem | Aqueous | 2–16 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Tarchonanthus camphoratus L. | Asteraceae | Leaves | Aqueous | 25–200 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Helichrysum paronychioides L. | Asteraceae | Whole Plant | Pet ether | 50–200 μg/mL | B. cereus | S. flexneri | C. glabrata, C. krusei, T. rubrum and T. tonsurans | [2] |
| Methanol | 50–200 μg/mL | |||||||
| Senecio longiflorus L. | Asteraceae | Stem and Leaves | Pet ether | 125–625 μg/mL | B. cereus | S. flexneri | C. glabrata, C. krusei, T. rubrum and T. tonsurans | [2] |
| Methanol | 50–200 μg/mL | |||||||
| Dahlia pinnata L. | Asteraceae | Leaves | Chloroform | 2–16 μg/mL | – | E. aerogenes, P. aeruginosa | – | [16] |
| Athrixia phylicoides DC. | Asteraceae | Leaves | Aqueous | 25–200 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/ml | |||||||
| Dicoma anomala Sond. | Asteraceae | Tuber | Aqueous | 50–200 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Vernonia natalensis Sch. Bip. exWalp. | Asteraceae | Leaves, Roots | Aqueous | 10–50 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Achillea millefolium L. | Asteraceae | Leaves | Ethanol | 100 μg/mL | S. aureus | P. aeruginosa S. typhi, E. coli | C. albicans | [29] |
| Blumea balsamifer (Linn.) D.C. | Asteraceae | Whole Plant | Ethanol | 250 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Impatiens balsamina L. | Balsaminaceae | Leaf | Ethanol | 50–200 μg/ml | methicillin-resistant S. aureus | - | - | [28] |
| Berberis chitria L. | Berberidaceae | Roots | Ethanol, | 5.5–6.5 mg/mL | S. aureus | E. coli | - | [33] |
| Methanol | 2.5–3.5 mg/mL | |||||||
| Alnus nepalensis D. Don. | Betulaceae | TBL | Ethanol | 50–200 μg/mL | Methicillin-resistant S. aureus | - | - | [32] |
| Tecoma capensis Lindl. | Bignoniaceae | Leaves, Stem | Aqueous, | 10–50 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 2.5 mg/mL | |||||||
| Spathodea campanulata L. | Bignoniaceae | Leaves | Ethanol | 221–254 μg/mL | B. subtilis, S. aureus, | E. coli, K. pneumonia, P. vulgaris, S. typhi, Pseudomonas spp., V. cholerae | - | [6,34,35] |
| Flowers | 156–173 μg/mL | |||||||
| Kigelia africana (Lam.) Benth. | Bignoniaceae | Fruit | Aqueous | 2–16 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Opuntia ficus-indica Mill. | Cactaceae | Leaves | Aqueous | 25–200 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane | 750–12,000 μg/mL | |||||||
| Methanol | ||||||||
| Senna italic L. | Caesalpiniaceae | Leaves | Acetone | 2.5 mg/mL | B. cereus, B. pumilus, B. subtilis, S. aureus, E. faecalis, | - | - | [36] |
| Cassia fistula L. | Caesalpiniaceae | Seeds | Aqueous | 780–6250 μg /mL | S. aureus | - | - | [6] |
| Ethanol | 2–16 μg/mL | |||||||
| Warburgia salutaris (G. Bertol.) Chiov. | Canellaceae | Bark, Twigs | Aqueous | 5.0–10 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| 50–200 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] | ||||
| Dichloromethane, Methanol | 750–12,000 μg/mL | |||||||
| Cadaba fruticosa L. | Capparaceae | Leaves | Acetone | 100–200 μg/mL | S. pyogens, S. aureus, B. subtilis | S. typhi, P. vulgaris, K. pneumoniae, P. aeruginosa, E. coli | - | [37] |
| Aqueous | 4–16 μg/mL | |||||||
| Benzene | 4–16 μg/mL | |||||||
| Butanol | 4–16 μg/mL | |||||||
| Chloroform | 4–16 μg/mL | |||||||
| Ethanol | 4–16 μg/mL | |||||||
| Boscia senegalensis Del. | Capparidaceae | Roots | Methanol | 10–20 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Celastrus orbiculatus Thunb. | Celastraceae | Vane | Ethanol | 1–2 mg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Euonymus fortunei (Turcz.); Hand. Mazz. | Celastraceae | Leaves | Ethanol | 10–40 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Chenopodium ambrosioides Bert. ex Steud. | Chenopodiaceae | Leaves | Aqueous | 2–16 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Garcinia mangostana L. | Clusiaceae | Fruit Shell | Ethanol | 25–200 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Garcinia morella Desr. | Clusiaceae | Whole Plant | Ethanol | 100–400 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Terminalia paniculata L. | Combretaceae | Stem Bark | Ethyl Acetate | 3.25, 3.5 mg/mL | S. aureus, B. subtilis | - | - | [38] |
| Methanol | 5–20 μg/mL | |||||||
| Terminalia sericea Burch. ex DC. | Combretaceae | Roots | Aqueous | 100–300 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Eupatorium odoratum L. | Compositae | Leaves | Benzene | 300–600 μg/mL | B. cereus, S. aureus | E. coli, K. pneumoniae, V. cholerae | C. albicans | [39] |
| Aqueous | 300–600 μg/mL | |||||||
| Acetone | 300–600 μg/mL | |||||||
| Acmella paniculata L. | Compositae | Whole Plant | Chloroform | 15 μg/mL | - | E. aerogenes | - | [40] |
| Pet. ether | 5–15 μg/mL | |||||||
| Methanol | 5–15 μg/mL | |||||||
| Cotyledon orbiculata L. | Crassulaceae | Leaves | Aqueous | 5–30 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane | 750–12,000 μg/mL | |||||||
| Methanol | ||||||||
| Cotyledono rbiculata Forssk. | Crassulaceae | Leaves | Aqueous | 25–200 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Mormodica basalmina L. | Cucurbitaceae | Whole Plant | Methanol | 500 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Coccinia grandis L. | Cucurbitaceae | Leaves | Aqueous | 500 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 2 mg/mL | |||||||
| Luffa acyntangula L. | Cucurbitaceae | Leaves | Aqueous | 5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane | 2 mg/mL | |||||||
| Methanol | ||||||||
| Mukia maderspatana L. | Cucurbitaceae | Leaves | Aqueous | 5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 1 mg/mL | |||||||
| Trichosanthes cucumerina L. | Cucurbitaceae | Leaves | Aqueous | 5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 1 mg/mL | |||||||
| Momordica balsami- na L. | Cucurbitaceae | Leaves, Roots | Acetone | 500 μg/mL | B. cereus, B. pumilus, B. subtilis, S. aureus, E. faecalis | E. coli, E. cloaceae, K. pneumoniae, P. aeruginosa, S. marcescens | - | [42] |
| Carex prainii C.B. Clarke | Cyperaceae | Whole Plant | Ethanol | 15–45 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Dioscorea dregeana T. Durand & Schinz. | Dioscoreaceae | Tuber | Aqueous | 5–30 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Sansevieria hyacinthoides L. | Dracaenaceae | Leaves, rhizome | Aqueous, | 1–4 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrataC. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Diospyros mespiliformis Hochst. exA. DC. | Ebenaceae | Leaves | Aqueous | 15–45 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Phyllanthus amarus Schum. Thonn. | Euphorbiaceae | Whole Plant | Methanol | 650–600 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Croton gratissimus Burch. | Euphorbiaceae | Leaves, Stem, | Aqueous | 5 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrataC. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Spirostachys africana Sond. | Euphorbiaceae | Leaves, Bark | Aqueous | 490 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrataC. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Acalypha indica L. | Euphorbiaceae | Leaves | Aqueous | 4% v/v | M. tuberculosis | - | - | [43] |
| Bridelia micrantha Baill. | Euphorbiaceae | Bark, Leaves | Aqueous | 5 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Emblica officinalis L. | Euphorbiaceae | Leaves | Benzene | 350–600 μg/mL | B. cereus, S. aureus | E. coli, K. pneumoniae, V. cholerae | C. albicans | [39] |
| Aqueous | 300–600 μg/mL | |||||||
| Acetone | 300–600 μg/mL | |||||||
| Hevea brasiliensis L. | Euphorbiaceae | Leaves | Benzene | 350–600 μg/mL | B. cereus, S. aureus | E. coli, K. pneumoniae, V. cholerae | C. albicans | [39] |
| Aqueous | 300–600 μg/mL | |||||||
| Acetone | 300–600 μg/mL | |||||||
| Mallotus yunnanensis Pax et. Hoffm. | Euphorbiaceae | Tender Branches & Leaves | Ethanol | 8–256 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Acacia albida Del. | Fabaceae | Stem Bark | Methanol | 50 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Acacia catechu (L. f.) Willd | Fabaceae | Wood | Ethanol | 100 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Peltophorum ptercarpum (DC.) | Fabaceae | Bark | Ethanol | 4% v/v | methicillin-resistant S. aureus | - | - | [28] |
| Acacia erioloba Edgew. | Fabaceae | Bark and Leaves | Aqueous | 1.56–3.12 mg/mL | S. aureus, methicillin– resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Dichrostachys cinerea L. | Fabaceae | Stem | Aqueous | 129 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Albizia odoratissima (L.f.) Benth | Fabaceae | Leaves | Hexane | 7.5–15 mg/mL | S. aureus | K. pneumoniae, E. coli, P. aeruginosa, P. vulgaris | - | [44] |
| Chloroform | 859–6875 μg/mL | |||||||
| Ethyl Acetate | 136–546 μg/mL | |||||||
| Methanol | 136–546 μg/mL | |||||||
| Prosopis juliflora L. | Fabaceae | Pod | Chloroform | 250 μg/mL | M. luteus, S. aureus, S. mutans | - | - | [36] |
| Bauhinia macranthera Benth. Ex Hemsl. | Fabaceae | Leaves | Aqueous, | 1.56–3.12 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Erythrina lysistemon Hutch. | Fabaceae | Leaves | Aqueous | 4 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes, S. mutans, S. sanguis, L. acidophilus L. casei | P. aeruginosa, P. gingivalis F. nucleatum | T. mentagrophytes, M. canis, C. albicans C. glabrata C. krusei | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Elephantorrhiza elephantina (Burch.) Skeels | Fabaceae | Leaves, roots and rhizomes | Aqueous | 1–4 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes, B. cereus | P. aeruginosa, S. flexneri | T. mentagrophytes, M. canis, C. glabrata, C. krusei, T. rubrum and T. tonsurans | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Albizia lebbeck L. | Fabaceae | Leaves | Benzene, Aqueous and Acetone | 350–600 μg/mL | B. cereus, S. aureus | E. coli, K. pneumoniae, V. cholera | C. albicans | [39] |
| Adenanthera pavonina L. | Fabaceae | Leaves | Aqueous | 5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 60 μg mg/mL | |||||||
| Alysicarpus vaginalis L. | Fabaceae | Leaves | Aqueous | 5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 2 mg/mL | |||||||
| Bauhinia acuminate L. | Fabaceae | Leaves | Aqueous | 5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 50 μg mg/mL | |||||||
| Bauhinia purpurea L. | Fabaceae | Leaves | Aqueous | 5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 1 mg/mL | |||||||
| Bauhinia racemose L. | Fabaceae | Leaves, Stem Bark | Aqueous | 500 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 500 μg/mL | |||||||
| Cassia alata L. | Fabaceae | Leaves | Aqueous | 250 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 500 μg/mL | |||||||
| Cassia auriculata L. | Fabaceae | Leaves | Aqueous | 1 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 4 mg/mL | |||||||
| Cassia fistula L. | Fabaceae | Root Bark, Stem Bark | Aqueous | 1–5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 500–1000 μg/mL | |||||||
| Cassia tora L. | Fabaceae | Leaves, Root Bark, Stem Bark | Aqueous | 250–4000 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 1000–4000 μg/mL | |||||||
| Crotalaria retusa L. | Fabaceae | Leaves | Aqueous | 4 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 60 μg/mL | |||||||
| Crotalaria verrucosa L. | Fabaceae | Leaves | Aqueous | 1 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 1 mg/mL | |||||||
| Derris Scandens L. | Fabaceae | Leaves | Aqueous | 100 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 4 mg/mL | |||||||
| Desmodiumtriflorum (L.) DC. var. majus Wight & Arn. | Fabaceae | Stem Bark | Aqueous | 1 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 25 μg/mL | |||||||
| Erythuria variegate L. | Fabaceae | Leaves, Stem Bark | Aqueous | 1–5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 250–1000 μg/mL | |||||||
| Indigofera tinctoria L. | Fabaceae | Leaves | Aqueous | 500 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 4 mg/mL | |||||||
| Mimosa pudica L. | Fabaceae | Stem Bark | Aqueous | 1–2 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 250–5000 μg/mL | |||||||
| Myroxylon balsamum L. | Fabaceae | Leaves | Aqueous | 1 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 500 μg/mL | |||||||
| Pterocarpus marsupium Roxb. | Fabaceae | Leaves | Aqueous | 4 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 250 μg/mL | |||||||
| Pterocarpus santalinus L. | Fabaceae | Leaves | Aqueous | 2 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 4 mg/mL | |||||||
| Saraca asoca (Roxb.) Willd | Fabaceae | Leaves | Aqueous | 120 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 5 mg/mL | |||||||
| Sesbania grandiflora (L.) Poiret | Fabaceae | Stem Bark, Root Bark, Leaves | Aqueous, | 2 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 100 μg/mL | |||||||
| Tamarindus indica L. | Fabaceae | Leaves | Aqueous | 250–500 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 60–100 μg/mL | |||||||
| Tephrosia purpurea L. Pers. | Fabaceae | Leaves | Aqueous | 5 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 5 mg/mL | |||||||
| Butea monosperma L. | Fabaceae | Leaves | Aqueous | 4 mg/mL | B. cereus, S. aureus, methicillin-resistant S. aureus | - | - | [41,45] |
| Dichloromethane/Methanol | 2 mg/mL | |||||||
| Ethanol | 100–200 μg/mL | |||||||
| Senna alata | Fabaceae | Leaf | Ethanol | 100 μg/mL | methicillin-resistant S. aureus | - | - | [46] |
| Quercus infectoria Olivier | Fagaceae | Nutgalls | Ethanol | 100–200 μg/mL | methicillin-resistant S. aureus | - | - | [16] |
| Cyclobalanopsis austroglauca Y.T. Chang | Fagaceae | TBL | Ethanol | 8–256 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Scaevola spinescens L. | Goodeniaceae | Aerial parts | Ethyl Acetate, Methanol | 500 μg/mL | S. pyogenes, S. aureus | - | - | [38] |
| Gunnera perpensa L. | Gunneraceae | Leaves, Rhizome | Aqueous, | 4 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Eucomis punctate L’Her. | Hyacinthaceae | Leaves | Aqueous, | 500 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Drimia sanguinea L. | Hyacinthaceae | Bulb | Pet ether | 18.75, 37.5, 300, 600, 1200 μg/mL | B. cereus | S. flexneri | C. glabrata, C. krusei, T. rubrum and T. tonsurans | [2] |
| Hypoxis hemerocallidea L. | Hypoxidaceae | Leaves | Pet ether | 195–12,500 μg/mL | B. cereus | S. flexneri | T. rubrum, T.urans, C. glabrata C. krusei | [47] |
| Methanol | 390–3125 μg/mL | |||||||
| Curculigo orchioides Gaertn. | Hypoxidaceae | Whole Plant | Ethanol | 8–256 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Illicium simonsii Maxim. | Illiciaceae | TBL | Ethanol | 8–256 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Aristea ecklonii Baker. | Iridaceae | Leaves and Roots | Aqueous | 129 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Tetradenia riparia Hochst. | Lamiaceae | Leaves, Stem | Aqueous | 200–755 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Thymus vulgaris L. | Lamiaceae | Leaves | Essential Oil | 50 μg/mL | methicillin-resistant S. aureus | - | - | [48] |
| Mentha aquatica L. | Lamiaceae | Aerial Parts | Methanol | 1.56–3.12 mg/mL | S. aureus | E. coli, P. aeruginosa, S. heidelberg, K. pneumoniae, E. aerogenes, M. morganii | - | [49] |
| Chloroform | 128 μg/mL | |||||||
| Acetone | 32–128 μg/mL | |||||||
| Stachys guyoniana Noë ex. Batt. | Lamiaceae | Leaves | n-Butanol | 4 mg/mL | S. aureus | E. coli, P. aeruginosa, S. heidelberg, K. pneumoniae, E. aerogenes, M. morganii | - | [49] |
| Ethyl Acetate | 128 μg/mL | |||||||
| Chloroform | 32–128 μg/mL | |||||||
| Ocimum basilicum L. | Lamiaceae | Stem, leaves | Ethanol | 1–4 mg/mL | S. aureus | - | - | [38] |
| Ocimum gratissimum L. | Lamiaceae | Leaves | Methanol | 780–6250 μg/mL | S. aureus | S. typhi, E. coli, S. paratyphi | - | [38] |
| Ocimum sanctum L. | Lamiaceae | Whole Plant | Methanol | 360 μg/mL | S. aureus, S. saprophyticus | S. typhi, E. coli, S. paratyphi | - | [6] |
| Mentha longifolia Huds. | Lamiaceae | Leaves | Aqueous | 150, 300, 600 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Melissa officinalis L. | Lamiaceae | Leaves | Ethanol | 49 μg/mL | - | K. pneumoniae | - | [42] |
| Ocimum americanum L. | Lamiaceae | Leaves | Acetone | 2.5 mg/mL | B. cereus, B. pumilus, B. subtilis, S. aureus, E. faecalis | - | - | [16] |
| Machilus salicina Hance. | Lauraceae | Tender Branches & Leaves | Ethanol | 500 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Meliosma squamulata Hance. | Lauraceae | TBL | Ethanol | 1–4 mg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Sophora alopecuroides | Leguminosae | Aerial Parts, Seeds | Ethanol | 129 mg/mL | B. subtilis, S. aureus, B. subtilis | P. aeruginosa | - | [50] |
| Acacia karroo Hayne. | Leguminosae | Leaves, Stem | Aqueous, | 200–755 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Acacia polyacantha Willd. | Leguminosae | Leaves, Stem | Aqueous | 50 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Dalbergia obovate E. Mey. | Leguminosae | Leaves, stem | Aqueous | 1.56–3.12 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Sophora jaubertii | Leguminosae | Aerial Parts, Seeds | Ethanol | 4 mg/mL | B. subtilis, P. aeruginosa, S. aureus | - | - | [38] |
| Glycyrrhiza glabra L. | Leguminosae | Leaves | Methanol | 1–4 mg/mL | K. kristinae, M. luteus, S. auricularis, B. megaterium | A. bohemicus, E. coli | - | [51] |
| Allium cepa L. | Liliaceae | Bulb | Aqueous | 780–6250 μg/mL | M. tuberculosis | - | - | [43] |
| Allium sativum L. | Liliaceae | Bulb | Aqueous | 4% v/v | M. tuberculosis | - | - | [43] |
| Allium vera L. | Liliaceae | Gel | Aqueous | 4% v/v | M. tuberculosis | - | - | [43] |
| Lobelia nicotianaefolia L. | Lobeliaceae | Root | Chloroform | 129 mg/mL | S. aureus | P. aeruginosa | - | [39] |
| Acetone | 6 mg/mL | |||||||
| Ethanol | 6 mg/mL | |||||||
| Woodfordia fruticose L. | Lythraceae | Flower | Aqueous | 200–755 mg/mL | S. aureus, B. cereus | S. typhi, E. coli, S. dysenteriae. V. cholerae | - | [37] |
| Dichloromethane/Methanol | 100 mg/mL | |||||||
| Manglietia hongheensis Y.m Shui et. W.H. Chen. | Magnoliaceae | TBL | Ethanol | 50 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Malva parviflora L. | Malvaceae | Leaves | Aqueous | 500 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Sida rhombifolia L. | Malvaceae | Stem | Chloroform | 162.2–665 mg/mL | S. lutea, B. subtilis, | E. coli, Shigella shiga | - | [38] |
| Walsura robusta L. | Meliaceae | Wood | Ethanol | 250 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Swietenia mahagoni | Meliaceae | Seed | Ethanol | 500 μg/mL | methicillin-resistant S. aureus | - | - | [52] |
| Azadirachta indica | Meliaceae | LeavesStem | MethanolAqueous | 1.56–3.12 mg/mL | M. luteusS. aureus, S. pyogenes | P. vulgarisE. coli, P. aeruginosa | -- | [53] |
| Ekebergia capensis Sparrm. | Meliaceae | Bark, Leaves | Aqueous | 1.59–25 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Trichilia emetica Vahl | Meliaceae | Leaves | Aqueous | 50–600 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Melia azedarach L. | Meliaceae | Leaves | Methanol | 3.33–33.3 mg/mL | B. cereus, S. aureus | E. coli, P. aeruginosa | A. niger, A. flavus, F. oxysporum, R. stolonifer | [16] |
| Ethanol | 500 μg/mL | |||||||
| Pet.ether | 1.56–3.12 mg/mL | |||||||
| Aqueous | 10–30 mg/mL | |||||||
| Melianthus comosus Vahl. | Melianthaceae | Leaves | Aqueous | 50 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes, methicillin-resistant S. aureus | P. aeruginosa | T. mentagrophytes, M. canis | [28] |
| Dichloromethane/Methanol | 4–64 mg/mL | |||||||
| Melianthus major L. | Melianthaceae | Leaves | Ethanol | 10–100 mg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Melianthus major L. | Melianthaceae | Leaves | Aqueous | 5–50 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Cissampelos torulosa E. Mey. Ex Harv. | Menispermaceae | Leaves, Stem | Aqueous | 25, 50, 100 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Tinospora crispa L. | Menispermaceae | Stem | Ethanol | 10 mg/mL | methicillin-resistant S. aureus | - | - | [21] |
| Cissampelos capensis Thunb. | Menispermaceae | Leaves | Aqueous | 3.33–33.3 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Ficus natalensis Hochst. | Moraceae | Leaves | Aqueous | 250 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Ficus sur Forssk. | Moraceae | Bark, Leaves | Aqueous, | 10–100 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Moringa oleifera Lam. | Moringacceae | Leaf | Ethanol | 5–50 mg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Myrothamnus flabellifolia Welw., | Myrothamnaceae | Leaves | Aqueous | 156–625 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Embelia ruminate (E. Mey.exA.Dc.) Mez | Myrsinaceae | leaves | Aqueous | 350–600 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Embelia burm f. | Myrsinaceae | Leaves | Ethanol | 500 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Callistemon rigidus R.Br. | Myrtaceae | Leaf | Methanol | 800 mg/disc | methicillin-resistant S. aureus | - | - | [28] |
| Psidium guajava L. | Myrtaceae | Leaf | Ethanol | 600, 1200 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Heteropyxis natalenesis Harv. | Myrtaceae | Leaves, Stem | Aqueous, | 5 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Eucalyptus camaldulensis Dehnh. | Myrtaceae | Bark | Aqueous | 9.375, 18.75, 37.5, 75, 150, 300, 600 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Eucalyptus deglupta | Myrtaceae | Leaves | Benzene | 37.5, 75, 150, 300, 600 μg/mL | B. cereus, S. aureus | E. coli, K. pneumoniae, V. cholerae | C. albicans | [39] |
| Aqueous | 4–8 mg/mL | |||||||
| Acetone | 6 mg/mL | |||||||
| Myrtus communis L. | Myrtaceae | Leaves | Ethanol | 12.5–50 mg/mL | B. cereus, L. monocytogenes | E. coli | C. albicans | [42] |
| Nelumbo nucifera L. | Nelumbonaceae | Flower | Ethanol | 8–32 mg/mL | B. subtilis, S. aureus, | E. coli, K. pneumonia, P. aeruginosa | - | [54] |
| Nymphaea lotus L. | Nymphaeaceae | Leaf | Ethanol | 500 μg/mL | methicillin-resistant S. aureus | - | - | [21] |
| Oxalis corniculata L. | Oxalidaceae | Leaves | Aqueous | 5 mg/mL | B. cereus, S. aureus | E. coli, K. pneumoniae, V. cholera | C. albicans | [39] |
| Benzene | 37.5, 75, 150, 300, 600 μg/mL | |||||||
| Acetone | 6 mg/mL | |||||||
| Paeonia lactiflora Pall. | Paeoniaceae | Leaves | Ethanol | 22.4–52.3 μg/mL | K. kristinae, M. luteus, S. auricularis, B. megaterium | A. bohemicus, E. coli | - | [51] |
| Argemone mexicana | Papaveraceae | Stem | Chloroform | 32.4–55.8 μg/mL | S. aureus | E. coli, P. aeruginosa, k. pneumoniae | - | [55] |
| Passiflora Mexicana L. | Passifloraceae | Aerial Parts | Ethanol | 33.7–58.3 μg/mL | S. aureus | - | - | [21] |
| Cleistanthus collinus | Phyllanthaceae | Leaves | Benzene | 100 mg/mL | B. cereus, S. aureus | E. coli, K. pneumoniae, V. cholerae | C. albicans | [39] |
| Aqueous | 4–8 mg/mL | |||||||
| Acetone | 5 mg/mL | |||||||
| Piper nigrum L. | Piperaceae | Bark, Seeds | Ethanol | 500 μg/mL | S. aureus, B. cereus, S. fecalis | P. aeruginosa, E. coli, S. typhi | - | [38] |
| Acetone | 6 mg/mL | |||||||
| Dichloromethane/Methanol | 12.5–50 μg/mL | |||||||
| Pittosporum viridiflorum Sims. | Pittosporaceae | Leaves | Aqueous | 600 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Spinifex littoreus | Poaceae | Grass | Acetone | 2.5 mg/mL | - | - | Dermatophytes | [27] |
| Polygonum molle D. Don. | Polygonaceae | Whole Plant | Ethanol | 25–50 μg/mL | Methicillin-resistant S. aureus | - | - | [32] |
| Eichhornia crassipes L. | Pontederiaceae | Leaves, Shoot | Ethanol | 500–4000 μg/mL | M. luteus | R. rubrum | M. ruber, A. fumigates | [56] |
| Chloroform | 32.4–55.8 μg/mL | |||||||
| Aqueous | 2.5–15 μg/mL | |||||||
| Punica granatum L. | Punicaceae | Fruit Shell | Ethanol | 70 mg/mL | Methicillin-resistant S. aureus | - | - | [28] |
| Clematis brachiate Thunb. | Ranunculaceae | Flower, Leaves, Stem, Root | Aqueous, | 1 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Ziziphus mucronata Willd. | Rhamnaceae | Bark, Leabes | Aqueous | 2.5 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes, S. mutans, S. sanguis, L. acidophilus L. casei | P. aeruginosa, P. gingivalis F. nucleatum | T. mentagrophytes, M. canis, C. albicans C. glabrata C. krusei | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Eriobotrya japonica (Thunb.) Lindl. | Rosaceae | Leaves | Ethanol | 2–16 μg/mL | K. kristinae, M. luteus, S. auricularis, B. megaterium | A. bohemicus, E. coli | - | [51] |
| Pavetta crassipes K. Schum. | Rubiaceae | Leaf | Methanol | 12.5–50 mg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Uncaria gambir (Hunter) Roxb. | Rubiaceae | Leaf, Stem | Ethanol | 8–32 mg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Vangueria spinose L. | Rubiaceae | Leaves | Ethyl Acetate | 500 μg/mL | S. aureus | E. coli, K. pneumoniae, P. aeruginosa | - | [57] |
| Pentanisia prunelloides Walp. | Rubiaceae | Root Bark | Aqueous | 5 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Rothmannia capensis Thunb. | Rubiaceae | Leaves | Aqueous | 22.4–52.3 μg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Geophila repens L. | Rubiaceae | Leaves, Stem Bark | Aqueous | 1 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/methanol | 250 μg/mL | |||||||
| Guettarda speciose L. | Rubiaceae | Leaves | Aqueous | 2 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 2 mg/mL | |||||||
| Haldina cordifolia L. | Rubiaceae | Leaves | Aqueous | 1 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 500 μg/mL | |||||||
| Hedyotis auricularia L. | Rubiaceae | Leaves | Aqueous | 300 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 250 μg/mL | |||||||
| Knoxia zeylanica L. | Rubiaceae | Leaves, Stem | Aqueous | 250 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 1 mg/mL | |||||||
| Mitragyna parvifolia L. | Rubiaceae | Leaves | Aqueous | 300 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 1 mg/mL | |||||||
| Morinda umbellate L. | Rubiaceae | Leaves, Stem Bark | Aqueous | 100 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 250 μg/mL | |||||||
| Nauclea orientalis L. | Rubiaceae | Leaves | Aqueous | 500 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 500 μg/mL | |||||||
| Oldenlandia biflora L. | Rubiaceae | Leaves | Aqueous | 2 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 5 mg/mL | |||||||
| Oldenlandia herbacea L. | Rubiaceae | Stem, Root | Aqueous | 5mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 60 μg/mL | |||||||
| Ophiorrhiza mungos L. | Rubiaceae | Leaves | Aqueous | 2 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 500 μg/mL | |||||||
| Paederia foetida L. | Rubiaceae | Leaves, Stem | Aqueous | 300 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 60 μg/mL | |||||||
| Pavetta lanceolate Eckl. | Rubiaceae | Leaves | Aqueous | 1 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 250 μg/mL | |||||||
| Spermacoce hispida L. | Rubiaceae | Leaves, Stem | Aqueous | 300 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 120 μg/mL | |||||||
| Wendlandia bicuspidate Wight & Arn. | Rubiaceae | Leaves | Aqueous | 60 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 5 mg/mL | |||||||
| Chassalia kolly | Rubiaceae | Whole Plant | Mthanol | 5 mg/mL | S. aureus | E. coli, P. aeruginosa, S. typhi, P. aeruginosa | - | [16] |
| Randia dumetorum L. | Rubiaceae | Fruits | Methanol | 9.375, 18.75, 37.5, 75, 150, 300, 600 μg/mL | S. aureus, S. epidermidis, B. subtilis | E. coli, S. typhi | - | [23] |
| Mitragyna speciosa L. | Rubiaceae | Leaves | Methanol | 37.5, 75, 150, 300, 600 μg/mL | S. typhi | [42] | ||
| Clausena anisate (Willd) Hook. f. ex. | Rutaceae | Leaves, Stem, Twigs | Aqueous | 12.5–50 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Zanthoxylum capense Harv. | Rutaceae | Stem | Aqueous | 8–32 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Aegle marmelos L. | Rutaceae | Leaves and Fruits | Methanol | 500 μg/ml | S.aureus, B. cereus | E. coli, S. typhi, P. aeruginosa, S. boydii, K. aerogenes, P.vulgaris, | [20] | |
| Evodia daneillii (Benn) Hemsl. | Rutaceae | Tender Branches & Leaves | Ethanol | 3.33–33.3 mg/mL | Methicillin-resistant S. aureus | - | - | [32] |
| Skimmia arborescens Anders. | Rutaceae | TBL | Ethanol | 250 mg/mL | Methicillin-resistant S. aureus | - | - | [32] |
| Salvadora australis | Salvadoraceae | Leaves | Acetone | 10–100 mg/mL | B. cereus, B. pumilus, B. subtilis, S. aureus, E. faecalis | - | - | [18] |
| Viscum capense L.f. | Santalaceae | Leaves | Aqueous | 5–50 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Dodonaea angustifolia (L.f.) Benth | Sapindaceae | Leaves | Ethanol | 156–625 μg/mL | methicillin-resistant S. aureus | - | - | [28] |
| Dodonaea viscosa Jacq. | Sapindaceae | Leaves, Stem | Aqueous | 350–600 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Cardiospermum halicacabum L. | Sapindaceae | Leaves | n-Butanol | 500 μg/mL | S. aureus, S. agalactiae | E. coli, S. typhimurium and K. pneumoniae | T. rubrum, C. albicans | [58] |
| Ethyl acetate | 60 μg/mL | |||||||
| Chloroform | 40 μg/mL | |||||||
| Dodonaea angustifolia L. f. | Sapindaceae | Leaves | Aqueous | 800 mg/disc | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Englerophytum magalismontanum Sonder. | Sapotaceae | Leaves, Stem | Aqueous | 600, 1200 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrataC. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Schisandra viridis A.c. Smith. | Schisandraceae | Vane | Ethanol | 5 mg/mL | Methicillin-resistant S. aureus | - | - | [32] |
| Halleria lucida L. | Scrophulariaceae | Leaves Stem | Aqueous | 1–8 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Brandisia hancei Hook.f. | Scrophulariaceae | Whole Plant | Ethanol | 3.33–33.3 mg/mL | Methicillin-resistant S. aureus | - | - | [32] |
| Selaginella tamariscina (Seauv.) Spring. | Selaginellaceae | Whole Plant | Ethanol | 250 mg/mL | Methicillin-resistant S. aureus | - | - | [32] |
| Datura stramonium L. | Solanaceae | Leaves, Stem, Fruit | Aqueous | 10–100 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Solanum incanum L | Solanaceae | Leaves | Aqueous | 5–50 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Solanum trilobatum L. | Solanaceae | Leaves | Acetone | 156–625 μg/mL | S. pyogens, S. aureus, B. subtilis | S. typhi, P. vulgaris, K. pneumoniae, P. aeruginosa, E. coli | - | [37] |
| Aqueous | 250 mg/mL | |||||||
| Benzene | 10–100 mg/mL | |||||||
| Butanol | 5–50 mg/mL | |||||||
| Chloroform | 60 μg/mL | |||||||
| Ethanol | 5 mg/mL | |||||||
| Datura metel L. | Solanaceae | Leaves | Aqueous | 350–600 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 1 mg/mL | |||||||
| Solanum macrocarpon L. | Solanaceae | Leaves, Stem | Aqueous | 500 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 60 μg/mL | |||||||
| Solanum melongena L. | Solanaceae | Leaves, Root Stem | Aqueous | 800 mg/disc | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 100 μg/mL | |||||||
| Solanum nigrum L. | Solanaceae | Leaves, Stem | Aqueous | 600, 1200 μg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 1 mg/mL | |||||||
| Solanum torvum Sw. | Solanaceae | Leaves | Aqueous | 3.33–33.3 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 60 μg/mL | |||||||
| Solanum virginianum L. | Solanaceae | Leaves, Stem, Root | Aqueous | 250 mg/mL | B. cereus, S. aureus | - | - | [41] |
| Dichloromethane/Methanol | 4 mg/mL | |||||||
| Withania somnifera (L.) Dunal | Solanaceae | Roots & Leaves | Aqueous | 10–100 mg/mL | B. cereus, S. aureus, methicillin-resistant S. aureus | - | - | [41,59] |
| Dichloromethane/Methanol | 1 mg/mL | |||||||
| Cola acuminate L. | Sterculiaceae | Stem | Acetone | 5–50 mg/mL | S. aureus | - | C. albicans | [16] |
| Methanol | 100 μg/mL | |||||||
| Schima sinensis (Hemsl. et. Wils) Airy-shaw. | Theaceae | Tbl | Ethanol | 156–625 μg/mL | methicillin-resistant S. aureus | - | - | [32] |
| Coriandrum sativum | Umbelliferae | Seeds | Aqueous | 350–600 μg/mL | S. aureus | K. pneumoniae, P. aeruginosa, | A. niger, P. lilacinum | [27] |
| Clerodendrum inerme L | Verbenaceae | Leaves | Methanol | 500 μg/mL | S. aureus | - | A. niger | [60] |
| Lantana rugosa Thunb. | Verbenaceae | Leaves | Aqueous | 800 mg/disc | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Lantana camara L. | Verbenaceae | Leaves, Flower | Chloroform | 600, 1200 μg/mL | S. aureus, B. cereus | E. coli, S. typhi, P. aeruginosa, K. aerogenes, P. vulgaris, S. Boydii, K. pneumoniae, V. cholerae | A. fumigatus, A. flavus, A. niger, C. albicans | [39] |
| Acetone | 5 mg/mL | |||||||
| Methanol | 1–8 mg/mL | |||||||
| Aqueous | 1–2 mg/mL | |||||||
| Lantana indica L. | Verbenaceae | Leaves | Methanol | 3.33–33.3 mg/mL | B. subtilis, S. aureus, S. pyogenes, | E. coli, P. vulgaris, K. pneumoniae | C.albicans, | [61] |
| Aqueous | 4 mg/mL | |||||||
| Cyphostemma lanigerum Harv. | Vitaceae | Leaves, Stem | Aqueous | 250 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Cyphostemma setosum Roxb. | Vitaceae | Leaves, Stem, Fruit | Aqueous | 10–100 mg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Aloe arborescens Mill. | Xanthorrhoeaceae | Leaves | Aqueous | 5–50 mg/mL | S. aureus, methicillin- resistant S. aureus, gentamycin– methicillin-resistant S. aureus, S. epidermidis, B. agri, P. acnes | P. aeruginosa | T. mentagrophytes, M. canis | [21] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Siphonochilus aethiopicus Schweinf., | Zingiberaceae | Leaves, Stem, Root | Aqueous | 156–625 μg/mL | S. mutans, S. sanguis, L. acidophilus L. casei | P. gingivalis F. nucleatum | C. albicans C. glabrata C. krusei | [23] |
| Dichloromethane/Methanol | 750–12,000 μg/mL | |||||||
| Curcuma xanthorrhiza | Zingiberaceae | Rhizome | Ethanol | 350–600 μg/mL | methicillin-resistant S. aureus | - | - | [46] |
| Kaempferia pandurata Roxb. | Zingiberaceae | Rhizome | Ethanol | 500 μg/mL | methicillin-resistant S. aureus | - | - | [46] |
| Peganum harmala L. | Zygophyllaceae | Seeds | Ethanol | 800 mg/disc | S. aureus | E. coli | - | [21] |
| Botanical Name | Family | Extracts | Bioactive Compounds | MIC * | Organism Inhibited | References |
|---|---|---|---|---|---|---|
| Allium sativum L. | Alliaceae | Methanol | Cyanidin-3-(6’-malonyl)-glucoside, vanillic acid caffeic acid, p-coumaric acid, ferulic acid, sinapic acid, L-alliin, alliin isomer and methiin | - | B. cereus, L. monocytogenes S. aureus, P. aeruginosa, E. coli | [11] |
| Searsia chirindensis (Baker f.) Moffett | Anacardiaceae | Ethanol | Methyl gallate | 30–130 μg/mL | C. jejuni, E. coli, S. flexneri, S. aureus | [86] |
| myricetin-3-O-arabinopyranoside | 60–250 μg/mL | |||||
| myricetrin-3-O-rhamnoside | 60–250 μg/mL | |||||
| kaempferol-3-O-rhamnoside | 130–250 μg/mL | |||||
| quercetin-3-O-arabinofuranoside | 250 μg/mL | |||||
| Dichloromethane/Methanol | 250–6250 μg/mL | |||||
| n-butanol | 130–3125 μg/mL | |||||
| Ethyl Acetate | 60–780 μg/mL | |||||
| Crude | 60–780 μg/mL | |||||
| Xylopia aethiopica (Dunal) A. Rich. | Annonaceae | Aqueous | 1R-a-Pinene, β-Pinene, 2-Carene, Cyclohexene,5-methyl-3-(1-methylethenyl)-trans-(-)- Bicyclo [3.1.0] hexane,6-isopropylidene-1-methyl-, Eucalyptol, Ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan-2-yl carbonate, Isogeraniol, ɑ-Campholenal, L-trans-Pinocarveol, Pinocarvone, Myrtenal, (-)-Spathulenol | 1–256 μg/mL | S. aureus, B. licheniformis, E. coli, K. pneumoniae | [87] |
| Polyalthia cerasoides | Annonaceae | Hexane | N-(4-hydroxy-β-phenethyl-4-hydroxy cinnamide | 64–128 μg/mL | C. diphtheria, B. subtilis, B. cereus, M. lutens | [88] |
| Dichloromethane | 32–256 μg/mL | |||||
| Unonopsis lindmanii R. E. Fries | Anonaceae | Hexane | Gallic acid, kaempferol, ellagic acid, epicatechin, vitexin, corilagin | 25–250 μg/mL | C.albicans | [89] |
| Allagoptera leucocalyx (Drude) Kuntze, | Arecaceae | Hexane | Gallic acid, kaempferol, ellagic acid, epicatechin, vitexin, corilagin | 162.2–665 mg/mL | C.albicans | [89] |
| Bactris glaucescens Drude | Arecaceae | Hexane | Gallic acid, kaempferol, ellagic acid, epicatechin, vitexin, corilagin | 200–755 mg/mL | C.albicans | [89] |
| Scheelea phalerata Mart | Arecaceae | Hexane | Gallic acid, kaempferol, ellagic acid, epicatechin, vitexin, corilagin | 129 mg/mL | C.albicans | [89] |
| Artemisia herba-alba Asso | Asteraceae | Aqueous | 1,8-cineole, β-thujone, α-thujone, camphor | 640–2500 μg/mL | T. rubrum and E. floccosum | [90] |
| Vernonia adoensis Sch. Bip. ex Walp. | Asteraceae | Acetone | Chondrillasterol | 50 μg/mL | S. aureus, K. pneumonia, P. aeruginosa | [1] |
| Matricaria chamomilla | Asteraceae | Ethanol | Phenolic acid | 1.56–3.12 mg/mL | S. typhimurium | [19] |
| Solidago graminifolia L. Salisb. | Asteraceae | Ethanol | di-C-glycosylflavones (schaftoside, isoschaftoside), caftaric acid, gentisic acid, chlorogenic acid, p-coumaric acid, ferulic acid, hyperoside, rutin, quercitrin, quercetin, Luteolin, kaempferol, gallic acid, protocatechuic acid, vanillic acid, syringic acid, rosmarinic acid | 40–3120 μg/mL | S. aureus, C. albicans, C. parapsilosis. | [12] |
| Methanol | 90–3120 μg/mL | |||||
| Aqueous | 190–6250 μg/mL | |||||
| Baccharis trimera | Asteraceae | Crude | Polyphenols, flavonoids, alkaloids, and terpenes | 7.8–500 μg/mL | E. coli, S. aureus, P. aeruginosa, C. albicans, C. tropicalis, C. parapsilosis, Epicoccum sp., C. sphaerospermum, C. neoformans, P. brasiliensis, C. gatti, Pestalotiopsis sp., C. lunatus, Nigrospora sp. | [88] |
| Tecoma stans | Bignoniaceae | Aqueous | Phenolic compounds | 50–600 μg/mL | S. aureus | [91] |
| Bixa orellana L. | Bixaceae | Aqueous | Bixin, catechin, chlorogenic acid, chrysin, butein, hypolaetin, licochalcone A, and xanthohumol. | 16–32 μg/mL | B. cereus, S. aureus | [9] |
| Trichodesma indicum | Boraginaceae | Ethanol | Lanast-5-en-3β-D- glucopyranosyl-21(24)-oilde | 2.4–19.2 μg/mL | S. aureus | [92] |
| Boswellia dalzielii Hutch. | Burseraceae | Crude | Oleic acid, squalene and n-hexadecanoic acid | - | S. pyogenes, S. aureus, E. coli, E. faecalis, K. pneumonia, P. aeruginosa, P. mirabilis, S. typhi, and C. albicans | [93] |
| Caesalpinia coriaria (Jacq) Willd | Caesalpiniaceae | Aqueous | Methyl gallate and gallic acid | 1.56–25 mg/mL | S. typhi, E. coli, P. aeruginosa, L. monocytogenes, S. aureus. | [94] |
| Ethanol | 390–6250 μg/mL | |||||
| Senna aculeate (Bth.) Irw et Barn | Ceasalpinioideae | Hexane | Gallic acid, kaempferol, ellagic acid, epicatechin, vitexin, corilagin | 25, 50, 100 mg/mL | C.albicans | [89] |
| Kochia scoparia | Chenopodiaceae | Crude | Polyphenols, flavonoids, alkaloids, and terpenes | 3.125 mg/mL | C. graminicola, T. deformans, A. flavus, H. carbonum, C. zeaemaydis, C. macrocarpum, P. innundatus, S. japonicas, E. ficariae, P. herbarum, M. verticillata, Rhisoclosmatium sp., S. pseudodichotomus, S. kneipii, R. solani, P. sojae. | [8] |
| Buchenavia tomentosa (Mart) Eichler | Combretaceae | Hexane | Gallic acid, Kaempferol, Ellagic acid, epicatechin, Vitexin, Corilagin | 10 mg/mL | C.albicans | [89] |
| Terminalia phanerophlebia Engl. & Diels | Combretaceae | Crude | Methyl gallate (methyl-3,4,5-trihydroxybenzoate) and a phenylpropanoid glucoside, 1,6-di-O-coumaroyl glucopyranoside | 125 μg/mL | M. aurum, M. tuberculosis, S. aureus, K. pneumoniae | [95] |
| Dichloromethane | 16–250 μg/mL | |||||
| Hexane | 31–250 μg/mL | |||||
| Ethyl Acetate | 8–125 μg/mL | |||||
| n-butanol | 31–250 μg/mL | |||||
| Buchenavia tomentosa L. | Combretaceae | Crude | Gallic acid, quinic acid, kaempferol, (-) epicatechin, ellagic acid, buchenavianine, eschweilenol b, eschweilenol c, vitexin, corilagin, 1α,23β-dihydroxy-12-oleanen-29-oicacid-23β-o-α-l-4-acetylramnopiranoside and punicalin | 200–12500 μg/mL | Candida albicans, Candida tropicalis, Candida parapsilosis, Candida glabrata, Candida krusei and Candida dubliniensis. | [96] |
| Diadema setosum f. depressa Dollfus & Roman. | Diadematidae | Acetone | Polyunsaturated fatty acids (PUFAs) and β-carotene | 500–4000 μg/mL | S. typhi, S. typhimurium, S. flexneri, P. aeruginosa, A. hydrophila, Acinetobacter sp, C. freundii and K. pneumonia, B. subtilis, S. epidermidis S. aureus | [1] |
| Monotes kerstingii Gilg | Dipterocarpaceae | Crude | Stilbene-coumarin derivative, coumarin-carbinol and fatty glycoside | 1–8 mg/mL | B. subtilis, Septoria tritici Desm | [7] |
| Croton doctoris S Moore | Euphorbiaceae | Hexane | Gallic acid, kaempferol, ellagic acid, epicatechin, vitexin, corilagin | 500 μg/mL | C.albicans | [89] |
| Jatropha weddelliana Baillon | Euphorbiaceae | Hexane | Gallic acid, kaempferol, ellagic acid, epicatechin, vitexin, corilagin | 4–32 μg/mL | C.albicans | [89] |
| Cassia alata | Fabaceae | Ethanol | 4-butylamine, cannabinoid, dronabinol, methyl-6-hydroxy | 1.25, 1.5 mg/mL | S. aureus, E. coli, P. aeruginosa, C. albicans | [28] |
| Dalbergia scandens Roxb., Corom. | Fabaceae | Ethanol | Dalpanitin, vicenin-2 and 3, rutin | 780–6250 mg/mL | B. cereus, S. aureus, E. coli, P. aeruginosa, C. albicans | [41] |
| Acacia nilotica | Fabaceae | Crude | Alkaloids | 600–1200 μg/mL | S. aureus | [27] |
| Salvia sessei Benth | Lamiaceae | Hexane | Sessein, isosessein | 12.5–100 μg/mL | S. haemolyticus, S. hominis, E. faecalis, S. epidermis, S. pyogenes, S.aureus | [14] |
| Dichloromethane | 100 μg/mL | |||||
| Methanol | 12.5–100 μg/mL | |||||
| Mentha piperita | Lamiaceae | Methanol | 1,1-diphenyl-2-picrylhydazyl-hydrate | 1–4 mg/mL | S. aureus, E. coli, C. albicans | [97] |
| Ocimum basilicum L. | Lamiaceae | Ethanol | Gallic acid, 3,4-dihydroxy benzoic acid, 4-hydroxy benzoic acid, 2,5 dihydroxybenzoic acid, chlorogenic acid, vanillic acid, Epicatechin, caffeic acid, p-coumaric acid, ferulic acid, rutin, ellagic acid, naringin, quercetin, cinnamic acid, α-pinene, camphene, sabinene, β-pinene, myrcene, 3-octanol, α-terpinene, p-cymene, limonene, 1,8-cineole, (Z)-β-ocimene, (E)-β-ocimene, γ-terpinene, cis-sabinene hydrate, terpinolene, linalool, nonanal, pentylisovalerate, 1-octen-3-yl acetate, cis-p-menth-2-en-1-ol, 3-octyl acetate, α-campholenal, camphor, trans-verbenol, δ-terpineol, 4-terpineol, α-terpineol, cis-dihydrocarvone, trans-carveol, (Z)-3-hexenyl isovalerate, pulegone, neral, carvone, linalyl acetate, bornyl acetate, dihydroedulan IA, isodihydrocarvyl acetate, α-terpinyl acetate, cis-carvyl acetate, neryl acetate, geranyl acetate, β-elemene, (Z)-jasmone, β-caryophyllene, β-copaene, aromadendrene, α-humulene, (E)-β-farnesene, cis-muurola-4(14), 5-diene germacrene D, bicyclogermacrene, germacrene A, δ-cadinene, (E)-α-bisabolene, (E)-nerolidol, Spathulenol, caryophyllene oxide, viridiflorol, 1, 10-di-epi-cubenol, T-cadinol, T-muurolol, monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, apocarotenes non-terpene derivatives | 16–256 μg/mL | S. epidermidis S. aureus, B. subtilis, E. coli, P. aeruginosa, K. pneumoniae, C. glabrata, C. albicans | [98] |
| Thymus algeriensis Boiss. & Reut | Lamiaceae | Ethanol | Gallic acid, 3,4-dihydroxy benzoic acid, 4-hydroxy benzoic acid, 2,5 dihydroxybenzoic acid, chlorogenic acid, vanillic acid, epicatechin, caffeic acid, p-coumaric acid, ferulic acid, rutin, ellagic acid, naringin, quercetin, cinnamic acid, α-pinene, camphene, sabinene, β-pinene, myrcene, 3-octanol, α-terpinene, p-cymene, limonene, 1,8-cineole, (Z)-β-ocimene, (E)-β-ocimene, γ-terpinene, cis-sabinene hydrate, terpinolene, linalool, nonanal, pentylisovalerate, 1-octen-3-yl acetate, cis-p-menth-2-en-1-ol, 3-octyl acetate, α-campholenal, camphor, trans-verbenol, δ-terpineol, 4-terpineol, α-terpineol, cis-dihydrocarvone, trans-carveol, (Z)-3-hexenyl isovalerate, pulegone, neral, carvone, linalyl acetate, bornyl acetate, dihydroedulan IA, isodihydrocarvyl acetate, α-terpinyl acetate, cis-carvyl acetate, neryl acetate, geranyl acetate, β-elemene, (Z)-jasmone, β-caryophyllene, β-copaene, aromadendrene, α-humulene, (E)-β-farnesene, cis-muurola-4(14), 5-diene germacrene D, bicyclogermacrene, germacrene A, δ-cadinene, (E)-α-bisabolene, (E)-nerolidol, spathulenol, caryophyllene oxide, viridiflorol, 1, 10-di-epi-cubenol, T-cadinol, T-muurolol, monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, apocarotenes non-terpene derivatives | 32–512 μg/mL | S. epidermidis S. aureus, B. subtilis, E. coli, P. aeruginosa, K. pneumoniae, C. glabrata, C. albicans | [98] |
| Cinnamomun inerme | Lauraceae | Ethyl Acetate | 5-(1,5-dimethyl-2-4-hexenyl)- methyl phenol) | 100–800 μg/mL | S. aureus, E. coli | [99] |
| Hexane | 8000 μg/mL | |||||
| Acetone | 8000 μg/mL | |||||
| n-butanol | 100–800 μg/mL | |||||
| Allium sativam | Liliaceae | Crude | Allicin | 49 μg/mL | C. albicans | [100] |
| Strychnos nigritana Baker | Loganiaceae | Crude | Nigritanine, Speciociliatine, Mytragine Paynantheine Rhyncophylline | 128–256 μg/mL | S. aureus | [10] |
| Mascagnia benthamiana (Gries) WR Anderson | Malpighiaceae | Hexane | Gallic acid, kaempferol, ellagic acid, epicatechin, vitexin, corilagin | 17.84 mg/mL | C.albicans | [89] |
| Mouriri elliptica Mart | Memecylaceae | Hexane | Gallic acid, kaempferol, ellagic acid, epicatechin, vitexin, corilagin | 100 μg/mL | C.albicans | [89] |
| Artocarpus communis | Moraceae | Crude | Atonin E, 2-(3,5-dihydroxy)-(Z)-4-(3 methyl but-1-etnyl | 4–512 μg/mL | P. aeruginosa, S.typhi, S.aureus, K.pneumoniae | [101] |
| Myrtus nivellei Batt. & Trab. | Myrtaceae | Crude | 1,8-cineole, limonene, isoamylcyclopentane, di-nor-sesquiterpenoids | 5 mg/mL | C. neoformans | [102] |
| Myrtus communis L | Myrtaceae | Crude | α-pinene, 1,8-cineole, linalool, and linalyl acetate | 156–625 μg/mL | E. floccosum, M. canis, T. rubrum | [102] |
| Piper nigrum | Piperaceae | Aqueous | Piperine | 500–1000 μg/mL | E. coli, M. luteus | [91] |
| Citrus aurantium L. | Rutaceae | Ethanol | Polyphenols, flavonoids, alkaloids, and terpenes | 1562–6250 μg/mL | Amoxycillin resistant B. cereus | [13] |
| Salix babylonica L. | Salicaceae | Hydroalcoholic | Luteolin, luteolin 7-O-glucoside | 1.56–100 mg/mL | E. coli, S. aureus and L. monocytogenes | [103] |
| Verbascum glabratum subsp. bosnense (K. Malý) Murb | Scrophulariaceae | Ethanol | quercitrin and rosmarinic acid, 4-hydroxybenzoic acid, salicylic acid, morin, and apigenin | 600, 1200 μg/mL | E. coli, S. aureus, Candida albicans | [17] |
| Simaba ferruginea A. St.-Hil | Simaroubaceae | Methanol | Canthin-6-one, indole β-carboxylic | 12.5–200 μg/mL | S. flexneri, S. aureus and S. aureus | [91] |
| Camellia sinensis | Theaceae | Aqueous | Catechin | 7.81–31.25 μg/mL | S. mutans | [104] |
| Talaromyces sp. | Trichocomaceae | Aqueous | Talaropeptide A and B | 5 mg/mL | B. subtilis | [18] |
| Hybanthus enneasperm us | Violaceae | Crude | Flavonoids, Tannins | 37.5, 75, 150, 300, 600 μg/mL | P. vulgaris, V. cholera | [100] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. https://doi.org/10.3390/antibiotics8040257
Mickymaray S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics. 2019; 8(4):257. https://doi.org/10.3390/antibiotics8040257
Chicago/Turabian StyleMickymaray, Suresh. 2019. "Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens" Antibiotics 8, no. 4: 257. https://doi.org/10.3390/antibiotics8040257
APA StyleMickymaray, S. (2019). Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics, 8(4), 257. https://doi.org/10.3390/antibiotics8040257




