Antibacterial Effects of Quinazolin-4(3H)-One Functionalized-Conjugated Silver Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Quinazolinone Derivatives Did Not Show Bactericidal Effects
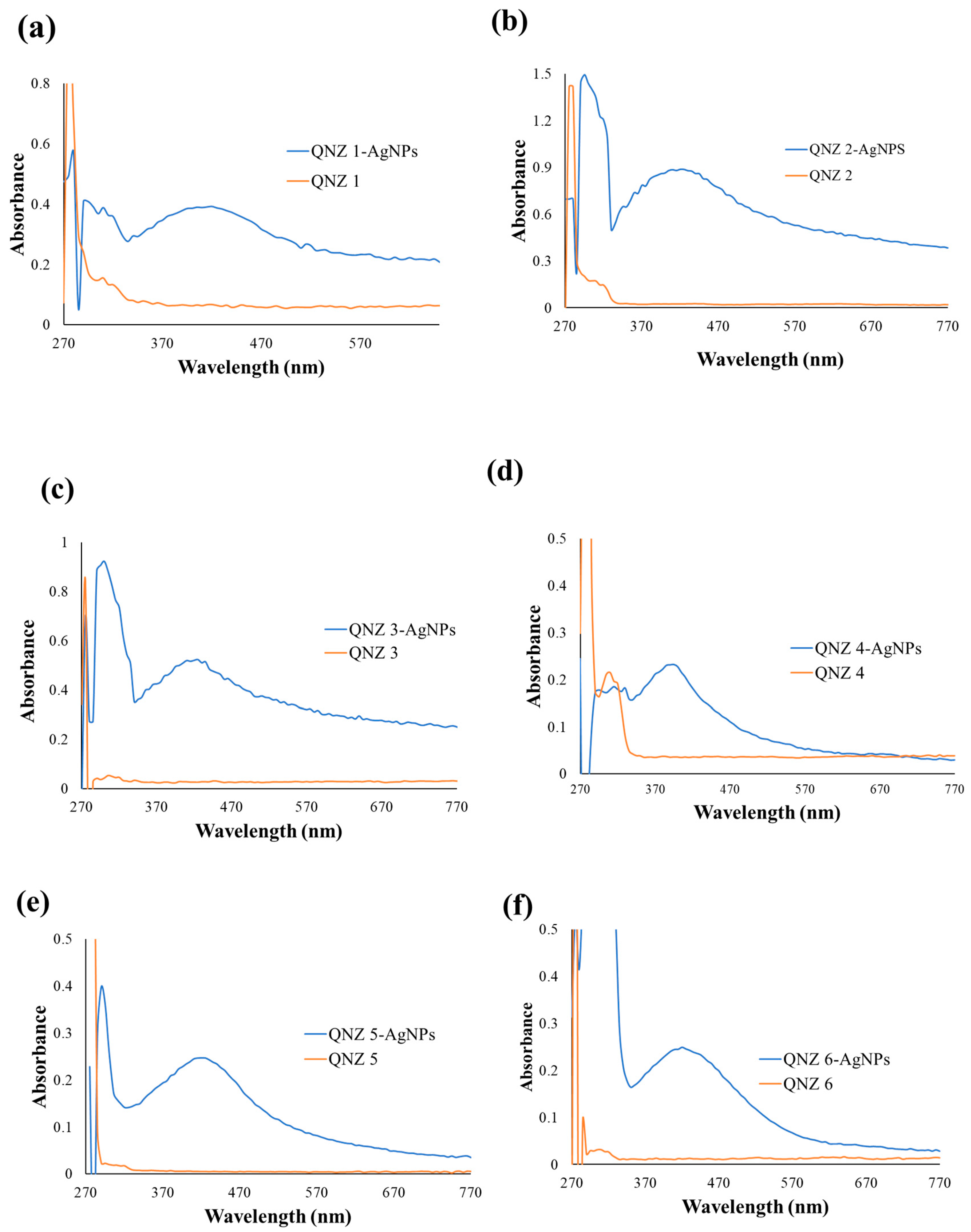
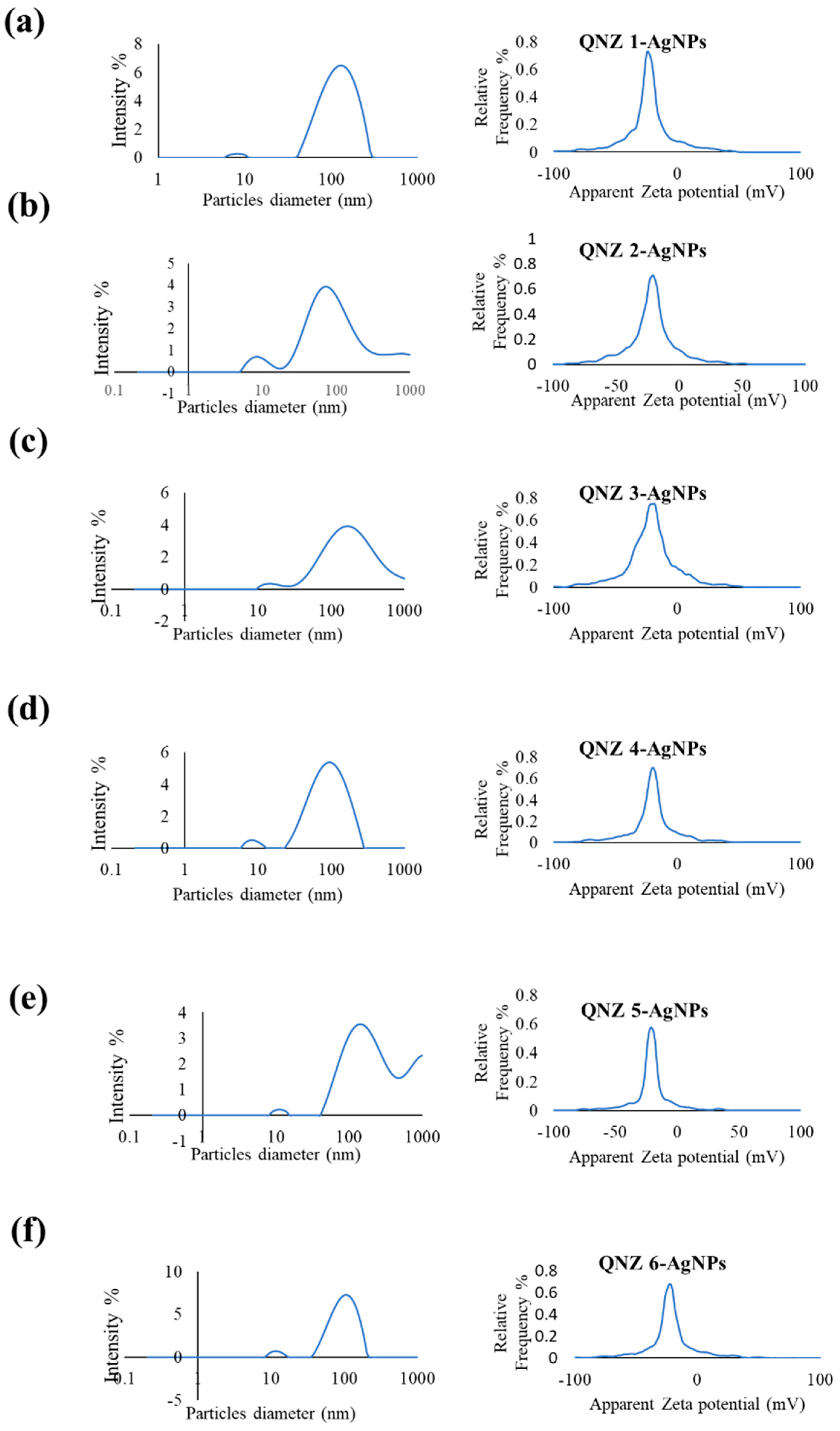
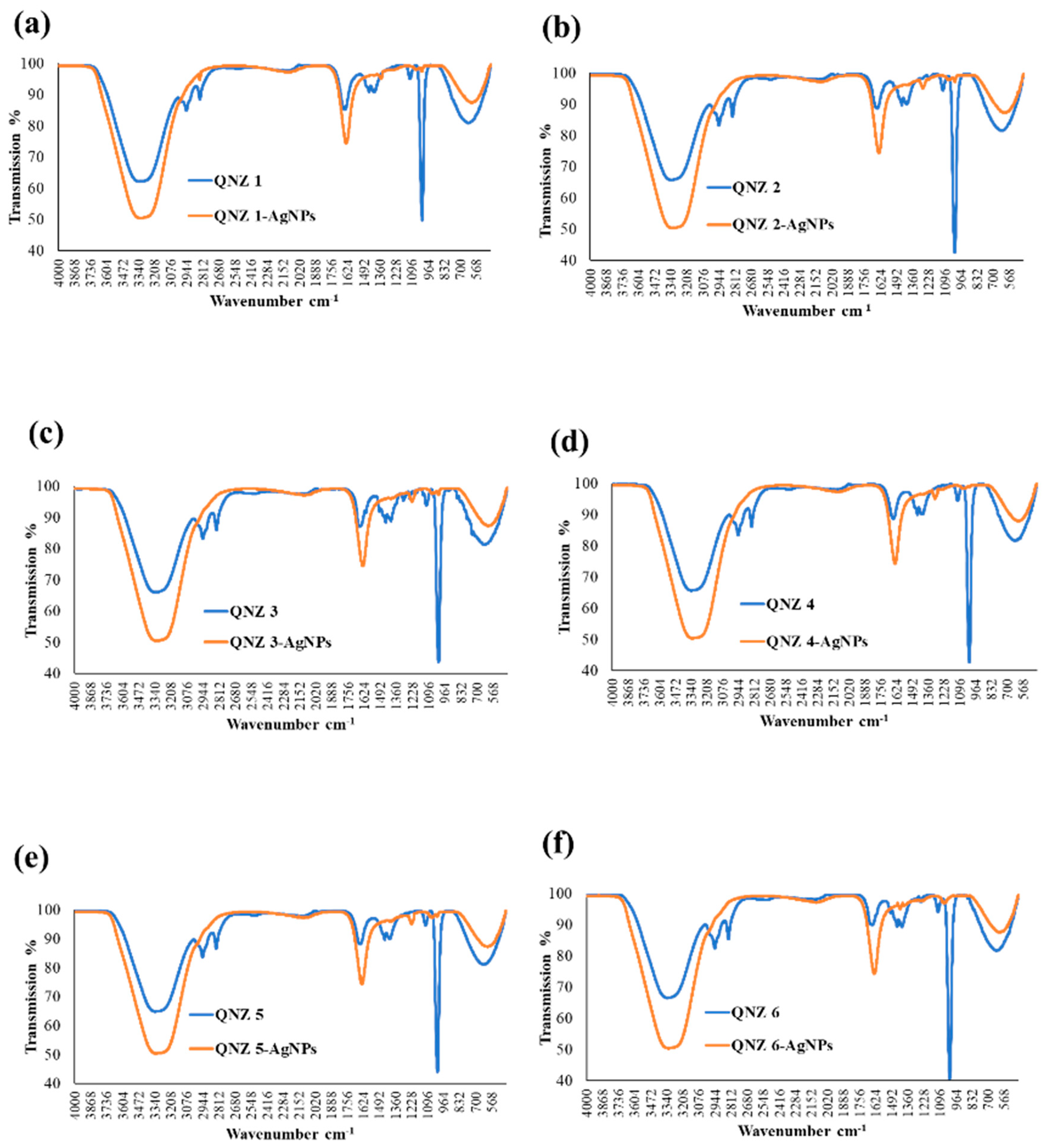
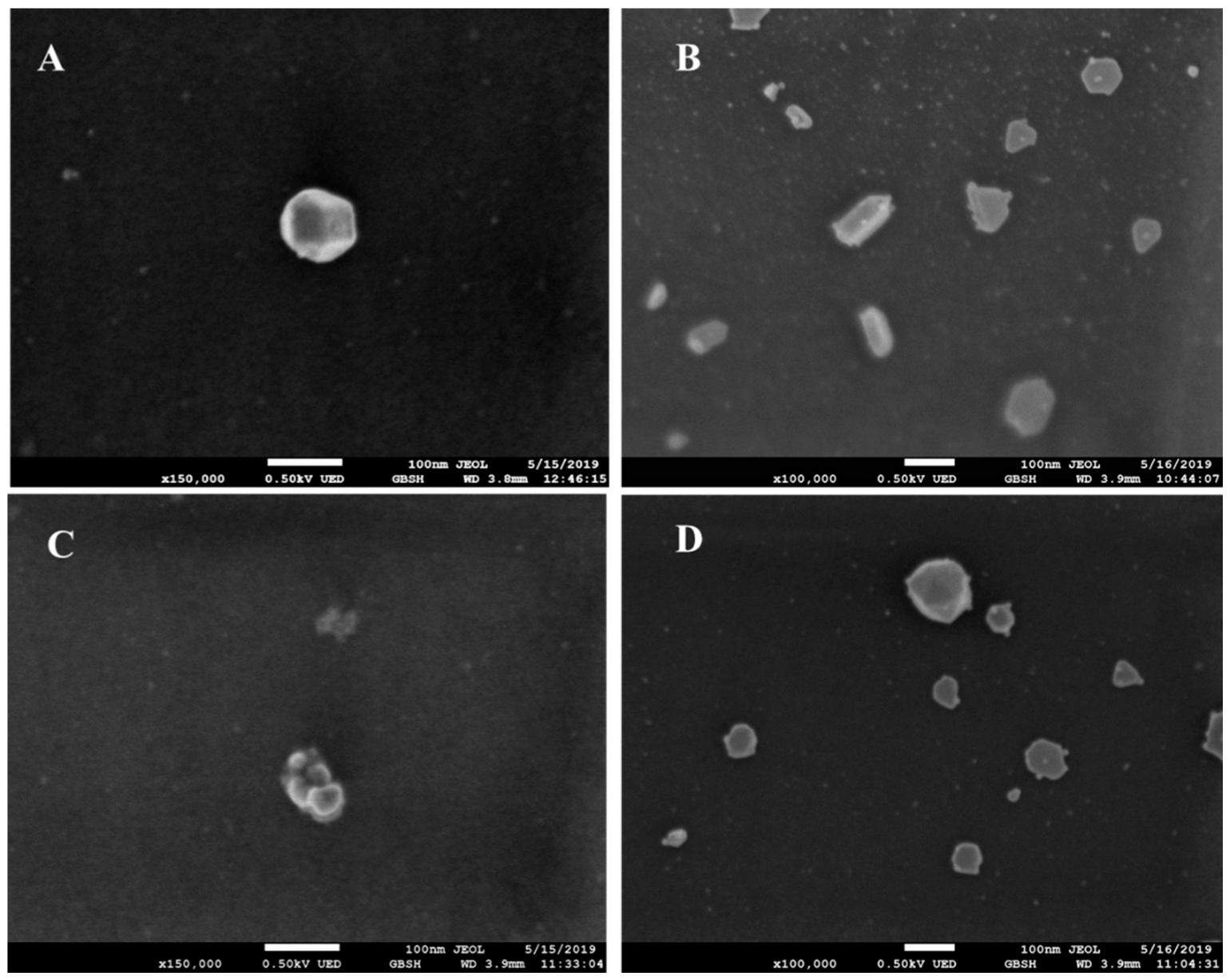
2.2. Characterization of QNZ-AgNPs
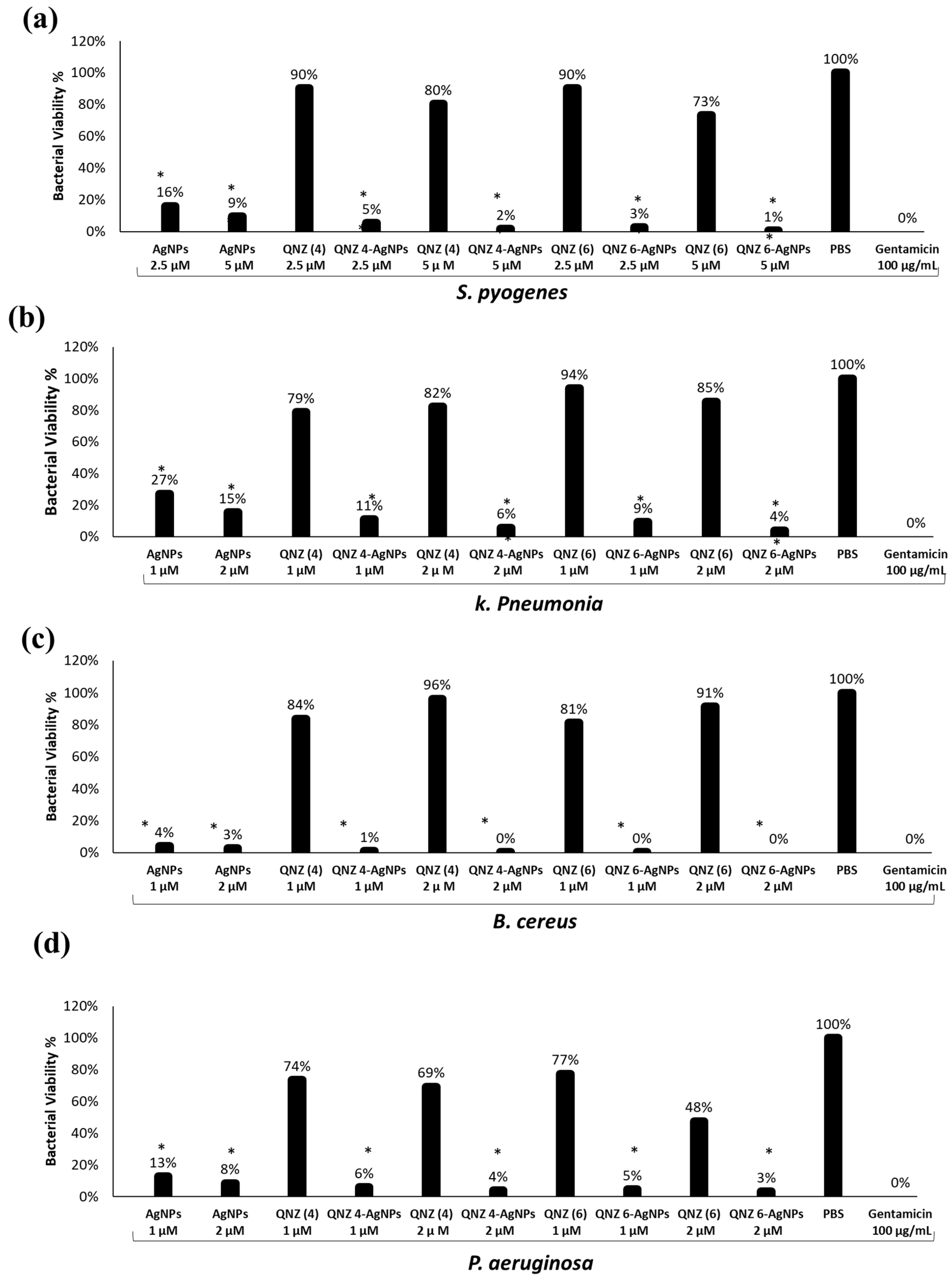
2.3. QNZ 4 and QNZ 6 Conjugated Silver Nanoparticles Exhibited Bactericidal Effects
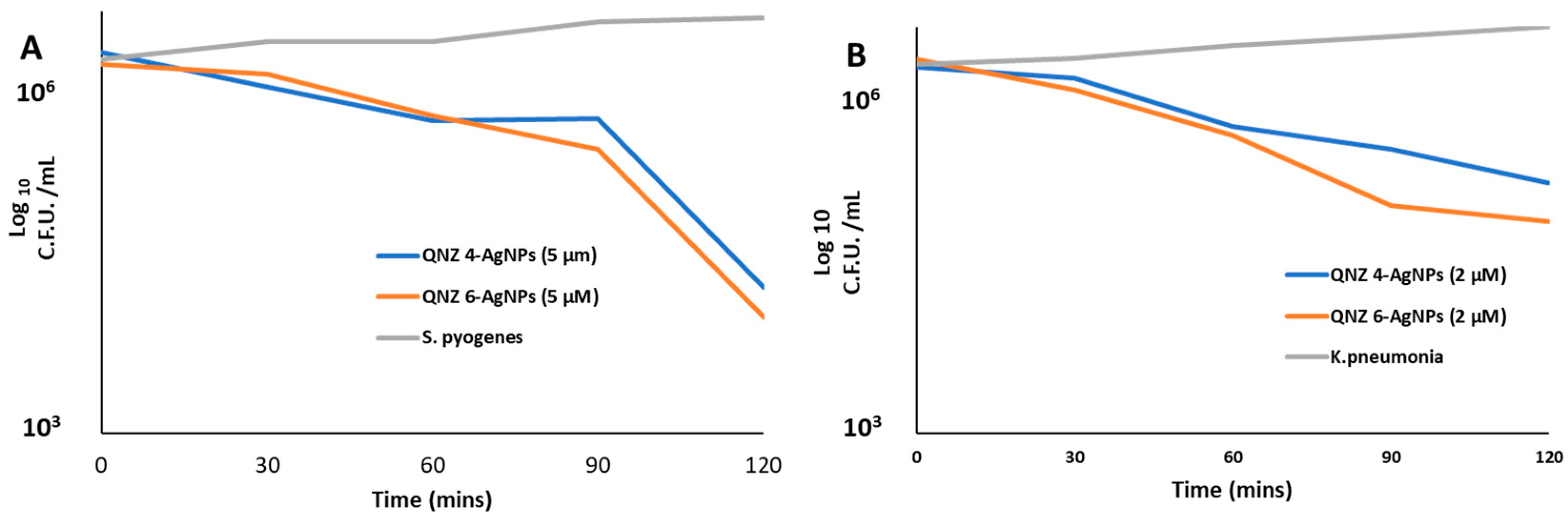
2.4. Effect of Incubation Time
2.5. QNZs Displayed Minimum Cytotoxicity to Human Cells and Abolished Bacterial Mediated Cytotoxicity to Human Cells
3. Discussion
4. Materials and Methods
4.1. Synthesis of Quinazolinones
4.2. Characterization of Quinazolinones
4.3. Bacterial Cultures
4.4. Human Keratinocyte (HaCaT) Cells Culture
4.5. Synthesis of AgNPs Coated with Quinazolinione
4.6. Characterization of Nanoparticles
4.7. Bactericidal Assay
4.8. Time-Kill Kinetics
4.9. Cytotoxicity Assay
4.10. Bacteria Mediated Host Cell Cytotoxicity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gualerzi, C.O.; Brandi, L. Antibiotics Targets, Mechanisms and Resistance, 5th ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 87–88. [Google Scholar]
- Ali, S.M.; Siddiqui, R.; Ong, S.K.; Shah, M.R.; Anwar, A.; Heard, P.J.; Khan, N.A. Identification and characterization of antibacterial compound(s) of cockroaches. Appl. Microbiol. Biotechnol. 2017, 1, 253–286. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Hiasa, H.; Kerns, R.; Malik, M.; Mustaev, A. Quinolones: Action and Resistance Updated. Med. Chem. 2009, 9, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Bedi, P.M.S.; Kumar, V.; Mahajan, M.P. Synthesis and biological activity of novel antibacterial quinazolines. Biorganic Med. Chem. Lett. 2004, 14, 5211–5213. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Oshero, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Sadjadi, S.; Sadjadi, S.; Oskooie, H.A.; Hekmat, S.R.; Bamoharram, F.F. Silica-supported preyssler nanoparticles as new catalysts in the synthesis of 4 (3H)-quinazolinones. S. Afr. J. Chem. 2009, 62, 1–4. [Google Scholar]
- Rakesh, K.P.; Darshini, N.; Shubhavathi, T.; Mallesha, N. Biological Applications of Quinazolinone Analogues: A Review. Org. Med. Chem. Int. J. 2017, 2, 1–5. [Google Scholar]
- Yu, L.; Wang, M.; Li, P.; Wang, L. Fe3O4 nanoparticle-supported copper (I): Magnetically recoverable and reusable catalyst for the synthesis of quinazolinones and bicyclic pyrimidinones. Appl. Organomet. Chem. 2012, 26, 576–582. [Google Scholar] [CrossRef]
- Shaabani, A.; Hezarkhani, Z. Ferrite nanoparticles supported on natural wool in one-pot tandem oxidative reactions: Strategy to synthesize benzimidazole, quinazolinone and quinoxaline derivatives. Appl. Organomet. Chem. 2017, 31, 3542. [Google Scholar] [CrossRef]
- Khalafi, A.; Divar, M.; Panahi, F. Magnetic nanoparticles-supported tungstic acid (MNP-TA): An efficient magnetic recyclable catalyst for the one-pot synthesis of spirooxindoles in water. RSC Adv. 2015, 5, 2223–2230. [Google Scholar] [CrossRef]
- Salahuddin, N.; Elbarbary, A.A.; Alkabes, H.A. Quinazolinone Derivatives Loaded Polypyrrole/Chitosan Core—Shell Nanoparticles with Different Morphologies: Antibacterial and Anticancer Activities. NANO: Brief Rep. Rev. 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Salahuddin, N.; Elbarbary, A.A.; Alkabes, H.A. Antibacterial and antitumor activities of 3-amino-phenyl-4(3H)-quinazolinone/polypyrrole chitosan core shell nanoparticles. Polym. Bull. 2016, 16. [Google Scholar] [CrossRef]
- Anwar, A.; Masri, A.; Rao, K.; Rajendran, K.; Khan, N.A. Antimicrobial activities of green synthesized gums-stabilized nanoparticles loaded with flavonoids. Sci. Rep. 2019, 5, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Anwar, A.; Ahmed, D.; Siddiqui, R.; Shah, M.R.; Khan, N. Silver Nanoparticle Conjugation-Enhanced Antibacterial Efficacy of Clinically Approved Drugs Cephradine and Vildagliptin. Antibiotics 2018, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Divar, M.; Zomorodian, K.; Bastan, S.; Yazdanpanah, S.; Khabnadideh, S. Synthesis of some quinazolinone derivatives using magnetic nanoparticles—Supported tungstic acid as antimicrobial agents. J. Iran. Chem. Soc. 2018, 15, 1457–1466. [Google Scholar] [CrossRef]
- Rohini, R.; Reddy, P.M.; Shanker, K.; Hu, A.; Ravinder, V. Antimicrobial study of newly synthesized 6-substituted indolo [1, 2-c] quinazolines. Eur. J. Med. Chem. 2010, 45, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Kumar, A.; Yadav, R. Synthesis and Biological Activity of Peptide Derivatives of Iodoquinazolinones/Nitroimidazoles. Molecules 2008, 13, 958–976. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.M.; Desai, K.R. Synthesis, characterization, and pharmacological evaluation 3-phenyl ureas. Med. Chem. Res. 2013, 22, 225–233. [Google Scholar] [CrossRef]
- Ugale, V.G.; Bari, S.B. Quinazolines: New horizons in anticonvulsant therapy. Eur. J. Med. Chem. 2014, 80, 447–501. [Google Scholar] [CrossRef]
- Salahuddin, N.; Elbarbary, A.A.; Alkabes, H.A. Antibacterial and anticancer activity of loaded quinazolinone Polypyrrole/chitosan silver chloride nanocomposite. J. Polym. Bull. 2016, 1, 1–37. [Google Scholar] [CrossRef]
- Choi, O.; Kanjun, K.; Kim, N.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Ma, L.; Gilbert, B.; Shi, Ќ.H.; Yeh, I.; Zink, J.I.; Nel, A. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. Am. Chem. Soc. 2008, 10, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M. Fluoroquinolones: Mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Siddiqui, R.; Iqbal, M.; Sagathevan, K.; Khan, N.A. Gut bacteria of cockroaches are a potential source of antibacterial compound(s). Lett. Appl. Microbiol. 2018, 5, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Jeyamogan, S.; Khan, N.A.; Anwar, A.; Shah, M.R.; Siddiqui, R. Cytotoxic effects of Benzodioxane, Naphthalene diimide, Porphyrin and Acetamol derivatives on HeLa cells. SAGE Open Med. 2018, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Siddiqui, R. Brain-Eating Amoebae: Silver Nanoparticle Conjugation Enhanced Efficacy of Anti-Amoebic Drugs against Naegleria fowleri. Neuroscience 2017, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Khalid, S.; Perveen, S.; Shakil, A.; Siddiqui, R.; Khan, N.A.; Shah, M.R. Synthesis of 4-(dimethylamino)pyridine propylthioacetate coated gold nanoparticles and their antibacterial and photophysical activity. J. Nanobiotechnol. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Naqvi, S.Z.H.; Urooj, K.; Ishtiaq, A.M.; Asif, J.; Abdul, H.; Safia, A.; Naeem, A. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef]
- Khan, N.A.; Osman, K.; Goldsworthy, G.J. Lysates of Locusta migratoria brain exhibit potent broad-spectrum antibacterial activity. J. Antimicrob. Chemother. 2008, 3, 634–635. [Google Scholar] [CrossRef]
- He, Y.; Ingudam, S.; Reed, S.; Gehring, A.; Strobaugh, T.P.; Irwin, P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J. Nanobiotechnol. 2016, 14, 36–54. [Google Scholar] [CrossRef] [PubMed]




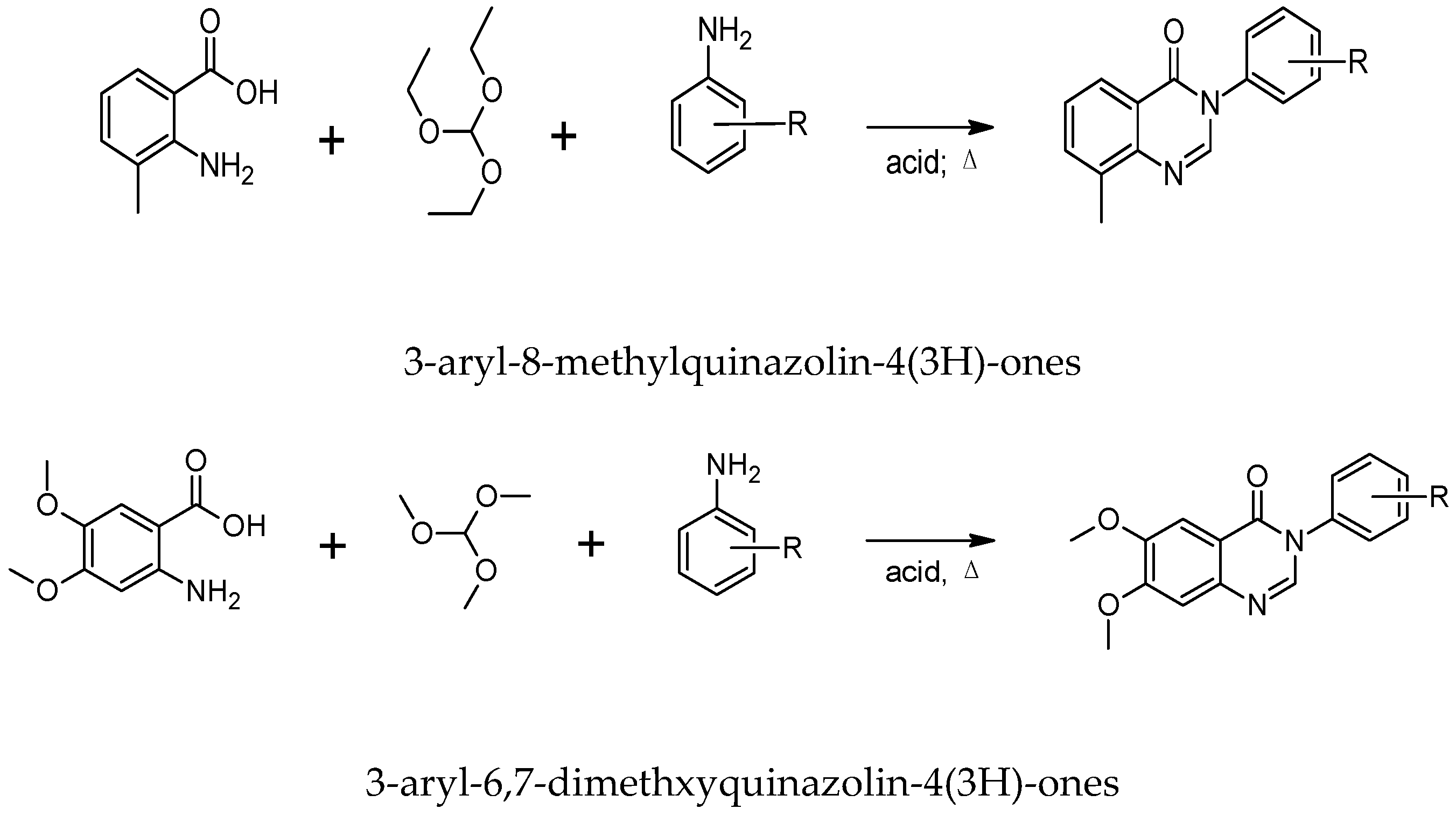
| Nanoparticles | ||||||
|---|---|---|---|---|---|---|
| Bacterial Viability % | AgNPs | QNZ 4-AgNPs | QNZ 6-AgNPs | |||
| 2.5 µM | 5 µM | 2.5 µM | 5 µM | 2.5 µM | 5 µM | |
| E. coli K1 | 11% | 5% | 3% | 1% | 2% | 1% |
| S. pyogenes | 16% | 9% | 5% | 2% | 3% | 1% |
| MRSA | No activity | |||||
| 1 µM | 2 µM | 1 µM | 2 µM | 1 µM | 2 µM | |
| K. pneumonia | 27% | 15% | 11% | 6% | 9% | 4% |
| B. cereus | 4% | 3% | 1% | 0% | 0% | 0% |
| P. aeruginosa | 13% | 8% | 6% | 4% | 5% | 3% |
| Code | Structure | International Union of Pure and Applied Chemistry (IUPAC) Name | Molecular Formula | Molecular Weight |
|---|---|---|---|---|
| QNZ-1 | 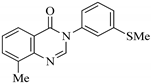 | 8-methyl-3-(3-(methylthio)phenyl)quinazolin-4(3H)-one | C16H14N2OS | 282.36 |
| QNZ-2 | 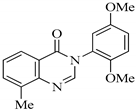 | 3-(2,5-dimethoxyphenyl)-8-methylquinazolin-4(3H)-one | C17H16N2O3 | 296.32 |
| QNZ-3 | 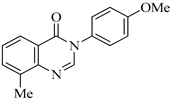 | 3-(4-methoxyphenyl)-8-methylquinazolin-4(3H)-one | C16H14N2O2 | 266.29 |
| QNZ-4 | 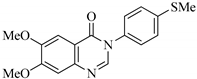 | 6,7-dimethoxy-3-(4-(methylthio)phenyl)quinazolin-4(3H)-one | C17H16N2O3S | 328.39 |
| QNZ-5 |  | 3-(4-butylphenyl)-6,7-dimethoxyquinazolin-4(3H)-one | C20H22N2O3 | 338.40 |
| QNZ-6 |  | 6,7-dimethoxy-3-(4-methoxyphenyl)quinazolin-4(3H)-one | C17H16N2O4 | 312.32 |
| Code | IUPAC Name | Chemical Characterization |
|---|---|---|
| QNZ-1 | 8-methyl-3-(3-(methylthio)phenyl)quinazolin-4(3H)-one | Yield: 65%; Rf: 0.76 (ethyl acetate/hexane, 3:7); 1H-NMR: (400 MHz, DMSO-d6): δH 8.36 (s, 1H, H-2), 8.04 (d, J5,6 = 8.0 Hz, 1H, H-5), 7.75 (d, J7,6 = 7.2 Hz, 1H, H-7), 7.50 (t, J6(5,7) = 8.0 Hz, 1H, H-6), 7.49 (t, J5′(6′,4′) = 7.6 Hz, 1H, H-5′), 7.43 (s, 1H, H-2′), 7.39 (d, J6′,5′ = 8.0 Hz, 1H, H-6′); 7.30 (d, J4′,5′ = 7.6 Hz, 1H, H-4′), 2.57 (s, 3H, 8-CH3), 2.50 (s, 3H, 3′-SCH3); EI-MS: m/z (rel. abund. %), 282 [M]+ (100), 267 (5), 254 (3), 123 (4), 105 (15); Anal. calcd. for C16H14N2OS: C, 68.06; H, 5.00; N, 9.92; O, 5.67; S, 11.36; found: C, 68.08; H, 5.04; N, 9.95; O, 5.62; S, 11.34. |
| QNZ-2 | 3-(2,5-dimethoxyphenyl)-8-methylquinazolin-4(3H)-one | Yield: 81%; Rf: 0.52 (ethyl acetate/hexane, 3:7); 1H-NMR: (300 MHz, DMSO-d6): δH 8.20 (s, 1H, H-2), 8.02 (d, J5,6 = 9.0 Hz, 1H, H-5), 7.74 (d, J7,6 = 7.2 Hz, 1H, H-7), 7.48 (t, J6(5,7) = 7.5 Hz, 1H, H-6), 7.19 (d, J3′,4′ = 9.0 Hz, 1H, H-3′), 7.12 (d, J6′,4′ = 3.0 Hz, 1H, H-6′), 7.09 (dd, J4′,3′ = 8.7 Hz, J4′,6′ = 3.0 Hz, 1H, H-4′), 3.74 (s, 3H, 2′-OCH3), 3.70 (s, 3H, 5′-OCH3) 2.57 (s, 3H, 8-CH3); EI-MS: m/z (rel. abund. %), 296 [M]+ (38), 265 (100), 250 (5); Anal. calcd. for C17H16N2O3: C, 68.91; H, 5.44; N, 9.45; O, 16.20; found: C, 68.94; H, 5.42; N, 9.44; O, 16.23. |
| QNZ-3 | 3-(4-methoxyphenyl)-8-methylquinazolin-4(3H)-one | Yield: 84%; Rf: 0.62 (ethyl acetate/hexane, 3:7); 1H-NMR: (300 MHz, DMSO-d6): δH 8.32 (s, 1H, H-2), 8.03 (d, J5,6 = 7.8 Hz, 1H, H-5), 7.74 (d, J7,6 = 7.2 Hz, 1H, H-7), 7.48 (t, J6(5,7) = 7.8 Hz, 1H, H-6), 7.45 (d, 2H, J2′,3′ = J6′,5′ = 9.0 Hz, H-2′, H-6′), 7.10 (d, 2H, J3′,2′ = J5′,6′ = 9.0 Hz, H-2′, H-6′), 3.82 (s, 3H, 4′-OCH3), 2.57 (s, 3H, 8-CH3); EI-MS: m/z (rel. abund. %), 266 [M]+ (100), 251 (14), 235 (3); Anal. calcd. for C16H14N2O2: C, 72.16; H, 5.30; N, 10.52; O, 12.02; found: C, 72.18; H, 5.33; N, 10.50; O, 12.00. |
| QNZ-4 | 6,7-dimethoxy-3-(4-(methylthio)phenyl)quinazolin-4(3H)-one | Yield: 45.45%; Rf: 0.69 (ethyl acetate/hexanes, 7:3); 1H-NMR: (400 MHz, DMSO-d6): δH 8.21 (s, 1H, H-2), 7.49 (s, 1H, H-5), 7.43 (d, J2′,3′ = J6′,5′ = 7.6 Hz, 2H, H-2′, H-6′), 7.41 (d, J3′,2′ = J5′,6′ = 7.6 Hz, 2H, H-3′, H-5′), 7.19 (s, 1H, H-8), 3.92 (s, 3H, 6-OCH3), 3.87 (s, 3H, 7-OCH3), 1.54 (s, 3H, 4′-SCH3); Anal. calcd. for C17H16N2O3S: C, 62.18; H, 4.91; N, 8.53; O, 14.62; S, 9.76; found: C, 62.15; H, 4.93; N, 8.56; O, 14.64; S, 9.74. |
| QNZ-5 | 3-(4-butylphenyl)-6,7-dimethoxyquinazolin-4(3H)-one | Yield: 28.63%; Rf: 0.80 (ethyl acetate/hexanes, 7:3); 1H-NMR: (400 MHz, DMSO-d6): δH 8.21 (s, 1H, H-2), 7.49 (s, 1H, H-5), 7.40 (d, J2′,3′ = J6′,5′ = 8.4 Hz, 2H, H-2′, H-6′), 7.36 (d, J3′,2′ = J5′,6′ = 8.4 Hz, 2H, H-3′, H-5′), 7.19 (s, 1H, H-8), 3.92 (s, 3H, 6-OCH3), 3.87 (s, 3H, 7-OCH3), 2.67 (t, JCH2,CH2 = 7.6 Hz, 2H, 4′-CH2CH2CH2CH3), 1.63 (m, 2H, 4′-CH2CH2CH2CH3), 1.39 (m, 2H, 4′-CH2CH2CH2CH3), 2.67 (t, JCH3,CH2 = 7.2 Hz, 3H, 4′-CH2CH2CH2CH3); Anal. calcd. for C20H22N2O3: C, 70.99; H, 6.55; N, 8.28; O, 14.18; found: C, 70.97; H, 6.57; N, 8.24; O, 14.15. |
| QNZ-6 | 6,7-dimethoxy-3-(4-methoxyphenyl)quinazolin-4(3H)-one | Yield: 57.78%; Rf: 0.57 (ethyl acetate/hexanes, 7:3); 1H-NMR: (400 MHz, DMSO-d6): δH 8.18 (s, 1H, H-2), 7.48 (s, 1H, H-5), 7.42 (d, J2′,3′ = J6′,5′ = 8.8 Hz, 2H, H-2′, H-6′), 7.18 (s, 1H, H-8), 7.08 (d, J3′,2′ = J5′,6′ = 8.8 Hz, 2H, H-3′, H-5′), 3.92 (s, 3H, 6-OCH3), 3.87 (s, 3H, 7-OCH3), 3.81 (s, 3H, 4′-OCH3); Anal. calcd. for C17H16N2O4: C, 65.38; H, 5.16; N, 8.97; O, 20.49; found: C, 65.36; H, 5.13; N, 8.95; O, 20.44. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masri, A.; Anwar, A.; Khan, N.A.; Shahbaz, M.S.; Khan, K.M.; Shahabuddin, S.; Siddiqui, R. Antibacterial Effects of Quinazolin-4(3H)-One Functionalized-Conjugated Silver Nanoparticles. Antibiotics 2019, 8, 179. https://doi.org/10.3390/antibiotics8040179
Masri A, Anwar A, Khan NA, Shahbaz MS, Khan KM, Shahabuddin S, Siddiqui R. Antibacterial Effects of Quinazolin-4(3H)-One Functionalized-Conjugated Silver Nanoparticles. Antibiotics. 2019; 8(4):179. https://doi.org/10.3390/antibiotics8040179
Chicago/Turabian StyleMasri, Abdulkader, Ayaz Anwar, Naveed Ahmed Khan, Muhammad Saquib Shahbaz, Khalid Mohammed Khan, Syed Shahabuddin, and Ruqaiyyah Siddiqui. 2019. "Antibacterial Effects of Quinazolin-4(3H)-One Functionalized-Conjugated Silver Nanoparticles" Antibiotics 8, no. 4: 179. https://doi.org/10.3390/antibiotics8040179
APA StyleMasri, A., Anwar, A., Khan, N. A., Shahbaz, M. S., Khan, K. M., Shahabuddin, S., & Siddiqui, R. (2019). Antibacterial Effects of Quinazolin-4(3H)-One Functionalized-Conjugated Silver Nanoparticles. Antibiotics, 8(4), 179. https://doi.org/10.3390/antibiotics8040179






