Pseudomonas aeruginosa Coharboring BlaKPC-2 and BlaVIM-2 Carbapenemase Genes
Abstract
1. Introduction
2. Results
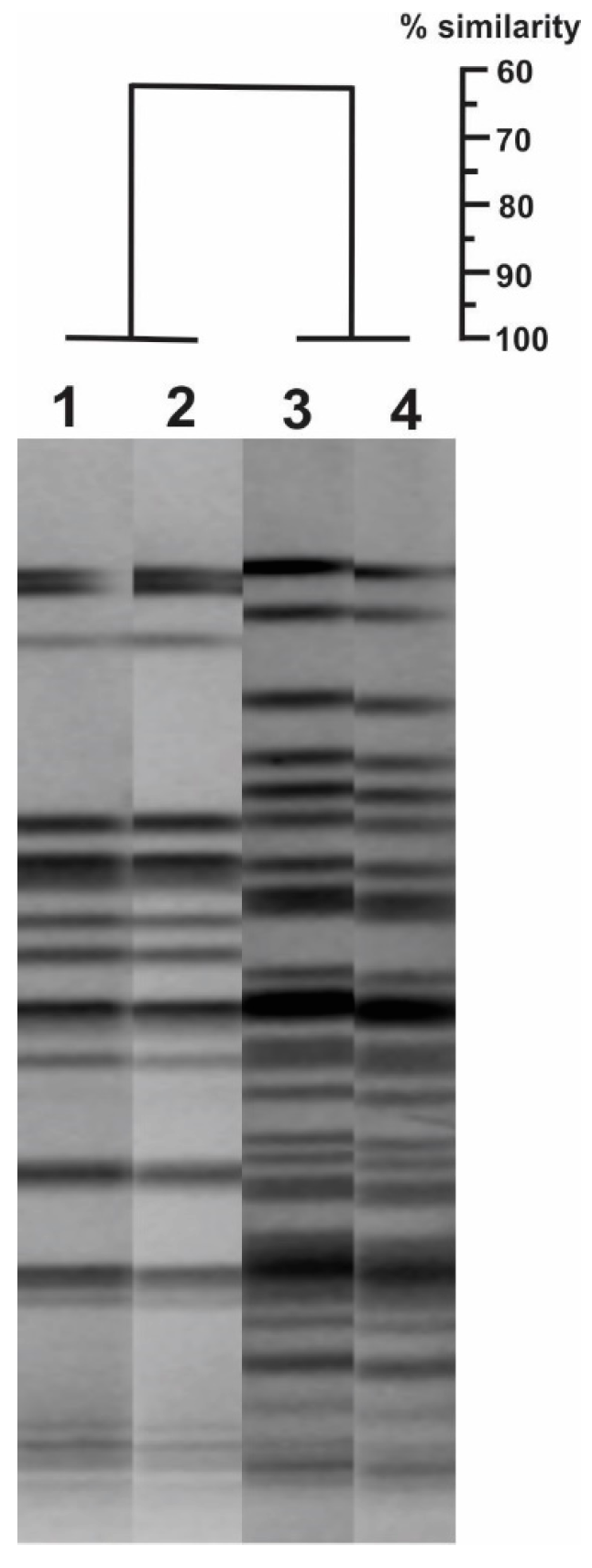
2.1. Case 1
2.2. Case 2
2.3. Case 3
2.4. Case 4
2.5. Case 5
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates and Susceptibility Profile
4.2. Detection of Resistance Genes
4.3. Establishment of the Genetic Relationship by Pulsed-Field Gel Electrophoresis (PFGE)
4.4. Assessment of the Genetic Platforms Mobilizing the Blakpc Gene
4.5. Ethical Approvals
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, I.; Hackel, M.; Badal, R.; Bouchillon, S.; Hawser, S.; Biedenbach, D. A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals 2013, 6, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.C.; Castanheira, M.; Jones, R.N.; Sader, H.S. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: Results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn. Microbiol. Infect. Dis. 2012, 73, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Duarte, C.; Gonzalez, N.; Saavedra, S. Co-producciones de carbapenemasas un fenómeno en aumento y de difícil detección en el laboratorio de microbiología con pruebas fenotípicas. Boletín Informativo GREBO 2016, 8, 36–39. [Google Scholar]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Rossolini, G.M.; Mantengoli, E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2005, 11, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Drenkard, E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 2003, 5, 1213–1219. [Google Scholar] [CrossRef]
- Livermore, D.M. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 2009, 64, i29–i36. [Google Scholar] [CrossRef]
- Jacoby, G.A. Beta-lactamase nomenclature. Antimicrob. Agents Chemother. 2006, 50, 1123–1129. [Google Scholar] [CrossRef]
- Poole, K. Pseudomonas aeruginosa: Resistance to the max. Front. Microbiol. 2011, 2, 65. [Google Scholar] [CrossRef]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.V.; Lolans, K.; Correa, A.; Kattan, J.N.; Lopez, J.A.; Quinn, J.P.; Colombian Nosocomial Resistance Study Group. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob. Agents Chemother. 2007, 51, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Akpaka, P.E.; Swanston, W.H.; Ihemere, H.N.; Correa, A.; Torres, J.A.; Tafur, J.D.; Montealegre, M.C.; Quinn, J.P.; Villegas, M.V. Emergence of KPC-producing Pseudomonas aeruginosa in Trinidad and Tobago. J. Clin. Microbiol. 2009, 47, 2670–2671. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P.; Lagrutta, E.; Cleary, T.; Munoz-Price, L.S. Emergence of KPC-producing Pseudomonas aeruginosa in the United States. Antimicrob. Agents Chemother. 2010, 54, 3072. [Google Scholar] [CrossRef] [PubMed]
- Robledo, I.E.; Aquino, E.E.; Vazquez, G.J. Detection of the KPC gene in Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii during a PCR-based nosocomial surveillance study in Puerto Rico. Antimicrob. Agents Chemother. 2011, 55, 2968–2970. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Wei, Z.; Jiang, Y.; Shen, P.; Yu, Y.; Li, L. Identification of KPC-2-producing Pseudomonas aeruginosa isolates in China. J. Antimicrob. Chemother. 2011, 66, 1184–1186. [Google Scholar] [CrossRef]
- Jacome, P.R.; Alves, L.R.; Cabral, A.B.; Lopes, A.C.; Maciel, M.A. First report of KPC-producing Pseudomonas aeruginosa in Brazil. Antimicrob. Agents Chemother. 2012, 56, 4990. [Google Scholar] [CrossRef]
- Cuzon, G.; Naas, T.; Nordmann, P. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob. Agents Chemother. 2011, 55, 5370–5373. [Google Scholar] [CrossRef]
- Falco, A.; Ramos, Y.; Franco, E.; Guzman, A.; Takiff, H. A cluster of KPC-2 and VIM-2-producing Klebsiella pneumoniae ST833 isolates from the pediatric service of a Venezuelan Hospital. BMC Infect. Dis. 2016, 16, 595. [Google Scholar] [CrossRef]
- Correa, A.; Montealegre, M.C.; Mojica, M.F.; Maya, J.J.; Rojas, L.J.; De La Cadena, E.P.; Ruiz, S.J.; Recalde, M.; Rosso, F.; Quinn, J.P.; et al. First report of a Pseudomonas aeruginosa isolate coharboring KPC and VIM carbapenemases. Antimicrob. Agents Chemother. 2012, 56, 5422–5423. [Google Scholar] [CrossRef]
- Vanegas, J.M.; Cienfuegos, A.V.; Ocampo, A.M.; Lopez, L.; del Corral, H.; Roncancio, G.; Sierra, P.; Echeverri-Toro, L.; Ospina, S.; Maldonado, N.; et al. Similar frequencies of Pseudomonas aeruginosa isolates producing KPC and VIM carbapenemases in diverse genetic clones at tertiary-care hospitals in Medellin, Colombia. J. Clin. Microbiol. 2014, 52, 3978–3986. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Biedenbach, D.J.; Hackel, M.; Rabine, S.; de Jonge, B.L.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. Global Dissemination of blaKPC into Bacterial Species beyond Klebsiella pneumoniae and In Vitro Susceptibility to Ceftazidime-Avibactam and Aztreonam-Avibactam. Antimicrob. Agents Chemother. 2016, 60, 4490–4500. [Google Scholar] [CrossRef]
- Martinez, T.; Vazquez, G.J.; Aquino, E.E.; Ramirez-Ronda, R.; Robledo, I.E. First report of a Pseudomonas aeruginosa clinical isolate co-harbouring KPC-2 and IMP-18 carbapenemases. Int. J. Antimicrob. Agents 2012, 39, 542–543. [Google Scholar] [CrossRef]
- Correa, A.; Del Campo, R.; Perenguez, M.; Blanco, V.M.; Rodriguez-Banos, M.; Perez, F.; Maya, J.J.; Rojas, L.; Canton, R.; Arias, C.A.; et al. Dissemination of high-risk clones of extensively drug-resistant Pseudomonas aeruginosa in Colombia. Antimicrob. Agents Chemother. 2015, 59, 2421–2425. [Google Scholar] [CrossRef]
- Cuzon, G.; Naas, T.; Truong, H.; Villegas, M.V.; Wisell, K.T.; Carmeli, Y.; Gales, A.C.; Venezia, S.N.; Quinn, J.P.; Nordmann, P. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 2010, 16, 1349–1356. [Google Scholar] [CrossRef]
- Giakkoupi, P.; Pappa, O.; Polemis, M.; Vatopoulos, A.C.; Miriagou, V.; Zioga, A.; Papagiannitsis, C.C.; Tzouvelekis, L.S. Emerging Klebsiella pneumoniae isolates coproducing KPC-2 and VIM-1 carbapenemases. Antimicrob. Agents Chemother. 2009, 53, 4048–4050. [Google Scholar] [CrossRef]
- Saavedra, S.; Duarte, C.; Gonzalez, N.; Realpe, M. Caracterización de aislamientos de Pseudomonas aeruginosa productores de carbapenemasas de siete departamentos de Colombia. Biomedica 2014, 34, 217–223. [Google Scholar] [CrossRef][Green Version]
- Patel, G.; Bonomo, R.A. “Stormy waters ahead”: Global emergence of carbapenemases. Front. Microbiol. 2013, 4, 48. [Google Scholar] [CrossRef]
- Escandon-Vargas, K.; Reyes, S.; Gutierrez, S.; Villegas, M.V. The epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev. Anti-Infect. Ther. 2017, 15, 277–297. [Google Scholar] [CrossRef]
- Plant, A.J.; Dunn, A.; Porter, R.J. Ceftolozane-tazobactam resistance induced in vivo during the treatment of MDR Pseudomonas aeruginosa pneumonia. Expert Rev. Anti-Infect. Ther. 2018, 16, 367–368. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Rybak, J.M.; Rybak, M.J. The beta-Lactams Strike Back: Ceftazidime-Avibactam. Pharmacotherapy 2015, 35, 755–770. [Google Scholar] [CrossRef]
- Leber, A.L. Clinical Microbiology Procedures Handbook, 4th ed.; American Society of Microbiology: Washington, DC, USA, 2016. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 2018. Available online: https://clsi.org/media/1928/m07ed11_sample.pdf (accessed on 5 May 2019).
- Monstein, H.J.; Ostholm-Balkhed, A.; Nilsson, M.V.; Nilsson, M.; Dornbusch, K.; Nilsson, L.E. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 2007, 115, 1400–1408. [Google Scholar] [CrossRef]
- Monteiro, J.; Widen, R.H.; Pignatari, A.C.; Kubasek, C.; Silbert, S. Rapid detection of carbapenemase genes by multiplex real-time PCR. J. Antimicrob. Chemother. 2012, 67, 906–909. [Google Scholar] [CrossRef]
- Woodford, N.; Ellington, M.J.; Coelho, J.M.; Turton, J.F.; Ward, M.E.; Brown, S.; Amyes, S.G.; Livermore, D.M. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2006, 27, 351–353. [Google Scholar] [CrossRef]
- Higgins, P.G.; Lehmann, M.; Seifert, H. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2010, 35, 305. [Google Scholar] [CrossRef]
- Robicsek, A.; Strahilevitz, J.; Sahm, D.F.; Jacoby, G.A.; Hooper, D.C. QNR prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 2006, 50, 2872–2874. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Marquez, C.; Labbate, M.; Raymondo, C.; Fernandez, J.; Gestal, A.M.; Holley, M.; Borthagaray, G.; Stokes, H.W. Urinary tract infections in a South American population: Dynamic spread of class 1 integrons and multidrug resistance by homologous and site-specific recombination. J. Clin. Microbiol. 2008, 46, 3417–3425. [Google Scholar] [CrossRef]
- Herschleb, J.; Ananiev, G.; Schwartz, D.C. Pulsed-field gel electrophoresis. Nat. Protoc. 2007, 2, 677–684. [Google Scholar] [CrossRef]
- Naas, T.; Bonnin, R.A.; Cuzon, G.; Villegas, M.V.; Nordmann, P. Complete sequence of two KPC-harbouring plasmids from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2013, 68, 1757–1762. [Google Scholar] [CrossRef]
- Dai, X.; Zhou, D.; Xiong, W.; Feng, J.; Luo, W.; Luo, G.; Wang, H.; Sun, F.; Zhou, X. The IncP-6 Plasmid p10265-KPC from Pseudomonas aeruginosa Carries a Novel DeltaISEc33-Associated bla KPC-2 Gene Cluster. Front. Microbiol. 2016, 7, 310. [Google Scholar] [CrossRef]
- Abril, D.; Marquez-Ortiz, R.A.; Castro-Cardozo, B.; Moncayo-Ortiz, J.I.; Olarte Escobar, N.M.; Corredor Rozo, Z.L.; Reyes, N.; Tovar, C.; Sanchez, H.F.; Castellanos, J.; et al. Genome plasticity favours double chromosomal Tn4401b-blaKPC-2 transposon insertion in the Pseudomonas aeruginosa ST235 clone. BMC Microbiol. 2019, 19, 45. [Google Scholar] [CrossRef]
| Patient | Date of Isolation | Age (Years) | Gender | Comorbidities | Site of Infection | Treatment | Death (Yes/No) | MIC * (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEM | DOR | CAZ | TZP | GEN | CIP | SXT | CST | ||||||||
| 1 | 8 April 2017 | 66 | Male | None | Abdomen | Colistimethate + Doripenem | Yes | 1024 | 512 | >256 | >512 | >256 | 32 | >256 | 0.5 |
| 2 | 25 March 2017 | 56 | Male | Polytrauma | Bone | Colistimethate + Doripenem + Rifampin | No | 1024 | 512 | >256 | >512 | >256 | 16 | >256 | 0.5 |
| 3 | 18 April 2017 | 84 | Female | Total hip replacement | Bone | Colistimethate + Doripenem + Rifampin | Yes | 1024 | 512 | >256 | >512 | >256 | 32 | >256 | 0.5 |
| 4 | 22 April 2017 | 57 | Male | Benign prostatic hyperplasia | Blood | Colistimethate + Doripenem | No | 1024 | 512 | >256 | >512 | >256 | 32 | >256 | 0.5 |
| 5 | 12 July 2017 | 29 | Male | Epilepsy, Down Syndrome | Urine | Colistimethate + Doripenem + Fosfomycin | Yes | 1024 | 512 | >256 | >512 | >256 | 32 | >256 | 0.5 |
| MIC of ATCC27853 | - | 0.25 | 0.12 | 4 | 4 | 1 | 0.12 | 16 | 0.5 | ||||||
| Code | DNA Sequence | Amplicon Size (bp) | Specific Target | Accession Number (GenBank) |
|---|---|---|---|---|
| GN634 | AAACGTGAACCTGGCTTTGT | 183 | Orf6-IncP-6 | KC609323.1 |
| GN635 | CGCATCCACAAATGACAATC | Orf6-IncP-6 | ||
| GN636 | TCCGCCTTTTGCTTCTCGAT | 545 | repA-IncU | KC609322.1 |
| GN637 | GAGCAGATGCCAACAGTCCT | repA-IncU | ||
| GN626 | GCAGCAAGAACTGGGACGA | 835 | arsC3 | NZ_CP029605 |
| GN656 | TTTGGTGCGTGTTGCGAAG | tnpR | ||
| GN628 | GATGAAACGGCTGATTGCCC | 953 | tnpA | |
| GN657 | TACAGGCCGACCGATACCA | acr3 | ||
| GN630 | TACAGCGTGTCGTACTGCTT | 960 * | parB-like gene | |
| GN658 | ACCTACTTTGAGGCCGATGAG | 994 ** | acrA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, T.; Bustos-Cruz, R.H.; Abril, D.; Arias, S.; Uribe, L.; Rincón, J.; García, J.-C.; Escobar-Perez, J. Pseudomonas aeruginosa Coharboring BlaKPC-2 and BlaVIM-2 Carbapenemase Genes. Antibiotics 2019, 8, 98. https://doi.org/10.3390/antibiotics8030098
Pacheco T, Bustos-Cruz RH, Abril D, Arias S, Uribe L, Rincón J, García J-C, Escobar-Perez J. Pseudomonas aeruginosa Coharboring BlaKPC-2 and BlaVIM-2 Carbapenemase Genes. Antibiotics. 2019; 8(3):98. https://doi.org/10.3390/antibiotics8030098
Chicago/Turabian StylePacheco, Tatiana, Rosa Helena Bustos-Cruz, Deisy Abril, Sara Arias, Lina Uribe, Jenny Rincón, Julio-Cesar García, and Javier Escobar-Perez. 2019. "Pseudomonas aeruginosa Coharboring BlaKPC-2 and BlaVIM-2 Carbapenemase Genes" Antibiotics 8, no. 3: 98. https://doi.org/10.3390/antibiotics8030098
APA StylePacheco, T., Bustos-Cruz, R. H., Abril, D., Arias, S., Uribe, L., Rincón, J., García, J.-C., & Escobar-Perez, J. (2019). Pseudomonas aeruginosa Coharboring BlaKPC-2 and BlaVIM-2 Carbapenemase Genes. Antibiotics, 8(3), 98. https://doi.org/10.3390/antibiotics8030098






