Abstract
Antimicrobial resistance to antibiotic treatment has significantly increased during recent years, causing this to become a worldwide public health problem. More than 70% of pathogenic bacteria are resistant to at least one of the currently used antibiotics. Polymyxin E (colistin) has recently been used as a “last line” therapy when treating Gram-negative multi-resistant bacteria. However, little is known about these molecules’ pharmacological use as they have been discontinued because of their high toxicity. Recent research has been focused on determining colistimethate sodium’s pharmacokinetic parameters to find the optimal dose for maintaining a suitable benefit–risk balance. This review has thus been aimed at describing the use of colistin on patients infected by multi-drug resistant bacteria and the importance of measuring this drug’s plasma levels in such patients.
1. Introduction
Antimicrobial resistance (AMR) has represented a growing threat for several decades now regarding the effective treatment of an increasing range of infections caused by bacteria, parasites, viruses and fungi [1]. The development of AMR is a normal evolutionary process for all microorganisms, but it has undergone historic acceleration due to selective pressure exerted by the widespread/excessive use of antibacterial drugs. Several new antibacterial drugs had been developed by the 1970s to which most pathogens were completely susceptible. However, increasing AMR has been observed for the latest new families of antibiotics discovered around the 1980s, thereby leading to the rediscovery of old antibiotics such as colistin.
The World Health Organization (WHO) and the United States (US) Centres for Disease Control and Prevention (CDC) coincide in defining AMR as a microorganism’s natural or acquired ability to resist the effects of drugs having bactericide or bacteriostatic properties. Resistant bacteria survive exposure to such antibiotics and continue multiplying, causing more damage and their consequent spread to other hosts [2]. The aim of this article is to show the importance of therapeutic drug monitoring (TDM) regarding colistin.
2. Colistin Used in Managing MDR Infection
2.1. Colistin’s Chemical Structure
Polymyxins are natural products [3] and were isolated from soil bacterium Paenibacillus polymyxa subsp. Colistinus in 1947 [4] and identified as polymyxins produced by Bacillus polymyxa var. Colistinus. This drug has been available since 1959 for treating infection caused by Gram-negative bacteria.
Five main chemically different members have been recognised and designated polymyxins A, B, C, D and E [5]; two types of polymyxin are available for clinical use: polymyxin E (PME, i.e., colistin) and polymyxin B (PMB) [6]. PME has two particular forms, colistin sulphate (for oral and topical use) and negatively-charged methanesulfonate (MSA) salt of colistin, known as colistin methanesulfonate (CMS), or sodium colistimethate (SCM) in aerosol and injectable forms [5]. CMS is a poly-methanosulfonylated inactive prodrug of colistin and is not active microbiologically; it hydrolyses spontaneously to release PME [7].
Colistin’s chemical structure (Figure 1) defines its mechanism of action (MoA) and so understanding its structure-activity relationship (SAR) is an essential precursor for developing and discovering modern antibiotics [3]. Both PMB and PME are non-ribosomal secondary peptides from metabolites produced by soil bacterium Bacillus polymyxa which are highly bactericidal for Gram/negative bacteria. PMB is considered one of the most efficient permeabilising cell components [8]. The structural difference between both polymyxins occurs in position 6 which is occupied by D-Phe in PMB and D-Leu in colistin [9,10]. The polymyxin molecule has a hydrophobic structure consisting of an N-terminal fatty acid chain, five L-α, γ-diaminobutyric (Dab) acid residues, a linear tripeptíde segment, another hydrophobic structure in positions R6 and R7 on the cyclic heptapeptide ring and a heptapeptide main chain [3].
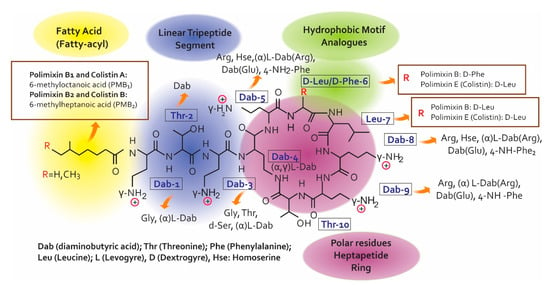
Figure 1.
Chemical structure for colistin and polymyxin B [3,6,7,9,11].
The decapeptide sequence contains the intramolecular cyclic heptapeptide in the Dab residue N-amino acid in position 4 and in the C-terminal threonine residue carboxyl group in position 10 [6]. It can be said from the description of polymyxin chemical structure that they are similar to that of the cationic antimicrobial peptides (CAMPs), representing the first line of defence against bacterial colonisation of eukaryotic cells [11]. Seven individual PMB have been identified to date. Six of the seven lipopeptides contain branched and unbranched N-terminal fatty acid groups, being structurally different regarding length by 7 to 9 carbons, called polymyxins B1–B6.
According to the SAR, polymyxins’ electrostatic and hydrophobic interactions with the lipid A component of Gram-negative bacteria’s outer membrane (OM) lipopolysaccharides (LPS) are essential in antimicrobial activity [12]. The polymyxin pharmacophore indicates that the positive charges of Dab 1, 3, 5, 8, 9 side chains represent the key points for such interaction; the pharmacophore is defined by the essential, steric, electronic and determination points of the function necessary for an optimal interaction with the pharmacological objective). The N-acyl fatty acid chain region and positions 6 and 7 on the heptapeptide cyclic ring form a fundamental part of the pharmacophore group. The positions of amino acids 2–5 and 8–10 show that they can become hydrogen donors. Another interesting aspect of polymyxin’s pharmacophore model is that it can be divided into polar (Dab and Thr residues) and hydrophobic domains (hydrophobic Na fatty acid chain and the D-Phe6-L-Leu7 motif).
Polymyxins are produced by fermentation, mixtures being obtained which have more than 30 components [10]; there are differences between pharmacopoeias regarding some components’ limits. The European Pharmacopoeia (Ph. Eur.) has limits for some colistin and polymyxin components [13,14], whilst US Pharmacopeia (USP) has no limits [15,16]. Colistin’s inherent toxicity is due to the hydrophobicity of the N-terminal fatty acyl segment, thereby greatly influencing colistin-s antimicrobial activity.
2.2. Mechanism of Action
Knowledge of Gram/negative bacteria’s architecture is necessary when talking about the polymyxins’ MOA. The plasma membrane is the barrier protecting cells from agents which can be harmful for them, including numerous antimicrobials. Polymyxins’ antimicrobial action is exerted through direct interaction with the lipid A component of the LPS. Although several models based on biophysical studies have been proposed to date, the consensus reached so far has had to do with the interaction of lipid A as polymyxin binding target [6]. Colistin and PMB antibiotic activity is concentration-dependent and has little or no post-antibiotic effect. The MoA is based on disrupting the bacteria’s plasmatic membrane and an ability to bind to the LPS, thereby preventing the release of LPS components causing destruction outside/beyond the membrane [17].
Regarding the first mechanism, the polymyxin target for Gram-negative bacteria is outside the membrane [11]. The polymyxins act as detergent agents and disrupt membrane integrity, thereby causing structural changes due to its amphipathic nature, i.e., the hydrophilic portion provided by the peptides while the hydrophobic portion provides the acyl-fatty portion and the final part of the chain. Cell membrane damage increases permeability, causing all bacterial cells’ content to become lost, ending in cell lysis. The electrostatic interaction occurs between LPS anions and the antibiotic cations donated by Dab residues, causing release of divalent cations (Ca2+ and Mg2+) outside the membrane [11,18].
The second MoA is related to the antibiotic’s ability to bind to the LPS and endotoxin release. Considering that the LPS have an important function concerning bacteria, such as endotoxin release, the polymyxins neutralise LPS lipid A component [19]. Such neutralisation mainly arises from fatty acyls binding to the final part of the polymyxin structure’s tripeptide side chain. Polymyxins also neutralise LPS inhibitory effect on mononuclear cells’ transcriptional activity, thereby reducing proinflammatory cytokine release. This antibiotic also causes mast cell degranulation due to histamine release, thus facilitating cell apoptosis [17]. It has been observed that polymyxin causes sensitisation of Gram/negative bacteria’s cell membrane to other types of antibiotics such as fusidic acid, novobiocin, rifampicin and erythromycin [17].
2.3. Colistin Resistance Mechanisms
Excessive colistin use has caused bacterial resistance to this antibiotic (i.e., in bacteria which were where normally susceptible to it). Research on colistin has increased dramatically during the last decade [20]. Polymyxin resistance mechanisms have not all been elucidated and involve a number of regulatory systems; however, colistin resistance regarding Gram-negative bacteria has been attributed to different factors. The most documented colistin resistance mechanism concerning Gram-negative bacteria has been attributed to LPS modifications via diverse routes. The lipid A head groups reduce initial electrostatic interaction with Escherichia coli, Salmonella enterica serovar Typhimurium, K. pneumoniae, and P. aeruginosa. Modifying the phosphates of lipid A positively-charged groups, such 4-amino-4-deoxy-L–arabinose (L-Ara4N) and/or phosphoethanolamine (PE), decrease lipid A net negative charge, thereby producing resistance to polymyxins [6,9]. This effect is produced by other types of Gram-negative bacterial species (i.e., S. enterica, E. coli) and the regulatory systems of two-component PhoP-PhoQ and PmrA-PmrB (response regulator/sensor kinase), involving the pmrAB, pmrD, phoPQ, parRS and mcr genes/determinants (Klebsiella pneumoniae CG43). These systems regulate cationic AMR due to low environmental Mg2+ and Ca2+.
Other two-component systems, such as ParR/ParS, ColR/ColS, and CprR/CprS [11,21], and alteration in the mgrB gene in K. pneumonia [22,23] (encoding a negative PhoP/Q regulator), cause structural modifications of the lipid A subunit, thereby affecting LPS charge and decreasing the electrostatic interaction between colistin’s negative and positive charges. Furthermore, the PmrA-PmrB system activates the expression of genes regulating lipid A modification enzymes. Another type of lipid A modification is associated with the loss of LPS, thus avoiding polymyxin fixation. Similarly, a loss of polymyxin target is caused by alteration in the lpxA, lpxC, and lpxD [24] genes, causing the inactivation of lipid A biosynthesis. It has been observed recently that a mutation in CrrB (crrC) is associated with Klebsiella pneumonia regarding colistin resistance [25]. Other genes have been identified as conferring resistance to polymyxin, such as mcr-1 to mcr-7 (E. Coli and K. pneumonia) [25,26,27].
Another polymyxin repulsion mechanism results from adding D-alanine (D-Ala) to teichoic acids, thereby increasing net positive charge due to the dlt-ABCD genes. Adding D-Ala to an OM has been described in other bacteria, regulating graXSR, dra/dlt, liaSR and CiaR operons (Staphylococcus aureus, Bordetella pertussis, Streptococcus gordonii, Listeria monocytogenes, Group B Streptococcus) [26,27]. Additional mechanisms have been described inducing modifications on cell surface regarding electrostatic repulsion of colistin, such as lipid A deacylation and hydroxylation by genes encoding Pag, PagL, LpxM and LpxO enzymes and decreasing membrane fluidity/permeability [25]. Additional lipid A mechanisms include phosphorylation, dephosphorylation, glycylation and glucosylation [28], putative hopanoid and staphyloxanthin biosynthesis by Bmul_2133/Bmul_2134 (Burkholderia multivorans) and genes involved in staphyloxanthin biosynthesis.
Associated membrane remodelling resistance mechanisms include loss of polymyxin target and capsule polysaccharide (CPS) overproduction, thereby hiding polymyxin binding sites in Neisseria meningitidis, Klebsiella pneumoniae and Salmonella enterica. Altered membrane composition (virB, suhB Bc, bvrRS, epsC-N, cgh, vacJ, waaL, rfbA, ompW, micF, pilMNOPQ operon, parRS, rsmA, bveA, ydeI (omdA), ompD (nmpC), ygiW (visP), ompF, rcs genes), altered membrane integrity (cas9, tracrRNA, scaRNA, Lol, TolQRA genes), lipooligosaccharide (LOS) and LPS modification (spgM, pgm, hldA, hldD, oprH, cj1136, waaF, lgtF, galT, cstII, galU genes) and loss of LPS and, consequently, loss of polymyxin target (lpxACD, lptD) are other membrane remodelling mechanisms [29].
Specific modifications to OM porins and overexpression of efflux pump systems have also been described. Various environmental factors such as oxidative stress, high temperature or salicylate affect porin expression through micF regulation [30,31]. Mutations in outer membrane porins (OmpU, OmpA and PorB) are associated with resistance to polymyxin B ([32]. Several types of multidrug efflux pumps (MtrC–MtrD–MtrE, RosAB, AcrAB–TolC (Escherichia coli), NorM, KpnEF and VexAB) play an important role in tolerance toward polymyxin B (Neisseria gonorrhoeae). The marRAB operon acts through interactions with Rob and up-regulation of the AcrAB–TolC efflux pumps. Other efflux pumps have been described in polymyxin resistance, such as AdeABC, HlyD Mex pumps [33].
The collection of all antibiotic resistance genes (resistome) has been defined since 2006 as a framework for understanding the evolution of and emergency regarding resistance [34]. Recent colistin resistance studies have highlighted the gene (dedA) encoding putative integral membrane protein (DedA) as playing an important role in membrane homeostasis (Escherichia coli) [35]. DedA proteins are essential for bacterial viability; thus inhibiting DedA function may provide the basis for new antibiotic development [36,37]. Table 1 summarizes the main mechanisms of resistance of bacteria and their associated genes.

Table 1.
Strategies employed by bacteria for achieving resistance to colistin.
3. Clinical Management of Colistin
3.1. Pharmacokinetics (PK)
One of the most important historical challenges in relation to colistin has been acquiring knowledge concerning its PK. Despite the discovery of this molecule more than six decades ago, and its resurgence due to the absence of a new therapeutic arsenal for MDR infections, the pharmacokinetic panorama is still not entirely clear. In this regard, about 70 articles are available in the pertinent literature, most of them dealing with (and coinciding on) this antibiotic’s pharmacokinetic variability which, associated with being a prodrug having a chemically complex structure and narrow therapeutic window, has thereby hampered in vivo and in vitro studies aimed at fully elucidating its behaviour. Figure 2 and Table 2; Table 3 summarise colistin distribution and protein binding.
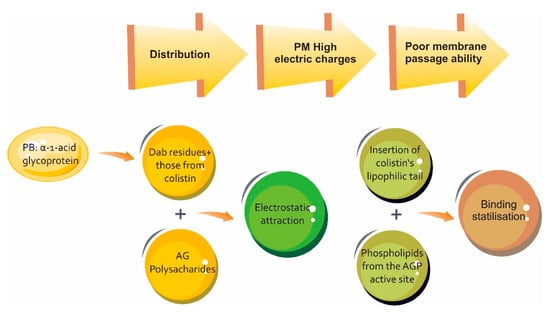
Figure 2.
Schematic representation of binding properties of colistin for human α-1-acid glycoprotein (AGP) and the impact in the distribution process. PB: prote in binding [55].

Table 2.
Colistin binding to proteins reported to date.

Table 3.
Colistin distribution in tissues.
Colistin elimination routes remain mostly unknown. Considering its peptide structure, colistin must be eliminated by hydrolysis, but the enzymes involved and their location are still unknown [53]. The blood, the liver and the kidneys are important sites for colistin elimination because they contain large amounts of proteases and peptidases; however, due to these enzymes’ ubiquitous availability throughout the body, colistin’s proteolytic degradation should not just be limited to the classical elimination organs. It is worth noting that colistin’s cyclical structure helps protect it from proteolytic endopeptidases and the acyl hydrophobic chain helps protect against exopeptidases, thereby explaining why colistin’s half-life is longer than that of many peptides [53]. This drug’s pharmacokinetic behaviour is specific for each type of population. The pertinent literature available in each case is summarised below.
3.1.1. Healthy Volunteers
Only two studies have been published to date regarding this population. Couet et al. (2011) characterised CMS and colistin’s PK after intravenous (IV) administration of CMS in healthy young volunteers at the University Hospital of Poitiers’ Clinical Research Centre (France) [54]. The study involved twelve healthy young male volunteers aged 29.5 ± 5.5 years-old on average, having 72.7 ± 9.1 kg average body weight. The exclusion criteria consisted of heart, lung, liver, kidney, haematological, neurological and psychiatric diseases and severe obesity (defined as > 30 kg/m2 BMI (body mass index)). A single dose of 1 MIU (80 mg) CMS infused in 1 hour was used; multiple blood samples were taken from 0.5 h to 18 h after the start of infusion and urine samples between 0 and 12, 24 h after starting the dose [54]. The concentration profiles related to time elapsed in this study were parallel for CMS and colistin (Figure 2); however, it should be noted that colistin’s average lifespan was longer than that for CMS which would mean that colistin elimination was not limited by the speed of its formation [54]. Table 4 summarises this study’s findings.

Table 4.
Colistin’s pharmacokinetic parameters regarding healthy volunteers [54].
Zhao et al., have also described colistin PK in healthy subjects in China; 24 volunteers were enrolled in their study which revealed that steady-state was rapidly achieved for colistin in healthy Chinese subjects using a 2.5 mg colistin base activity (CBA)/kg dose every 12 h. CMS half-life was much shorter than that for colistin. No significant CMS or colistin accumulation in plasma was observed within 1 week. This study characterised CMS and colistin urinary PK after 7-day treatment in humans; the very high concentration of colistin in urine strongly supported the use of IV CMS for serious urinary tract infections [65].
3.1.2. Critically-ill Patients
This molecule’s PK behaviour is more variable in this population. Table 5 summarises some general observations made in the pertinent literature.

Table 5.
General PK aspects regarding maintenance dose in critically-ill patients.
Significant discrepancies have arisen between available studies regarding this special population. Gregoire et al., observed typical Cmax values after the first dose of 2 MIU (2 mg/L) CMS [67], whereas Plachouras et al., deduced 0.6 mg/L Cmax for colistin after a first dose of 3 MIU CMS [68]. As can be observed, Cmax values have been reached in around 3 h in a study by Gregoire et al. [67] unlike that of Plachouras et al., in around 8 h [68]. There have been fewer discrepancies between studies regarding steady-state; average steady-state colistin (Css,avg) was calculated as 1.5 to 3.5 mg/L for a patient having 82 mL/min creatinine clearance being treated with 3 MIU CMS every 8 h, depending on the study [68]. The aforementioned study was the first to highlight the difficulties in achieving an average 2 mg/L Css for patients having 80 mL/min creatinine clearance. This study thereby showed the probable relevance of the loading dose in reaching steady state in less time (i.e., a function of the drug’s elimination half-life - t1/2) and such theory has been corroborated in two further studies [58,69]. Average colistin Cmax values were 1.3 mg/L (0.3–2.6 mg/L range) 8 h after dosing and t1/2 was 18.5 after administering 6 MIU CMS to ten critically-ill patients in the first of them [58]. After administering a 9 MIU loading dose to 19 critically-ill patients in the second study, colistin Cmax values were also highly variable (mean 2.65 mg/L and 0.9–5.1 mg/L range) with 11.2 h t1/2 [69]. Menna et al., also concluded that a dose-intensified CMS regimen for critically ill patients suffering acute kidney injury (AKI) and continuous renal replacement therapy (CRRT) results in high and long-lasting colistin plasma levels [70]. These PK parameters have enabled dosage algorithms to be constructed; one of the most recognised in the literature is that which has been that proposed by Garonzik et al. [66].
3.1.3. Patients having Extremely Impaired Renal Function
CMS is excreted sparingly in the urine and the dose fraction available for conversion to colistin is therefore higher [33]. Consequently, colistin exposure is generally three times greater in critically-ill patients requiring haemodialysis on days without a haemodialysis session than in patients having preserved renal function and being treated with the same dose of the drug [33]. Considering their molecular weights, CMS and colistin fractions not bound in plasma can thus pass freely through dialysis membranes [33]. Furthermore, colistin could also be adsorbed by dialysis membranes, especially those used for continuous renal replacement therapy, which could contribute towards the colistin clearance mechanism [31]. Table 6 summarises colistin and CMS clearance regarding dialysis mode.

Table 6.
Average colistin and CMS clearance by dialysis mode.
3.1.4. Cystic Fibrosis Patients
Cystic fibrosis patients have been amongst those most studied to date due to this drug’s extended use in this population. Table 7 summarises some of the most important findings.

Table 7.
Pharmacokinetic parameters regarding cystic fibrosis patients.
3.1.5. Burn Patients
Lee et al., have reported a 6.6 h colistin half-life following IV administration of 5 MIU CMS every 12 h, such clearance being comparable to that of critically-ill patients and healthy volunteers. This would suggest that it was not affected by this patient population’s hypermetabolism [74], as colistin Vd was slightly higher than that reported for healthy volunteers [74].
The currently available literature states that colistin PK vary widely which, associated with colistin being a drug having a narrow therapeutic index (NTI), makes the use of strategies such as monitoring plasma levels relevant after IV administration to ensure its proper use. The data regarding polymyxin safety is controversial. This antibiotic family has been historically associated with numerous adverse events (AE), including mortality, nephrotoxicity, neurotoxicity and hypersensitivity reactions [75].
One of the first reports regarding this drug’s safety was published in 1962, describing potentially serious reactions to colistin in 3 adults suffering renal failure and in a child who received ten times the recommended dose [76]. The first major study was published in June 1970 which evaluated adverse reactions in 317 courses of CMS therapy; prior to this, it was considered that CMS toxicity was low. Some researchers declared in the 1960s that using this drug did not induce nephrotoxicity; however, this investigation’s results alerted the global medical community, adverse reactions being seen in 1 out of every 3 patients [77].
From the above and other findings around the same time, compounds from this class of antibiotic were gradually withdrawn from clinical practice and replaced by newer antibiotics having the same or broader antibacterial spectra and whose toxicity reports were lower [78].
The association between polymyxins and mortality represents one of the most controversial issues regarding this topic; two major meta-analyses in this regard have assessed mortality in two pneumonia patient populations associated with mechanical ventilation. One sampled 1167 and the other 796 patients; no differences were found in both regarding all causes of death when comparing patients treated with colistin to those treated with other antibiotics. However, in neither study was it clear whether polymyxins can contribute to total mortality through their nephrotoxicity [79,80]. Later, in 2010, Falagas et al., and Elias et al., found a protective polymyxin dose effect, despite the development of acute kidney injury [81,82]. This has been corroborated by other studies showing greater area under curve (AUC)/minimum inhibitory concentration (MIC) exposure at higher polymyxin doses, ultimately predicting better activity for these drugs [83,84]. However, studies are required having a better methodological design involving significant variables affecting mortality after 30 days’ treatment [75].
Renal toxicity is the most commonly occurring AE related to polymyxin use, ranging from proteinuria to acute renal injury requiring interruption of therapy and even the initiation of renal replacement therapy; overall incidence has high (20% to 76%) variability based on updated data [75]. Recent studies have shown lower incidence regarding this AE than that thought in previous decades. The Greek research group led by Falagas has shown that deterioration in patients having normal basal creatinine was not significant during prolonged colistin administration, thereby contrasting with other available studies; they did not even find these doses’ influence on the renal function of patients having prior dysfunction [85]. Hartzell et al., have shown that 21% of cases have required cessation of therapy, but that no patient had to undergo renal replacement therapy [86].
Several theories have been advanced regarding polymyxins’ renal toxicity. It has been assumed that this may have been partly due to their content in D-amino acid isomeric form and the acid fatty component. Experimental studies have shown increased trans-epithelial conductance through the bladder’s epithelium [87]; CMS can then induce greater membrane permeability, resulting in cell lysis [87,88]. Between 49% and 78% of cases of nephrotoxicity occur within the first 5 to 7 days of therapy [89]. The risk factors related to this AE are numerous; however, most data comes from retrospective studies and must be interpreted carefully. One of the main controversies has thus concerned the dose; some studies have confirmed a relationship between nephrotoxicity and high total cumulative doses or longer-lasting therapy whilst others have failed to confirm such observations [75]. Other risk factors identified so far have been related to patients’ age [90] and the concomitant use of other nephrotoxic drugs [91]. Kady Phe (2014) compared and validated the performance of several models for predicting the risk of colistin-associated nephrotoxicity, identifying age, therapy duration and daily dose according to ideal weight as independent risk factors. Interestingly, cystic fibrosis was found to be a protective factor, no association being found with the concomitant use of other nephrotoxic agents [92]. Mathematical prediction models will undoubtedly prove useful for correctly identifying high-risk patients so that strategies aimed at minimising such AE can be selectively introduced; however, studies having better methodological design are required to unify data regarding polymyxin-associated nephrotoxicity.
A rather less-reported relevant AE in the literature has been neurological toxicity; its overall incidence is less than 7% and even recent multiple studies have not reported any cases [75]. Paraesthesia has been the most frequently reported neurotoxic effect amongst the wide range of such effects (7% to 23%), being even higher in cystic fibrosis patients [78,93]. Reports of other neurotoxic events are scarce, only three cases of respiratory/ventilatory failure having been reported after 1970 [94]. This could have been related to the difficulty regarding an objective interpretation of neurological symptoms which would have influenced clinical nephrotoxicity and neurotoxicity reports in not identifying or reporting polymyxin-associated neurotoxicity [75]. Direct interaction between polymyxins and neurons is considered the cause of neurotoxicity; this could lead to inhibiting acetylcholine action regarding neuromuscular binding, increase depolarisation and induce histamine release. However, the precise mechanism has not yet been completely elucidated [75]. Renal dysfunction accompanied by concomitant neurological diseases, such as myasthenia gravis, have been identified as risk factors [95].
The available literature regarding polymyxins’ other toxic effects is scarce; it is not clear however, whether their low incidence is real or whether one is dealing with these events’ slight clinical relevance or bias in the available retrospective studies [75].
4. Colistin Plasma Level Measurements
This drug’s dose and levels in blood must be controlled due to the increasing use of last-line antibiotics, especially polymyxins and mainly colistin [96]. Colistin dosing regimens derived from cases of acute Gram-negative bacterial infection have been based on clinical experience for decades now, thereby leading to doses not being defined by the antibiotic’s pharmacodynamic (PD) and PK properties [97], due to the lack of specific and reliable methods for taking measurements [98]. Robust assays have thus become necessary to enable the antibiotic to be quantified for determining its dose and mitigate its AEs regarding patients’ health by introducing/using techniques enabling this drug to be measured using different matrices, like milk, saliva, blood, plasma and tissue [99].
4.1. Analysis Methods
Several techniques have been described so far which have focused on quantifying CMS in plasma, including microbiological bioassays mainly based on the drug inhibiting microorganisms using pathogenic bacteria from the Bordetella bronchiseptica and Escherichia coli genera as indicator [100,101]. High-performance liquid chromatography (HPLC) is currently one of the most used analysis techniques; it has a high degree of accuracy and precision based on separating a sample’s components, involving different types of chemical interaction [100]. Mass spectrometry (MS) is a highly selective and sensitive technique, thereby enabling colistin quantification in different matrices, especially human plasma [102]. This drug’s molecule has little ultraviolet (UV) absorption and no fluorescence, which is why techniques such as UV spectroscopy are not frequently used for its quantification [103]. Table 8 shows the analytical method used for determining colistin in plasma.

Table 8.
Techniques used for quantifying colistin in plasma.
4.2. Difficulty Regarding Measurement
Difficulties have been encountered when measuring colistin effectiveness [104]. One of the main problems has arisen regarding colistin’s chemical characteristics as it is adsorbed by many materials used in the laboratory, such as plastic. Colistin’s structure is affected by an experiment’s physical-chemical conditions, mainly sample temperature, pH, incubation time and matrix, thereby leading to CMS degradation (hydrolysis) to colistin [97,104]. Such experimentation difficulties have led to false positives when quantifying the drug.
Some analysis- and quantification-related disadvantages must be highlighted regarding the techniques used to date for measuring colistin levels, thereby hampering reliable results being obtained. Microbiological bioassays represent one of the most inaccurate and least sensitive techniques because the incubation conditions interfere with molecule stability [100]. This technique involves using culture media which are difficult to obtain on the market; furthermore, the variables regarding microorganism growth can affect assay results [101].
HPLC has been widely used since it is a more sensitive and precise technique than a microbiological bioassay. However, its disadvantage lies in being a test which requires expensive equipment, high reagent consumption, sample pre-treatment, solid phase extraction and derivatisation which requires specialised personnel [108,109]. Difficulties have arisen regarding analysing and quantifying the mixture of CMS compounds in its commercial presentation, thereby hindering their separation [100].
MS is one of the most robust techniques for measuring colistin levels; however, it requires a specialised laboratory and trained personnel for analysing and quantifying samples, bearing in mind that the sample is destroyed during the process [68]. The molecules are derivatised when using other techniques, such as UV spectroscopy; reagents such as 9-fluorenylmethyl (FMOC-Cl) and ortho-phthalaldehyde (OPA) are used in their analysis and quantification [97].
4.3. The Importance of Measurement
Limited data regarding colistin PK and PD properties has led to confusion when determining patients’ doses, added to the uncontrolled increase of colistin-resistant bacteria and their neurotoxic and nephrotoxic effects [110]. The forgoing highlights the importance of monitoring colistin plasma levels to adjust the dose and dose interval, taking a particular patient’s clinical picture into account [111]. PK properties may become altered in critical patients because they are frequently prone to large oscillations regarding distribution volume, renal clearance fluctuations and protein binding variability. Likewise, these drugs’ antimicrobial activity is attenuated by the high bacterial load, as in pneumonia [112].
Different techniques, involving a high degree of sensitivity and precision, have thus been used for enabling colistin plasma concentrations to be quantified [113]. However, most are expensive and require specialised personnel and laboratories whilst matrix components and physico-chemical conditions can interfere with assay accuracy [114]. Nevertheless, this drug must be quantified for determining an appropriate dose mitigating resistance to it and its toxicity [97].
5. Conclusions
Although, there is still not enough evidence regarding adjusting an antibiotic dose for patients suffering multi-resistant bacterial infection, therapeutic monitoring of colistin could constitute good clinical practice to help administer a dose to patients according to infection levels and drug response. Clinical use of intravenous colistin is limited by its large interpatient PK variability; some dosing algorithms have been constructed in an attempt to define a desirable concentration. However, plasma concentrations overlapping for an antibacterial effect and those causing nephrotoxicity and neurotoxicity limit its feasible implementation.
Author Contributions
Conceptualization, T.P., R.H.B. and J.C.G.; writing—original draft preparation, T.P., R.H.B., D.G., V.G., J.C.G. and D.R. writing—review and editing, T.P. and R.H.B.; supervision, R.H.B.; project administration, R.H.B., J.C.G.; funding acquisition, R.H.B., J.C.G.
Funding
This research was funded by UNIVERSIDAD DE LA SABANA, grant number MED-213-2016; we would like to thank the Universidad de La Sabana for extending its full support for the project.
Conflicts of Interest
All the authors have contributed towards the writing of this article. The authors declare no conflict of interest.
References
- WHO. Antimicrobial resistance. Global Report on Surveillance. Bull. World Health Organ. 2014, 61, 383–394. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States; 2013 Centres for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2013. [Google Scholar]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Wang, J.; Thompson, P.E.; Li, J. Teaching ‘old’ polymyxins new tricks: New-generation lipopeptides targeting gram-negative ‘superbugs’. ACS Chem. Biol. 2014, 9, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.G.; Langlykke, A.F. Antibiotic activity of Bacillus polymyxa. J. Bacteriol. 1947, 54, 24. [Google Scholar] [PubMed]
- Brink, A.J.; Richards, G.A.; Colombo, G.; Bortolotti, F.; Colombo, P.; Jehl, F. Multicomponent antibiotic substances produced by fermentation: Implications for regulatory authorities, critically ill patients and generics. Int. J. Antimicrob. Agents 2014, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of polymyxins: New insights into an ‘old’ class of antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Godoy, A.; Muldoon, C.; Becker, B.; Elliott, A.G.; Lash, L.H.; Huang, J.X.; Butler, M.S.; Pelingon, R.; Kavanagh, A.M.; Ramu, S.; et al. Activity and Predicted Nephrotoxicity of Synthetic Antibiotics Based on Polymyxin B. J. Med. Chem. 2016, 59, 1068–1077. [Google Scholar] [CrossRef]
- Tsubery, H.; Ofek, I.; Cohen, S.; Fridkin, M. N-terminal modifications of Polymyxin B nonapeptide and their effect on antibacterial activity. Peptides 2001, 22, 1675–1681. [Google Scholar] [CrossRef]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure—Activity relationships of polymyxin antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef]
- Nation, R.L.; Velkov, T.; Li, J. Colistin and polymyxin B: Peas in a pod, or chalk and cheese? Clin. Infect. Dis. 2014, 59, 88–94. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef]
- Pristovšek, P.; Kidrič, J. Solution structure of polymyxins B and E and effect of binding to lipopolysaccharide: An NMR and molecular modeling study. J. Med. Chem. 1999, 42, 4604–4613. [Google Scholar] [CrossRef]
- European Pharmacopoeia 8.0. Monographs for Colistimethate Sodium and Colisitin Sulfate. Available online: http://online6.edqm.eu/ep800/ (accessed on 29 May 2019).
- European Pharmacopoeia 8.0. Monograph for Polymyxin B Sulfate. Available online: http://online6.edqm.eu/ep800/ (accessed on 21 February 2019).
- United States Pharmacopoeia 36. Monographs for Colistimethate Sodium and Colistin Sulfate. Available online: https://www.uspnf.com/official-text/proposal-statuscommentary/usp-36-nf-31 (accessed on 21 February 2019).
- United States Pharmacopoeia 36. Monograph for Polymyxin B Sulfate. Available online: https://www.uspnf.com/official-text/proposal-statuscommentary/usp-36-nf-31 (accessed on 21 February 2019).
- Kadar, B.; Kocsis, B.; Nagy, K.; Szabo, D. The renaissance of polymyxins. Curr. Med. Chem. 2013, 20, 3759–3773. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Chopra, I. Leakage of periplasmic proteins from Escherichia coli mediated by polymyxin B nonapeptide. Antimicrob. Agents Chemother. 1986, 29, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nation, R.L.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Beaudry, F.; Letellier, A. Resistance to colistin: What is the fate for this antibiotic in pig production? Int. J. Antimicrob. Agents 2016, 48, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Snesrud, E.; Maybank, R.; Kwak, Y.I.; Jones, A.R.; Hinkle, M.K.; McGann, P. Chromosomally Encoded mcr-5 in Colistin-Nonsusceptible Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018, 62, e00679-18. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Bontron, S.; Villegas, M.V.; Ozdamar, M.; Turkoglu, S.; Nordmann, P. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2015, 70, 75–80. [Google Scholar] [CrossRef]
- Shankar, C.; Pragasam, A.K.; Anandan, S.; Veeraraghavan, B. mgrB as Hotspot for Insertion Sequence Integration: Change Over from Multidrug-Resistant to Extensively Drug-Resistant Klebsiella pneumoniae? Microb. Drug Resist. 2019. [Google Scholar] [CrossRef]
- Nurtop, E.; Bayindir Bilman, F.; Menekse, S.; Kurt Azap, O.; Gonen, M.; Ergonul, O.; Can, F. Promoters of Colistin Resistance in Acinetobacter baumannii Infections. Microb. Drug Resist. 2019. [Google Scholar] [CrossRef]
- Mlynarcik, P.; Kolar, M. Molecular mechanisms of polymyxin resistance and detection of mcr genes. Biomed. Pap. 2019, 163, 28–38. [Google Scholar] [CrossRef]
- Mavrici, D.; Yambao, J.C.; Lee, B.G.; Quinones, B.; He, X. Screening for the presence of mcr-1/mcr-2 genes in Shiga toxin-producing Escherichia coli recovered from a major produce-production region in California. PLoS ONE 2017, 12, e0187827. [Google Scholar] [CrossRef]
- Poirel, L.; Larpin, Y.; Dobias, J.; Stephan, R.; Decousser, J.W.; Madec, J.Y.; Nordmann, P. Rapid Polymyxin NP test for the detection of polymyxin resistance mediated by the mcr-1/mcr-2 genes. Diagn. Microbiol. Infect. Dis. 2018, 90, 7–10. [Google Scholar] [CrossRef]
- Trimble, M.J.; Mlynarcik, P.; Kolar, M.; Hancock, R.E. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef]
- Markou, N.; Fousteri, M.; Markantonis, S.L.; Zidianakis, B.; Hroni, D.; Boutzouka, E.; Baltopoulos, G. Colistin pharmacokinetics in intensive care unit patients on continuous venovenous haemodiafiltration: An observational study. J. Antimicrob. Chemother. 2012, 67, 2459–2462. [Google Scholar] [CrossRef]
- Pinho, A.; Rocha, M.; Alves, G.; Falcão, A.; Fortuna, A. Development and validation of an HPLC-FLD technique for colistin quantification and its plasma monitoring in hospitalized patients. Anal. Methods 2018, 10, 389–396. [Google Scholar] [CrossRef]
- Jitmuang, A.; Nation, R.L.; Koomanachai, P.; Chen, G.; Lee, H.J.; Wasuwattakul, S.; Sritippayawan, S.; Li, J.; Thamlikitkul, V.; Landersdorfer, C.B. Extracorporeal clearance of colistin methanesulphonate and formed colistin in end-stage renal disease patients receiving intermittent haemodialysis: Implications for dosing. J. Antimicrob. Chemother. 2015, 70, 1804–1811. [Google Scholar] [CrossRef]
- Niece, K.L.; Akers, K.S. Preliminary Method for Direct Quantification of Colistin Methanesulfonate by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR FT-IR). Antimicrob. Agents Chemother. 2015, 59, 5542–5547. [Google Scholar] [CrossRef]
- Jacobs, M.; Gregoire, N.; Mégarbane, B.; Gobin, P.; Balayn, D.; Marchand, S.; Mimoz, O.; Couet, W. Population pharmacokinetics of colistin methanesulfonate and colistin in critically ill patients with acute renal failure requiring intermittent hemodialysis. Antimicrob. Agents Chemother. 2016, 60, 1788–1793. [Google Scholar] [CrossRef]
- Perry, J.A.; Westman, E.L.; Wright, G.D. The antibiotic resistome: what’s new? Curr. Opin. Microbiol. 2014, 21, 45–50. [Google Scholar] [CrossRef]
- Jana, B.; Cain, A.K.; Doerrler, W.T.; Boinett, C.J.; Fookes, M.C.; Parkhill, J.; Guardabassi, L. The secondary resistome of multidrug-resistant Klebsiella pneumoniae. Sci. Rep. 2017, 7, 42483. [Google Scholar] [CrossRef]
- Doerrler, W.T.; Sikdar, R.; Kumar, S.; Boughner, L.A. New functions for the ancient DedA membrane protein family. J. Bacteriol. 2013, 195, 3–11. [Google Scholar] [CrossRef]
- Kumar, S.; Doerrler, W.T. Members of the conserved DedA family are likely membrane transporters and are required for drug resistance in Escherichia coli. Antimicrob. Agents Chemother. 2014, 58, 923–930. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Nummila, K.; Kilpeläinen, I.; Zähringer, U.; Vaara, M.; Helander, I.M. Lipopolysaccharides of polymyxin B-resistant mutants of Escherichia coii are extensively substituted by 2-aminoethyl pyrophosphate and contain aminoarabinose in lipid A. Mol. Microbiol. 1995, 16, 271–278. [Google Scholar] [CrossRef]
- Aghapour, Z.; Gholizadeh, P.; Ganbarov, K.; Bialvaei, A.Z.; Mahmood, S.S.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Yousefi, B.; Kafil, H.S. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect. Drug Resist. 2019, 12, 965–975. [Google Scholar] [CrossRef]
- Jiang, S.S.; Liu, M.C.; Teng, L.J.; Wang, W.B.; Hsueh, P.R.; Liaw, S.J. Proteus mirabilis pmrI, an RppA-regulated gene necessary for polymyxin B resistance, biofilm formation, and urothelial cell invasion. Antimicrob. Agents Chemother. 2010, 54, 1564–1571. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Dia, N.M.; Gautret, P.; Benkouiten, S.; Belhouchat, K.; Drali, T.; Parola, P.; Brouqui, P.; Memish, Z.; Raoult, D.; et al. Acquisition of extended-spectrum cephalosporin- and colistin-resistant Salmonella enterica subsp. enterica serotype Newport by pilgrims during Hajj. Int. J. Antimicrob. Agents 2015, 45, 600–604. [Google Scholar] [CrossRef]
- Jayol, A.; Poirel, L.; Brink, A.; Villegas, M.V.; Yilmaz, M.; Nordmann, P. Resistance to colistin associated with a single amino acid change in protein PmrB among Klebsiella pneumoniae isolates of worldwide origin. Antimicrob. Agents Chemother. 2014, 58, 4762–4766. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Emergence of colistin-resistant bacteria in humans without colistin usage: A new worry and cause for vigilance. Int. J. Antimicrob. Agents 2016, 47, 1–3. [Google Scholar] [CrossRef]
- Kang, K.N.; Klein, D.R.; Kazi, M.I.; Guerin, F.; Cattoir, V.; Brodbelt, J.S.; Boll, J.M. Colistin heteroresistance in Enterobacter cloacae is regulated by PhoPQ-dependent 4-amino-4-deoxy-l-arabinose addition to lipid A. Mol. Microbiol. 2019, 111, 1604–1616. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Lin, T.L.; Lin, Y.T.; Wang, J.T. Amino Acid Substitutions of CrrB Responsible for Resistance to Colistin through CrrC in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2016, 60, 3709–3716. [Google Scholar] [CrossRef]
- Jaidane, N.; Bonnin, R.A.; Mansour, W.; Girlich, D.; Creton, E.; Cotellon, G.; Chaouch, C.; Boujaafar, N.; Bouallegue, O.; Naas, T. Genomic Insights into Colistin-Resistant Klebsiella pneumoniae from a Tunisian Teaching Hospital. Antimicrob. Agents Chemother. 2018, 62, e01601-17. [Google Scholar] [CrossRef]
- Da Silva, G.J.; Domingues, S. Interplay between Colistin Resistance, Virulence and Fitness in Acinetobacter baumannii. Antibiotics (Basel) 2017, 6, 28. [Google Scholar] [CrossRef]
- Zhou, Z.; Ribeiro, A.A.; Lin, S.; Cotter, R.J.; Miller, S.I.; Raetz, C.R. Lipid a modifications in polymyxin-resistant Salmonella typhimurium PmrA-dependent 4-amino-4-deoxy-l-arabinose, and phosphoethanolamine incorporation. J. Biol. Chem. 2001, 276, 43111–43121. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhou, Y.; Li, J.; Yin, W.; Wang, S.; Zhang, S.; Shen, J.; Shen, Z.; Wang, Y. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg. Microbes Infect. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Llobet, E.; Martinez-Moliner, V.; Moranta, D.; Dahlstrom, K.M.; Regueiro, V.; Tomas, A.; Cano, V.; Perez-Gutierrez, C.; Frank, C.G.; Fernandez-Carrasco, H.; et al. Deciphering tissue-induced Klebsiella pneumoniae lipid A structure. Proc. Natl. Acad. Sci. USA 2015, 112, E6369–E6378. [Google Scholar] [CrossRef]
- Malott, R.J.; Steen-Kinnaird, B.R.; Lee, T.D.; Speert, D.P. Identification of hopanoid biosynthesis genes involved in polymyxin resistance in Burkholderia multivorans. Antimicrob. Agents Chemother. 2012, 56, 464–471. [Google Scholar] [CrossRef]
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef]
- Couet, W.; Grégoire, N.; Gobin, P.; Saulnier, P.J.; Frasca, D.; Marchand, S.; Mimoz, O. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin. Pharmacol. Ther. 2011, 89, 875–879. [Google Scholar] [CrossRef]
- Azad, M.A.; Huang, J.X.; Cooper, M.A.; Roberts, K.D.; Thompson, P.E.; Nation, R.L.; Li, J.; Velkov, T. Structure-activity relationships for the binding of polymyxins with human α-1-acid glycoprotein. Biochem. Pharmacol. 2012, 84, 278–291. [Google Scholar] [CrossRef]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Smeaton, T.C.; Coulthard, K. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 2003, 47, 1766–1770. [Google Scholar] [CrossRef]
- Cheah, S.E.; Wang, J.; Nguyen, V.T.; Turnidge, J.D.; Li, J.; Nation, R.L. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 2015, 70, 3291–3297. [Google Scholar] [CrossRef]
- Mohamed, A.F.; Karaiskos, I.; Plachouras, D.; Karvanen, M.; Pontikis, K.; Jansson, B.; Papadomichelakis, E.; Antoniadou, A.; Giamarellou, H.; Armaganidis, A.; et al. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob. Agents Chemother. 2012, 56, 4241–4249. [Google Scholar] [CrossRef]
- Imberti, R.; Cusato, M.; Villani, P.; Carnevale, L.; Iotti, G.A.; Langer, M.; Regazzi, M. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest 2010, 138, 1333–1339. [Google Scholar] [CrossRef]
- Boisson, M.; Jacobs, M.; Grégoire, N.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob. Agents Chemother. 2014, 58, 7331–7339. [Google Scholar] [CrossRef]
- Yapa, S.W.S.; Li, J.; Patel, K.; Wilson, J.W.; Dooley, M.J.; George, J.; Clark, D.; Poole, S.; Williams, E.; Porter, C.J.; et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: Targeting advantage of inhalational administration. Antimicrob. Agents Chemother. 2014, 58, 2570–2579. [Google Scholar] [CrossRef]
- Markantonis, S.L.; Markou, N.; Fousteri, M.; Sakellaridis, N.; Karatzas, S.; Alamanos, I.; Dimopoulou, E.; Baltopoulos, G. Penetration of colistin into cerebrospinal fluid. Antimicrob. Agents Chemother. 2009, 53, 4907–4910. [Google Scholar] [CrossRef]
- Ziaka, M.; Markantonis, S.L.; Fousteri, M.; Zygoulis, P.; Panidis, D.; Karvouniaris, M.; Makris, D.; Zakynthinos, E. Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection. Antimicrob. Agents Chemother. 2013, 57, 1938–1940. [Google Scholar] [CrossRef]
- Imberti, R.; Cusato, M.; Accetta, G.; Marinò, V.; Procaccio, F.; Del Gaudio, A.; Iotti, G.A.; Regazzi, M. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate. Antimicrob. Agents Chemother. 2012, 56, 4416–4421. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, X.J.; Fan, Y.X.; Zhang, Y.Y.; Guo, B.N.; Yu, J.C.; Cao, G.Y.; Chen, Y.C.; Wu, J.F.; Shi, Y.G.; et al. Pharmacokinetics of colistin methanesulfonate (CMS) in healthy Chinese subjects after single and multiple intravenous doses. Int. J. Antimicrob. Agents 2018, 51, 714–720. [Google Scholar] [CrossRef]
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef]
- Grégoire, N.; Mimoz, O.; Mégarbane, B.; Comets, E.; Chatelier, D.; Lasocki, S.; Gauzit, R.; Balayn, D.; Gobin, P.; Marchand, S.; et al. New colistin population pharmacokinetic data in critically ill patients suggesting an alternative loading dose rationale. Antimicrob. Agents Chemother. 2014, 58, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Plachouras, D.; Karvanen, M.; Friberg, L.E.; Papadomichelakis, E.; Antoniadou, A.; Tsangaris, I.; Karaiskos, I.; Poulakou, G.; Kontopidou, F.; Armaganidis, A.; et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2009, 53, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Friberg, L.E.; Pontikis, K.; Ioannidis, K.; Tsagkari, V.; Galani, L.; Kostakou, E.; Baziaka, F.; Paskalis, C.; Koutsoukou, A.; et al. Colistin Population Pharmacokinetics after Application of a Loading Dose of 9 MU Colistin Methanesulfonate in Critically Ill Patients. Antimicrob. Agents Chemother. 2015, 59, 7240–7248. [Google Scholar] [CrossRef] [PubMed]
- Menna, P.; Salvatorelli, E.; Mattei, A.; Cappiello, D.; Minotti, G.; Carassiti, M. Modified Colistin Regimen for Critically Ill Patients with Acute Renal Impairment and Continuous Renal Replacement Therapy. Chemotherapy 2018, 63, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Marchand, S.; Frat, J.P.; Petitpas, F.; Lemaître, F.; Gobin, P.; Robert, R.; Mimoz, O.; Couet, W. Removal of colistin during intermittent haemodialysis in two critically ill patients. J. Antimicrob. Chemother. 2010, 65, 1836–1837. [Google Scholar] [CrossRef] [PubMed]
- Luque, S.; Sorli, L.; Li, J.; Collado, S.; Barbosa, F.; Berenguer, N.; Horcajada, J.P.; Grau, S. Effective removal of colistin methanesulphonate and formed colistin during intermittent haemodialysis in a patient infected by polymyxin-only-susceptible Pseudomonas aeruginosa. J. Chemother. 2014, 26, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Coulthard, K.; Milne, R.; Nation, R.L.; Conway, S.; Peckham, D.; Etherington, C.; Turnidge, J. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J. Antimicrob. Chemother. 2003, 52, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Han, S.; Jeon, S.; Hong, T.; Song, W.; Woo, H.; Yim, D.S. Population pharmacokinetic analysis of colistin in burn patients. Antimicrob. Agents Chemother. 2013, 57, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Falagas, M.E. The safety of polymyxin antibiotics. Expert Opin. Drug Saf. 2015, 14, 1687–1701. [Google Scholar] [CrossRef]
- Wolinsky, E.; Hines, J.D. Neurotoxic and nephrotoxic effects of colistin in patients with renal disease. N. Engl. J. Med. 1962, 266, 759–762. [Google Scholar] [CrossRef]
- Koch-Weser, J.A.N.; Sidel, V.W.; Federman, E.B.; Kanarek, P.; Finer, D.C.; Eaton, A.N.N.E. Adverse Effects of Sodium ColistimethateManifestations and Specific Reaction Rates During 317 Courses of Therapy. Ann. Intern. Med. 1970, 72, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [PubMed]
- Florescu, D.F.; Qiu, F.; McCartan, M.A.; Mindru, C.; Fey, P.D.; Kalil, A.C. What is the efficacy and safety of colistin for the treatment of ventilator-associated pneumonia? A systematic review and meta-regression. Clin. Infect. Dis. 2012, 54, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.J.; Wang, F.; Tang, L.; Bakker, J.; Liu, J.C. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2014, 44, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Rafailidis, P.I.; Ioannidou, E.; Alexiou, V.G.; Matthaiou, D.K.; Karageorgopoulos, D.E.; Kapaskelis, A.; Nikita, D.; Michalopoulos, A. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: A retrospective cohort study of 258 patients. Int. J. Antimicrob. Agents 2010, 35, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Elias, L.S.; Konzen, D.; Krebs, J.M.; Zavascki, A.P. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J. Antimicrob. Chemother. 2010, 65, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Sandri, A.M.; Landersdorfer, C.B.; Jacob, J.; Boniatti, M.M.; Dalarosa, M.G.; Falci, D.R.; Behle, T.F.; Bordinhão, R.C.; Wang, J.; Forrest, A.; et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: Implications for selection of dosage regimens. Clin. Infect. Dis. 2013, 57, 524–531. [Google Scholar] [CrossRef]
- Tam, V.H.; Schilling, A.N.; Vo, G.; Kabbara, S.; Kwa, A.L.; Wiederhold, N.P.; Lewis, R.E. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 3624–3630. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Rizos, M.; Bliziotis, I.A.; Rellos, K.; Kasiakou, S.K.; Michalopoulos, A. Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect. Dis. 2005, 5, 1. [Google Scholar] [CrossRef]
- Hartzell, J.D.; Neff, R.; Ake, J.; Howard, R.; Olson, S.; Paolino, K.; Vishnepolsky, M.; Weintrob, A.; Wortmann, G. Nephrotoxicity Associated with Intravenous Colistin (Colistimethate Sodium) Treatment at a Tertiary Care Medical Center. Clin. Infect. Dis. 2009, 48, 1724–1728. [Google Scholar] [CrossRef]
- Lewis, J.R.; Lewis, S.A. Colistin interactions with the mammalian urothelium. Am. J. Physiol. Cell Physiol. 2004, 286, C913–C922. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Johnson, W.W.; Roy, S. Colistin nephrotoxicity: Report of a case with light and electron microscopic studies. Acta Pathol. Jpn. 1969, 19, 55–67. [Google Scholar] [CrossRef]
- Deryke, C.A.; Crawford, A.J.; Uddin, N.; Wallace, M.R. Colistin Dosing and Nephrotoxicity in a Large Community Teaching Hospital. Antimicrob. Agents Chemother. 2010, 54, 4503–4505. [Google Scholar] [CrossRef] [PubMed]
- Phe, K.; Lee, Y.; McDaneld, P.M.; Prasad, N.; Yin, T.; Figueroa, D.A.; Musick, W.L.; Cottreau, J.M.; Hu, M.; Tam, V.H. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob. Agents Chemother. 2014, 58, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Haynes, K.; Gallagher, J.C. Emergent renal dysfunction with colistin pharmacotherapy. Pharmacotherapy 2013, 33, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Phe, K.; Johnson, M.L.; Palmer, H.R.; Tam, V.H. Validation of a model to predict the risk of nephrotoxicity in patients receiving colistin. Antimicrob. Agents Chemother. 2014, 58, 6946–6948. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Fragoulis, K.N.; Kasiakou, S.K.; Sermaidis, G.J.; Michalopoulos, A. Nephrotoxicity of intravenous colistin: A prospective evaluation. Int. J. Antimicrob. Agents 2005, 26, 504–507. [Google Scholar] [CrossRef]
- Shrestha, A.; Soriano, S.M.; Song, M.; Chihara, S. Intravenous colistin-induced acute respiratory failure: A case report and a review of literature. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 266–270. [Google Scholar] [CrossRef]
- Decker, D.A.; Fincham, R.W. Respiratory Arrest in Myasthenia Gravis with Colistimethate Therapy. Arch. Neurol. 1971, 25, 141–144. [Google Scholar] [CrossRef]
- Gales, A.C.; Reis, A.O.; Jones, R.N. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: Review of available interpretative criteria and quality control guidelines. J. Clin. Microbiol. 2001, 39, 183–190. [Google Scholar] [CrossRef]
- Jansson, B.; Karvanen, M.; Cars, O.; Plachouras, D.; Friberg, L.E. Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J. Pharm. Biomed. Anal. 2009, 49, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Decolin, D.; Leroy, P.; Nicolas, A.; Archimbault, P. Hyphenated liquid chromatographic method for the determination of colistin residues in bovine tissues. J. Chromatogr. Sci. 1997, 35, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Wootton, M.; Holt, H.; Macgowan, A. Development of a novel assay method for colistin sulphomethate. Clin. Microbiol. Infect. 2005, 11, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.J.; Yin, E.J. Microbioassay of antimicrobial agents. Appl. Microbiol. 1970, 19, 573–579. [Google Scholar] [PubMed]
- Gobin, P.; Lemaître, F.; Marchand, S.; Couet, W.; Olivier, J.-C. Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob. Agents Chemother. 2010, 54, 1941–1948. [Google Scholar] [CrossRef]
- Domes, C.; Domes, R.; Popp, J.; Pletz, M.W.; Frosch, T. Ultrasensitive Detection of Antiseptic Antibiotics in Aqueous Media and Human Urine Using Deep UV Resonance Raman Spectroscopy. Anal. Chem. 2017, 89, 9997–10003. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Clarot, I.; Storme-Paris, I.; Chaminade, P.; Estevenon, O.; Nicolas, A.; Rieutord, A. Simultaneous quantitation of tobramycin and colistin sulphate by HPLC with evaporative light scattering detection. J. Pharm. Biomed. Anal. 2009, 50, 64–67. [Google Scholar] [CrossRef]
- Grande, B.C.; Falcón, M.G.; Perez-Lamela, C.; Comesaña, M.R.; Gándara, J.S. Quantitative analysis of colistin and tiamulin in liquid and solid medicated premixes by HPLC with diode-array detection. Chromatographia 2001, 53, S460–S463. [Google Scholar] [CrossRef]
- Gikas, E.; Bazoti, F.N.; Katsimardou, M.; Anagnostopoulos, D.; Papanikolaou, K.; Inglezos, I.; Skoutelis, A.; Daikos, G.L.; Tsarbopoulos, A. Determination of colistin A and colistin B in human plasma by UPLC–ESI high resolution tandem MS: Application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2013, 83, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Morovján, G.; Csokan, P.; Nemeth-Konda, L. HPLC determination of colistin and aminoglycoside antibiotics in feeds by post-column derivatization and fluorescence detection. Chromatographia 1998, 48, 32–36. [Google Scholar] [CrossRef]
- Pfeifer, C.; Fassauer, G.; Gerecke, H.; Jira, T.; Remane, Y.; Frontini, R.; Byrne, J.; Reinhardt, R. Purity determination of amphotericin B, colistin sulfate and tobramycin sulfate in a hydrophilic suspension by HPLC. J. Chromatogr. B 2015, 990, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.M.; Ly, N.; Anderson, D.; Yang, J.C.; Macander, L.; Jarkowski, A., III; Forrest, A.; Bulitta, J.B.; Tsuji, B.T. Resurgence of colistin: A review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Chepyala, D.; Tsai, I.-L.; Sun, H.-Y.; Lin, S.-W.; Kuo, C.-H. Development and validation of a high-performance liquid chromatography-fluorescence detection method for the accurate quantification of colistin in human plasma. J. Chromatogr. B 2015, 980, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Spapen, H.; Jacobs, R.; Van Gorp, V.; Troubleyn, J.; Honoré, P.M. Renal and neurological side effects of colistin in critically ill patients. Ann. Intensive Care 2011, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Li, J. Colistin in the 21st century. Curr. Opin. Infect. Dis. 2009, 22, 535. [Google Scholar] [CrossRef]
- Ng, J.; Gosbell, I.B.; Kelly, J.A.; Boyle, M.J.; Ferguson, J.K. Cure of multiresistant Acinetobacter baumannii central nervous system infections with intraventricular or intrathecal colistin: Case series and literature review. J. Antimicrob. Chemother. 2006, 58, 1078–1081. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).