Antifungal Agents Based on Chitosan Oligomers, ε-polylysine and Streptomyces spp. Secondary Metabolites against Three Botryosphaeriaceae Species
Abstract
1. Introduction
2. Results
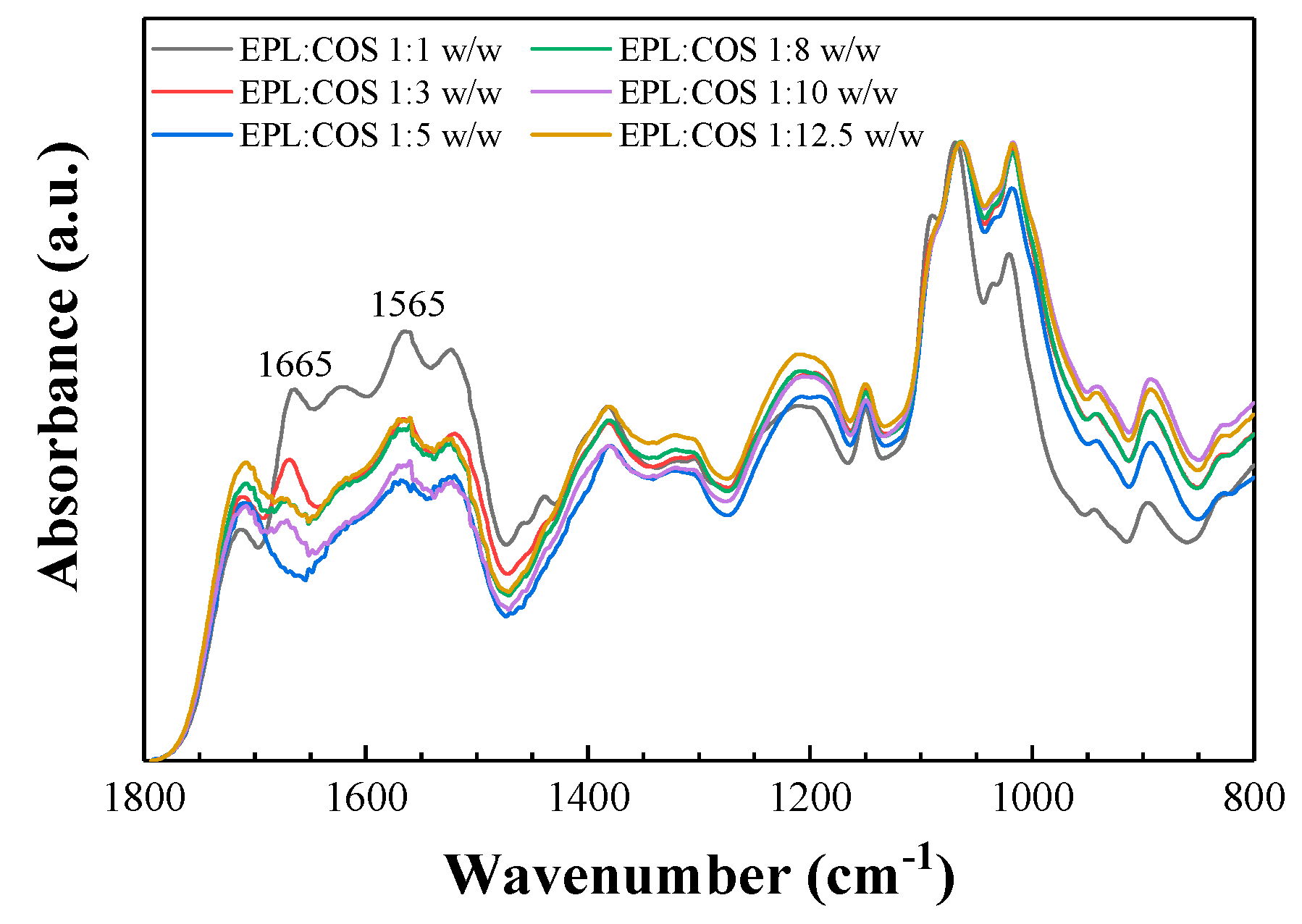
2.1. Vibrational Analysis of the ε-polylysine: Chitosan Conjugates
2.2. Mycelial Growth Inhibition Tests
3. Discussion
3.1. Efficacy of the Treatments
3.2. Mechanism of Action
3.3. Applicability to GTDs in vivo
3.4. Significance of the Reported Findings
4. Materials and Methods
4.1. Reagents, Bacteria and Fungi
4.2. Equipment
4.3. Preparation of Chitosan Oligomers
4.4. ε-polylysine Treatment
4.5. Synthesis of ε-polylysine: Chitosan Oligomers Conjugates
4.6. Secondary Metabolites Production from Streptomyces spp. Strains
4.7. Synthesis of Chitosan Oligomers-secondary Metabolites Inclusion Compounds
4.8. In vitro Mycelial Growth Inhibition Tests
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guerin, D.L.; Fontaine, F.; Mugnai, L. Grapevine trunk disease in European and Mediterranean vineyards: Occurrence, distribution and associated disease-affecting cultural factors. Phytopathol. Mediterr. 2019, 58, 49–71. [Google Scholar]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Nascimento, T.; Rego, C.; Oliveira, H. Potential use of chitosan in the control of grapevine trunk diseases. Phytopathol. Mediterr. 2007, 46, 218–224. [Google Scholar]
- Cobos, R.; Mateos, R.M.; Álvarez-Pérez, J.M.; Olego, M.A.; Sevillano, S.; González-García, S.; Garzón-Jimeno, E.; Coque, J.J.R.; Brakhage, A.A. Effectiveness of natural antifungal compounds in controlling infection by grapevine trunk disease pathogens through pruning wounds. Appl. Environ. Microbiol. 2015, 81, 6474–6483. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, E.R.; Wallis, C.M.; Baumgartner, K. Grapevine trunk pathogen Neofusicoccum parvum tolerates host phytoalexin phenolic compounds. Phytopathology 2018, 108, 46. [Google Scholar]
- Compant, S.; Brader, G.; Muzammil, S.; Sessitsch, A.; Lebrihi, A.; Mathieu, F. Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. BioControl 2012, 58, 435–455. [Google Scholar] [CrossRef]
- Liang, C.; Yuan, F.; Liu, F.; Wang, Y.; Gao, Y. Structure and antimicrobial mechanism of ε-polylysine—Chitosan conjugates through Maillard reaction. Int. J. Biol. Macromol. 2014, 70, 427–434. [Google Scholar] [CrossRef]
- Álvarez-Pérez, J.M.; González-García, S.; Cobos, R.; Olego, M.Á.; Ibañez, A.; Díez-Galán, A.; Garzón-Jimeno, E.; Coque, J.J.R.; Master, E.R. Use of endophytic and rhizosphere actinobacteria from grapevine plants to reduce nursery fungal graft infections that lead to young grapevine decline. Appl. Environ. Microbiol. 2017, 83, e01564-17. [Google Scholar] [CrossRef]
- Dalmas, F.; Astarita, L.; DeFilippis, L.; Magel, E.; Fiedler, H.P.; Bauer, R.; Hampp, R. Growth inhibition of an Araucaria angustifolia (Coniferopsida) fungal seed pathogen, Neofusicoccum parvum, by soil streptomycetes. BMC. Microbiol. 2013, 13, 168. [Google Scholar] [CrossRef]
- Andreolli, M.; Zapparoli, G.; Angelini, E.; Lucchetta, G.; Lampis, S.; Vallini, G. Pseudomonas protegens MP12: A plant growth-promoting endophytic bacterium with broad-spectrum antifungal activity against grapevine phytopathogens. Microbiol. Res. 2019, 219, 123–131. [Google Scholar] [CrossRef]
- Daraignes, L.; Gerbore, J.; Yacoub, A.; Dubois, L.; Romand, C.; Zekri, O.; Roudet, J.; Chambon, P.; Fermaud, M. Efficacy of P. oligandrum affected by its association with bacterial BCAs and rootstock effect in controlling grapevine trunk diseases. Biol. Control. 2018, 119, 59–67. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Abou-Mansour, E.; Courteaux, B.; Rabenoelina, F.; Clément, C.; Fontaine, F.; Aziz, A. Bacillus subtilis PTA-271 counteracts Botryosphaeria dieback in grapevine, triggering immune responses and detoxification of fungal phytotoxins. Front. Plant Sci. 2019, 10, 25. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Martín-García, J.; Diez, J.J.; Flores-Pacheco, J.A.; Martín-Gil, J.; Martín-Ramos, P. Potential control of forest diseases by solutions of chitosan oligomers, propolis and nanosilver. Eur. J. Plant Pathol. 2017, 150, 401–411. [Google Scholar] [CrossRef]
- Matei, P.M.; Martin-Ramos, P.; Sanchez-Bascones, M.; Hernandez-Navarro, S.; Correa-Guimaraes, A.; Navas-Gracia, L.M.; Rufino, C.A.; Ramos-Sanchez, M.C.; Martin-Gil, J. Synthesis of chitosan oligomers/propolis/silver nanoparticles composite systems and study of their activity against Diplodia seriata. Int. J. Polym. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer. 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Umemura, K.; Kawai, S. Modification of chitosan by the Maillard reaction using cellulose model compounds. Carbohydr. Polym. 2007, 68, 242–248. [Google Scholar] [CrossRef]
- Lambert, C.; Bisson, J.; Waffo-Téguo, P.; Papastamoulis, Y.; Richard, T.; Corio-Costet, M.-F.; Mérillon, J.-M.; Cluzet, S. Phenolics and their antifungal role in grapevine wood decay: Focus on the Botryosphaeriaceae family. J. Agric. Food. Chem. 2012, 60, 11859–11868. [Google Scholar] [CrossRef]
- Olmo, D.; Gramaje, D.; Armengol, J. Evaluation of fungicides to protect pruning wounds from Botryosphaeriaceae species infections on almond trees. Phytopathol. Mediterr. 2017, 56, 77–86. [Google Scholar]
- Pitt, W.M.; Sosnowski, M.R.; Huang, R.; Qiu, Y.; Steel, C.C.; Savocchia, S. Evaluation of fungicides for the management of Botryosphaeria canker of grapevines. Plant. Dis. 2012, 96, 1303–1308. [Google Scholar] [CrossRef]
- Tennakoon, K.M.S.; Ridgway, H.J.; Jaspers, M.V.; Langford, G.; Eirian Jones, E. Evaluation of fungicide efficacy against Neofusicoccum species causing dieback disease of blueberries in New Zealand. Australas. Plant Pathol. 2018, 48, 75–84. [Google Scholar] [CrossRef]
- Torres, C.; Latorre, B.A.; Undurraga, P.; Besoain, X. Evaluation of DMI fungicides against species of Diplodia and Neofusicoccum associated with Botryosphaeria canker of grapevine. Cienc. Investig. Agrar. 2013, 40, 131–138. [Google Scholar] [CrossRef]
- Ahmad, H.; Rajagopal, K.; Shah, A.H.; Bhat, A.H.; Venugopal, K. Study of bio-fabrication of iron nanoparticles and their fungicidal property against phytopathogens of apple orchards. IET. Nanobiotechnol. 2017, 11, 230–235. [Google Scholar] [CrossRef]
- Khatami, M.; Nejad, M.S.; Almani, P.G.N.; Salari, S. Plant-mediated green synthesis of silver nanoparticles using Trifolium resupinatum seed exudate and their antifungal efficacy on Neofusicoccum parvum and Rhizoctonia solani. IET. Nanobiotechnol. 2016, 10, 237–243. [Google Scholar] [CrossRef]
- Khatami, M.; Mortazavi, S.M.; Kishani-Farahani, Z.; Amini, A.; Amini, E.; Heli, H. Biosynthesis of silver nanoparticles using pine pollen and evaluation of the antifungal efficiency. Iran. J. Biotechnol. 2017, 15, 95–101. [Google Scholar] [CrossRef]
- Ammad, F.; Moumen, O.; Gasem, A.; Othmane, S.; Hisashi, K.N.; Zebib, B.; Merah, O. The potency of lemon (Citrus limon L.) essential oil to control some fungal diseases of grapevine wood. C. R. Biol. 2018, 341, 97–101. [Google Scholar] [CrossRef]
- Harada, S.; Okada, J.; Takeda, M.; Yamazaki, T. Inclusion compounds of lankacidin-group antibiotics with cyclodextrins. J. Antibiot. 1985, 38, 877–885. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs. 2010, 8, 1305–1322. [Google Scholar] [CrossRef]
- Wu, Q.X.; Lin, D.Q.; Yao, S.J. Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes. Mar. Drugs. 2014, 12, 6236–6253. [Google Scholar] [CrossRef]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs. 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, H.; Zhang, W.; Cui, G.; Zhu, J.; Duan, J. Chitosan coupling makes microbial biofilms susceptible to antibiotics. Sci. Rep. 2013, 3, 3364. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Vad, B.S.; Stenvang, M.; Otzen, D.E.; Meyer, R.L. The antimicrobial mechanism of action of epsilon-poly-l-lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770. [Google Scholar] [CrossRef]
- Ye, R.; Xu, H.; Wan, C.; Peng, S.; Wang, L.; Xu, H.; Aguilar, Z.P.; Xiong, Y.; Zeng, Z.; Wei, H. Antibacterial activity and mechanism of action of ε-poly-l-lysine. Biochem. Biophys. Res. Commun. 2013, 439, 148–153. [Google Scholar] [CrossRef]
- Mitchell, D.J.; Steinman, L.; Kim, D.T.; Fathman, C.G.; Rothbard, J.B. Polyarginine enters cells more efficiently than other polycationic homopolymers. Int. J. Pept. Res. Ther. 2000, 56, 318–325. [Google Scholar] [CrossRef]
- Mutschler, A.; Tallet, L.; Rabineau, M.; Dollinger, C.; Metz-Boutigue, M.H.; Schneider, F.; Senger, B.; Vrana, N.E.; Schaaf, P.; Lavalle, P. Unexpected bactericidal activity of poly(arginine)/hyaluronan nanolayered coatings. Chem. Mater. 2016, 28, 8700–8709. [Google Scholar] [CrossRef]
- Andreev, K.; Bianchi, C.; Laursen, J.S.; Citterio, L.; Hein-Kristensen, L.; Gram, L.; Kuzmenko, I.; Olsen, C.A.; Gidalevitz, D. Guanidino groups greatly enhance the action of antimicrobial peptidomimetics against bacterial cytoplasmic membranes. Biochim. Biophys. Acta. 2014, 1838, 2492–2502. [Google Scholar] [CrossRef]
- Cutrona, K.J.; Kaufman, B.A.; Figueroa, D.M.; Elmore, D.E. Role of arginine and lysine in the antimicrobial mechanism of histone-derived antimicrobial peptides. FEBS Lett. 2015, 589, 3915–3920. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop. Protect. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F.; Mugnai, L. First studies on the potential of a copper formulation for the control of leaf stripe disease within esca complex in grapevine. Phytopathol. Mediterr. 2011, 50, S300–S309. [Google Scholar]
- Darrieutort, G.; Lecomte, P. Evaluation of a trunk injection technique to control grapevine wood diseases. Phytopathol. Mediterr. 2007, 46, 50–57. [Google Scholar]
- Di Marco, S.; Mazzullo, A.; Calzarano, F.; Cesari, A. The control of esca: Status and perspectives. Phytopathol. Mediterr. 2000, 39, 232–240. [Google Scholar]
- Rolshausen, P.E.; Úrbez-Torres, J.R.; Rooney-Latham, S.; Eskalen, A.; Smith, R.J.; Gubler, W.D. Evaluation of pruning wound susceptibility and protection against fungi associated with grapevine trunk diseases. Am. J. Enol. Vitic. 2010, 61, 113–119. [Google Scholar]
- Roblin, G.; Luini, E.; Fleurat-Lessard, P.; Larignon, P.; Berjeaud, J.M. Towards a preventive and/or curative treatment of esca in grapevine trunk disease: General basis in the elaboration of treatments to control plant pathogen attacks. Crop Protect. 2019, 116, 156–169. [Google Scholar] [CrossRef]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Arjona-Girona, I.; Ruano-Rosa, D.; López-Herrera, C.J. Identification, pathogenicity and distribution of the causal agents of dieback in avocado orchards in Spain. Span. J. Agric. Res. 2019, 17, e1003. [Google Scholar] [CrossRef]
- Moral, J.; Agustí-Brisach, C.; Pérez-Rodríguez, M.; Xaviér, C.; Raya, M.C.; Rhouma, A.; Trapero, A. Identification of fungal species associated with branch dieback of olive and resistance of table cultivars to Neofusicoccum mediterraneum and Botryosphaeria dothidea. Plant. Dis. 2017, 101, 306–316. [Google Scholar] [CrossRef]
- Nunn, S.; Nishikida, K. Advanced ATR Correction Algorithm-Application Note 50581; Thermo Scientific: Madison, WI, USA, 2008; p. 4. [Google Scholar]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2017, 36, 57–67. [Google Scholar] [CrossRef]
- Babiker, E.E. Effect of chitosan conjugation on the functional properties and bactericidal activity of gluten peptides. Food. Chem. 2002, 79, 367–372. [Google Scholar] [CrossRef]
- Song, Y.; Babiker, E.E.; Usui, M.; Saito, A.; Kato, A. Emulsifying properties and bactericidal action of chitosan–lysozyme conjugates. Food. Res. Int. 2002, 35, 459–466. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Dehnad, A.; Shanebandi, D.; Khalili, I.; Razmarayii, N.; Namvaran, A.J.V.R.C. Identification and characterization of a Streptomyces sp. isolate exhibiting activity against multidrug-resistant coagulase-negative Staphylococci. Vet. Res. Commun. 2011, 35, 477–486. [Google Scholar] [CrossRef]
- Pazhanimurugan, R.; Radhakrishnan, M.; Shanmugasundaram, T.; Gopikrishnan, V.; Balagurunathan, R. Terpenoid bioactive compound from Streptomyces rochei (M32): Taxonomy, fermentation and biological activities. World. J. Microbiol. Biotechnol. 2016, 32, 161. [Google Scholar] [CrossRef]
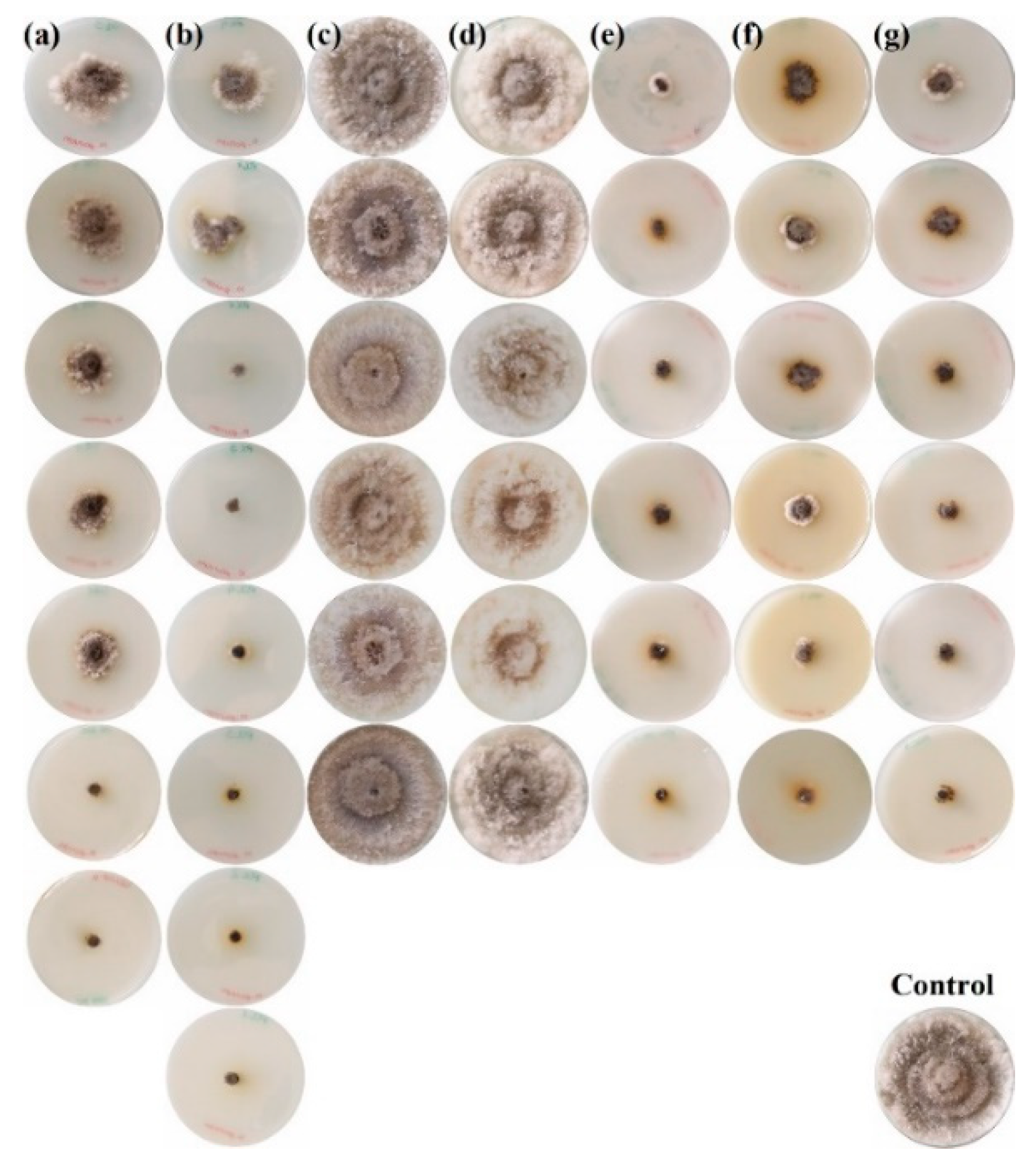
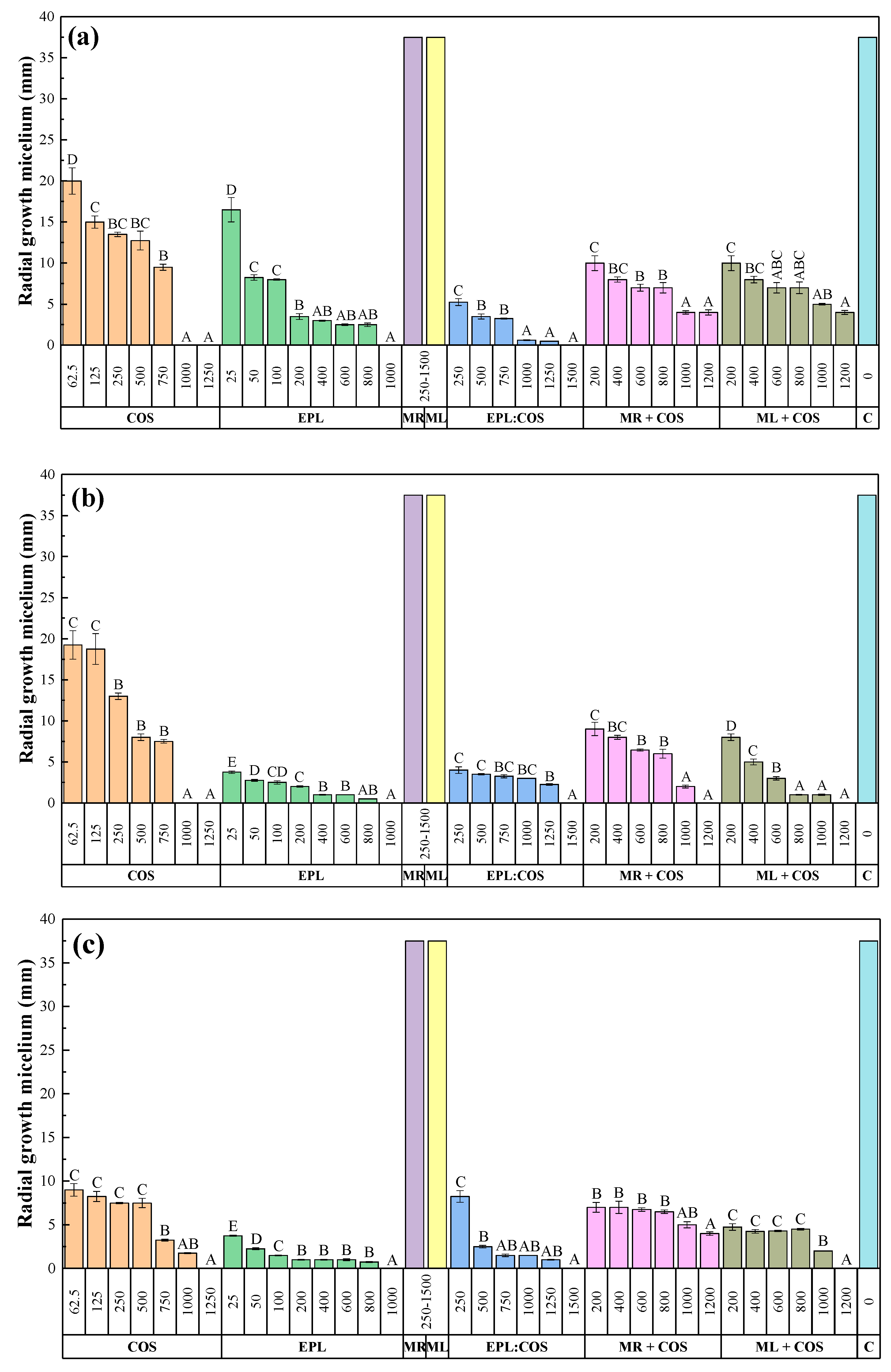
| Pathogen | Concentration (µg·mL−1) | Treatment | ||||
|---|---|---|---|---|---|---|
| COS | EPL | EPL:COS | MR + COS | ML + COS | ||
| N. parvum | EC50 | 60.7 | 16.0 | 11.2 | 67.2 | 46.7 |
| EC90 | 1270.0 | 227.0 | 507.5 | 2074.2 | 1101.7 | |
| D. seriata | EC50 | 94.3 | 0.3 | 11.6 | 45.1 | 30.7 |
| EC90 | 1120.7 | 26.9 | 580.2 | 906.9 | 498.2 | |
| B. dothidea | EC50 | 1.8 | 0.4 | 4.2 | 15.8 | 10.7 |
| EC90 | 689.5 | 22.5 | 497.4 | 1019.0 | 490.3 | |
| Fungicide | Fungal Species | Concentration (µg·mL−1) | Inhibition rate (%) | EC50(µg·mL−1) | Ref. |
|---|---|---|---|---|---|
| Tebuconazole | N. parvum | 90 | [18] | ||
| D. seriata | 150 | ||||
| Pyraclostrobin | N. parvum | 100 | |||
| D. seriata | 250 | ||||
| Carbendazim, tebuconazole, iprodione, fludioxonil, fluazinam, flusilazole, penconazole, procymidone, myclobutanil, pyraclostrobin | N. parvum | 360–440 * | [19] | ||
| D. seriata | 530–620 * | ||||
| B. dothidea | 450 * | ||||
| Carbendazim | N. parvum | 40 | [20] | ||
| Tebuconazole | 130 | ||||
| Iprodione | 750 | ||||
| Tecobunazole | D. seriata | 300 | [21] | ||
| Fe NPs (FeNPs + neem leaf extract) | D. seriata | 100 (FeNPs / FeNPs+neem 1:1) | 79/80.3 | [22] | |
| B. dothidea | 83/82.5 | ||||
| AgNPs | N. parvum | 40 | 84 | [23] | |
| AgNPs | 30 | 81 | [24] | ||
| Lemon essential oil (limonene, neral, β-pinene, and γ-terpinene) in DMSO | B. dothidea | 2500 | 48.1 | [25] | |
| Chitosan oligosaccharin (mol. wt. <3 kDa) | Botryosphaeria sp. | 1.56 | [3] | ||
| Chitosan oligosaccharides | D. seriata | 1000 | 100 | [4] | |
| Vanillin | 1000 | 89.8 | |||
| Garlic extract | 40000 | 75.3 |
| Treatment | Concentrations Assayed in the Mycelial Growth Inhibition Tests (μg∙mL−1) |
|---|---|
| COS | 62.5, 125, 250, 500, 750, 1000, 1250, 1500 |
| EPL | 25, 50, 100, 200, 400, 600, 800, 1000 |
| MR | 250, 500, 750, 1000, 1250, 1500 |
| ML | 250, 500, 750, 1000, 1250, 1500 |
| EPL:COS | 250, 500, 750, 1000, 1250, 1500 |
| MR+COS | 200, 400, 600, 800, 1000, 1200 |
| ML+COS | 200, 400, 600, 800, 1000, 1200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzón-Durán, L.; Martín-Gil, J.; Pérez-Lebeña, E.; Ruano-Rosa, D.; Revuelta, J.L.; Casanova-Gascón, J.; Ramos-Sánchez, M.C.; Martín-Ramos, P. Antifungal Agents Based on Chitosan Oligomers, ε-polylysine and Streptomyces spp. Secondary Metabolites against Three Botryosphaeriaceae Species. Antibiotics 2019, 8, 99. https://doi.org/10.3390/antibiotics8030099
Buzón-Durán L, Martín-Gil J, Pérez-Lebeña E, Ruano-Rosa D, Revuelta JL, Casanova-Gascón J, Ramos-Sánchez MC, Martín-Ramos P. Antifungal Agents Based on Chitosan Oligomers, ε-polylysine and Streptomyces spp. Secondary Metabolites against Three Botryosphaeriaceae Species. Antibiotics. 2019; 8(3):99. https://doi.org/10.3390/antibiotics8030099
Chicago/Turabian StyleBuzón-Durán, Laura, Jesús Martín-Gil, Eduardo Pérez-Lebeña, David Ruano-Rosa, José L. Revuelta, José Casanova-Gascón, M. Carmen Ramos-Sánchez, and Pablo Martín-Ramos. 2019. "Antifungal Agents Based on Chitosan Oligomers, ε-polylysine and Streptomyces spp. Secondary Metabolites against Three Botryosphaeriaceae Species" Antibiotics 8, no. 3: 99. https://doi.org/10.3390/antibiotics8030099
APA StyleBuzón-Durán, L., Martín-Gil, J., Pérez-Lebeña, E., Ruano-Rosa, D., Revuelta, J. L., Casanova-Gascón, J., Ramos-Sánchez, M. C., & Martín-Ramos, P. (2019). Antifungal Agents Based on Chitosan Oligomers, ε-polylysine and Streptomyces spp. Secondary Metabolites against Three Botryosphaeriaceae Species. Antibiotics, 8(3), 99. https://doi.org/10.3390/antibiotics8030099