Comparison of Antibiotic Resistance Profile and Biofilm Production of Staphylococcus aureus Isolates Derived from Human Specimens and Animal-Derived Samples
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Susceptibility of S. aureus Human Isolates
2.2. Antimicrobial Susceptibility of Animal-Derived S. aureus Isolates
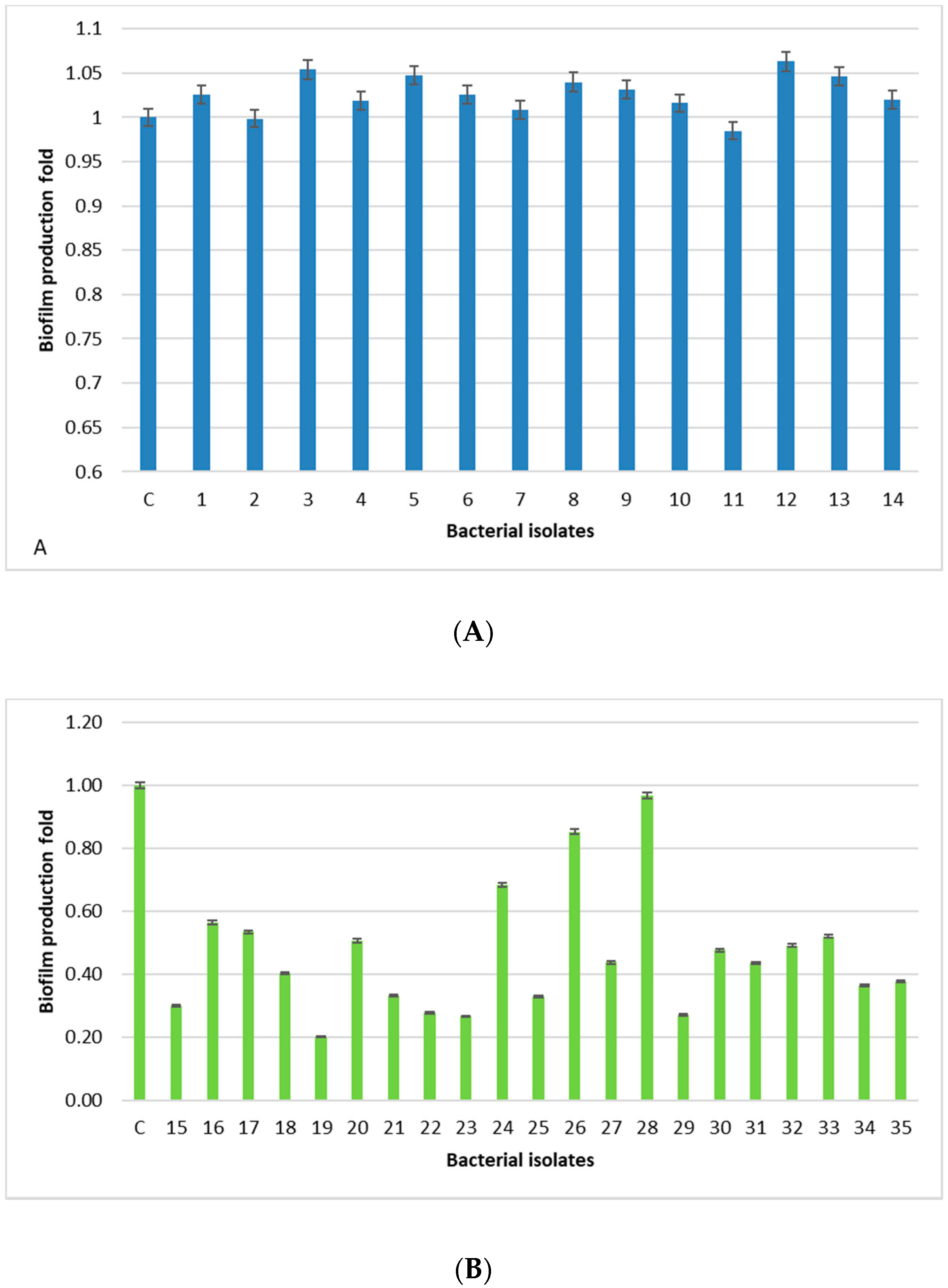
2.3. Biofilm Production
2.4. Detection of Virulence and Biofilm Related Genes of S. aureus
3. Discussion
4. Materials and Methods
4.1. Clinical Sampling
4.2. Antimicrobial Susceptibility Tests of Bacterial Isolates
4.3. Detection of SE (sea-see, seg-sei, sej, sep), tsst-1, eta, etb, mecA, and Biofilm Related Genes
4.4. Biofilm Formation Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plata, K.E.; Rosato, A.; Wegrzyn, G. Staphylococcus aureus as an infectious agent: Overview of biochemistry and molecular genetics of its pathogenicity. Acta Biochim. Pol. 2009, 56, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Falcão, C.; Viveiros, M.; Machado, D.; Martins, M.; Melo-Cristino, J.; Amaral, L.; Couto, I. Correction: Exploring the contribution of efflux on the resistance to fluoroquinolones in clinical isolates of Staphylococcus aureus. BMC Microbiol. 2011, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Paterson, G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Shang, W.; Hu, X. Staphylococcus aureus ST121: A globally disseminated hypervirulent clone. J. Med. Microbiol. 2015, 64, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Haasnoot, P.J.; De Vries, A. Staphylococcal scalded skin syndrome in a 4-year-old child: A case report. J. Med. Case Rep. 2018, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Scatassa, M.L.; Cardamone, C.; Oliveri, G.; Piraino, C.; Alduina, R.; Napoli, C. Staphylococcal Food Poisoning Case and Molecular Analysis of Toxin Genes in Staphylococcus aureus Strains Isolated from Food in Sicily, Italy. Foodborne Pathog. Dis. 2015, 12, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Seegers, H.; Fourichon, C. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.N.; Vélez, L.A.; Mediavilla, J.R.; Ocampo, A.M.; Vanegas, J.M.; Rodriguez, E.A.; Kreiswirth, B.N.; Correa, M.M. Livestock-associated Methicillin-Susceptible Staphylococcus aureus ST398 Infection in Woman, Colombia. Emerg. Infect. Dis. 2011, 17, 1970–1971. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A. Staphylococcus aureus as a Leading Cause of Foodborne Outbreaks Worldwide. In Staphylococcus aureus; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 129–146. [Google Scholar]
- O’Brien, A.M.; Hanson, B.M.; Farina, S.A.; Wu, J.Y.; Simmering, J.E.; Wardyn, S.E.; Forshey, B.M.; Kulick, M.E.; Wallinga, D.B.; Smith, T.C. MRSA in Conventional and Alternative Retail Pork Products. PLoS ONE 2012, 7, e30092. [Google Scholar] [CrossRef]
- Velasco, V.; Buyukcangaz, E.; Sherwood, J.S.; Stepan, R.M.; Koslofsky, R.J.; Logue, C.M. Characterization of Staphylococcus aureus from Humans and a Comparison with İsolates of Animal Origin, in North Dakota, United States. PLoS ONE 2015, 20, e0140497. [Google Scholar] [CrossRef]
- Asanin, J.; Misic, D.; Aksentijevic, K.; Tambur, Z.; Rakonjac, B.; Kovacevic, I.; Spergser, J.; Loncaric, I. Genetic Profiling and Comparison of Human and Animal Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Serbia. Antibiotics 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Khoramian, B.; Jabalameli, F.; Niasari-Naslaji, A.; Taherikalani, M.; Emaneini, M. Comparison of virulence factors and biofilm formation among Staphylococcus aureus strains isolated from human and bovine infections. Microb. Pathog. 2015, 88, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Normanno, G.; La Salandra, G.; Dambrosio, A.; Quaglia, N.; Corrente, M.; Parisi, A.; Santagada, G.; Firinu, A.; Crisetti, E.; Celano, G.V. Occurrence, characterization and antimicrobial resistance of enterotoxigenic Staphylococcus aureus isolated from meat and dairy products. Int. J. Food Microbiol. 2007, 115, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.E.; Contente-Cuomo, T.; Buchhagen, J.; Liu, C.M.; Watson, L.; Pearce, K.; Foster, J.T.; Bowers, J.; Driebe, E.M.; Engelthaler, D.M.; et al. Multidrug-Resistant Staphylococcus aureus in US Meat and Poultry. Clin. Infect. Dis. 2011, 52, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Antoci, E.; Pinzone, M.R.; Nunnari, G.; Stefani, S.; Cacopardo, B. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus (MRSA) among subjects working on bovine dairy farms. Infez. Med. 2013, 21, 125–129. [Google Scholar] [PubMed]
- Vitale, M.; Gaglio, S.; Galluzzo, P.; Cascone, G.; Piraino, C.; Presti, V.D.M.L.; Alduina, R. Antibiotic Resistance Profiling, Analysis of Virulence Aspects and Molecular Genotyping of Staphylococcus aureus Isolated in Sicily, Italy. Foodborne Pathog. Dis. 2018, 15, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Chowdhury, R.; Datta, M.; Chowdhury, G.; Mukhopadhyay, A.K. Characterization of the clonal profile of methicillin resistant Staphylococcus aureus isolated from patients with early post-operative orthopedic implant based infections. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Parisi, T.A.; Caruso, M.; Normanno, G.; Latorre, L.; Miccolupo, A.; Fraccalvieri, R.; Intini, F.; Manginelli, T.; Santagada, G. MRSA in swine, farmers and abattoir workers in Southern Italy. Food Microbiol. 2019, 82, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Campanile, F.; Bongiorno, D.; Perez, M.; Mongelli, G.; Sessa, L.; Benvenuto, S.; Gona, F.; AMCLI—S. aureus Survey Participants; Varaldo, P.E.; Stefani, S. Epidemiology of Staphylococcus aureus in Italy: First nationwide survey, 2012. J. Glob. Antimicrob. 2015, 3, 247–254. [Google Scholar] [CrossRef]
- Belmamoun, A.R.; Reguig, K.B.; Bouazza, S.; Mustapha, M.D. Subclinical mastitis on the raw milk as a risk factor for the transmission of Staphylococcus aureus and coagulase-negative staphylococci, multidrug resistance in Sidi Bel Abbes, Algeria. Adv. Environ. Biol. 2016, 10, 1–11. [Google Scholar]
- Ed-Dra, A.; Filali, F.R.; Bouymajane, A.; Benhallam, F.; El Allaoui, A.; Chaiba, A.; Giarratana, F. Antibiotic Susceptibility profile of Staphylococcus aureus isolated from sausages in Meknes, Morocco. Vet. World 2018, 11, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Matallah, A.M.; Bouayad, L.; Boudjellaba, S.; Mebkhout, F.; Hamdi, T.M.; Ramdani-Bouguessa, N. Staphylococcus aureus isolated from selected dairies of Algeria: Prevalence and susceptibility to antibiotics. Vet. World 2019, 12, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Cucarella, C.; Tormo, M.A.; Ubeda, C.; Trotonda, M.P.; Monzón, M.; Peris, C.; Amorena, B.; Lasa, I.; Penades, J.R. Role of Biofilm-Associated Protein Bap in the Pathogenesis of Bovine Staphylococcus aureus. Infect. Immun. 2004, 72, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.; Jularic, M.; Horsburgh, S.M.; Hirschhausen, N.; Neumann, C.; Bertling, A.; Schulte, A.; Foster, S.; Kehrel, B.E.; Peters, G.; et al. Molecular Characterization of a Novel Staphylococcus aureus Surface Protein (SasC) Involved in Cell Aggregation and Biofilm Accumulation. PLoS ONE 2009, 4, e7567. [Google Scholar] [CrossRef] [PubMed]
- Grispoldi, L.; Massetti, L.; Sechi, P.; Iulietto, M.F.; Ceccarelli, M.; Karama, M.; Popescu, P.A.; Pandolfi, F.; Cenci-Goga, B.T. Short communication: Characterization of enterotoxin-producing Staphylococcus aureus isolated from mastitic cows. J. Dairy Sci. 2019, 102, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Giardina, A.; Alduina, R.; Gottardi, E.; Di Caro, V.; Sussmuth, R.D.; Puglia, A.M. Two heterologously expressed Planobispora rosea proteins cooperatively induce Streptomyces lividans thiostrepton uptake and storage from the extracellular medium. Microb. Cell. Fact. 2010, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST), version 9.0. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf (accessed on 1 January 2019).
- Mehrotra, M.; Wang, G.; Johnson, W.M. Multiplex PCR for Detection of Genes for Staphylococcus aureus Enterotoxins, Exfoliative Toxins, Toxic Shock Syndrome Toxin 1, and Methicillin Resistance. J. Clin. Microbiol. 2000, 38, 1032–1035. [Google Scholar] [PubMed]
- Gilot, P.; Lina, G.; Cochard, T.; Poutrel, B. Analysis of the Genetic Variability of Genes Encoding the RNA III-Activating Components Agr and TRAP in a Population of Staphylococcus aureus Strains Isolated from Cows with Mastitis. J. Clin. Microbiol. 2002, 40, 4060–4067. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 30, 47. [Google Scholar]
- Shukla, S.K.; Rao, T.S. An Improved Crystal Violet Assay for Biofilm Quantification in 96-Well Microtitre Plate. Microbiology 2017. [Google Scholar] [CrossRef]
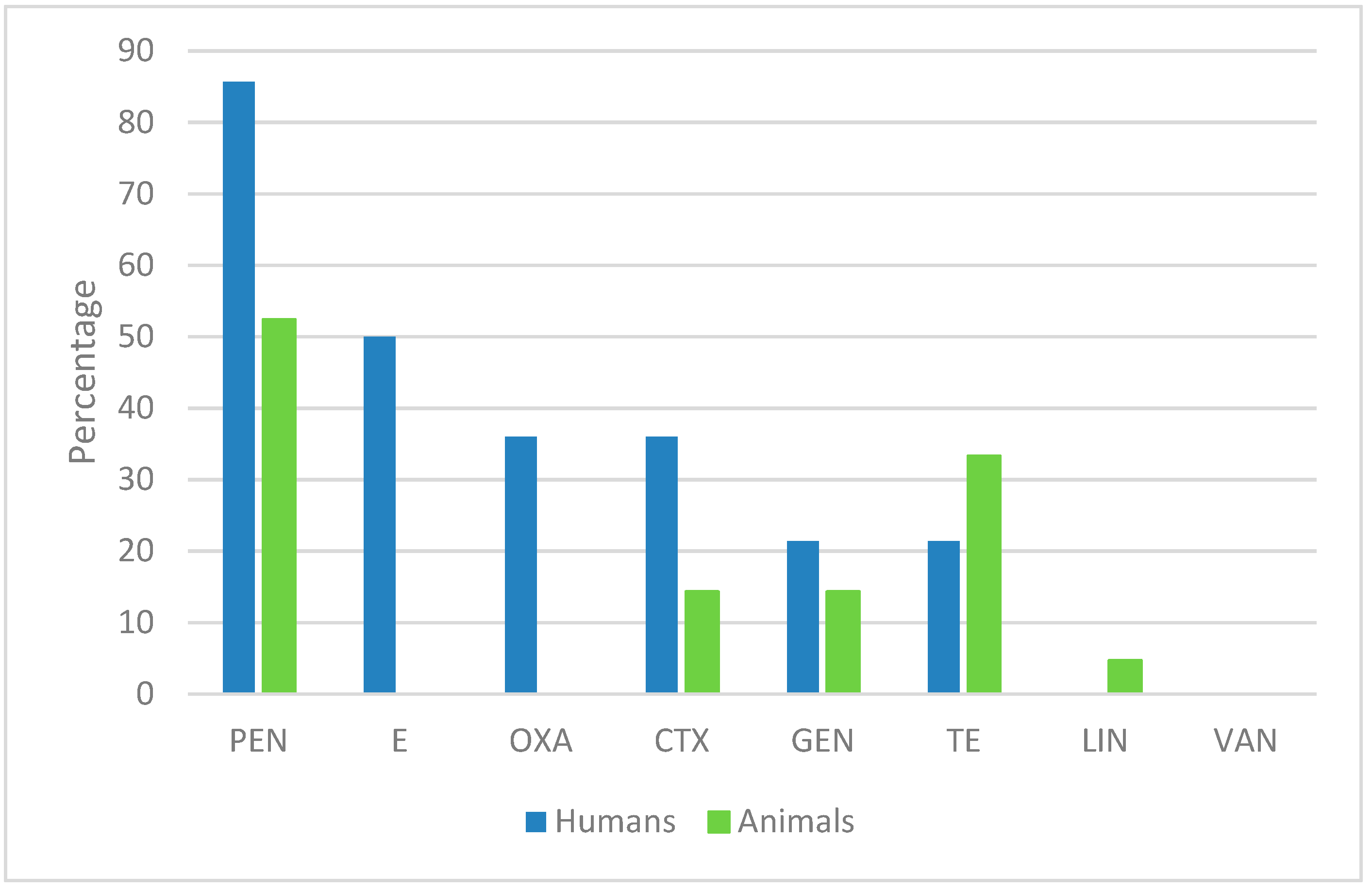
| Isolate | Sample | PEN | E | CLIN | OXA | FAC | CTX | CIP | MFA | AMC | GEN | TE | SXT | LZD | VAN | TIG | AF | Cefoxitin Screening |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nail injury | R | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | N |
| 2 | Generic swab | R | R | R | S | S | S | R | R | S | R | S | S | S | S | S | S | N |
| 3 | Endoarticular liquid | R | R | R | R | R | R | R | R | R | S | S | S | S | S | S | S | P |
| 4 | Urine culture | R | S | S | S | S | S | R | R | S | S | S | S | S | S | S | S | N |
| 5 | Sore | R | R | R | R | R | R | R | R | R | R | S | R | S | S | S | S | P |
| 6 | Sputum | S | R | R | S | S | S | S | S | S | R | S | S | S | S | S | S | N |
| 7 | Sputum | R | S | S | S | S | S | R | R | S | S | S | S | S | S | S | S | N |
| 8 | Pharyngeal swab | R | R | S | S | S | S | S | S | S | S | R | S | S | S | S | S | N |
| 9 | Pharyngeal swab | R | S | S | R | R | R | S | S | R | S | S | I | S | S | S | S | P |
| 10 | Pharyngeal swab | R | ND | S | S | S | S | S | S | S | S | S | S | S | S | S | S | N |
| 11 | Pharyngeal swab | R | R | R | R | R | R | S | S | R | S | R | S | S | S | S | S | P |
| 12 | Pharyngeal swab | R | R | R | R | R | R | S | S | R | S | R | S | S | S | S | S | P |
| 13 | Pharyngeal swab | R | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | N |
| 14 | Pharyngeal swab | S | ND | ND | ND | ND | ND | ND | ND | S | ND | S | ND | ND | S | ND | ND | ND |
| Isolate | Sample | PEN | E | OXA | CTX | GEN | TE | LIN | VAN |
|---|---|---|---|---|---|---|---|---|---|
| 15 | Cow milk | S | S | S | S | S | S | I | S |
| 16 | Cow milk | S | I | S | S | S | I | I | S |
| 17 | Cow milk | R | S | S | I | S | R | S | S |
| 18 | Goat milk | R | S | S | S | S | S | S | S |
| 19 | Goat milk | R | I | S | S | R | S | S | S |
| 20 | Sheep milk | S | I | S | S | S | S | S | S |
| 21 | Sheep milk | S | S | S | R | S | R | S | S |
| 22 | Sheep milk | R | I | S | I | S | R | S | S |
| 23 | Sheep milk | S | I | S | S | S | S | S | S |
| 24 | Sheep milk | R | I | S | R | I | I | I | S |
| 25 | Sheep milk | R | S | S | S | R | S | S | S |
| 26 | Sheep milk | R | S | S | I | S | R | S | S |
| 27 | Cheese | R | I | S | I | R | S | R | S |
| 28 | Cheese | R | S | S | S | S | R | S | S |
| 29 | Cheese | S | S | S | S | S | S | S | S |
| 30 | Cheese | R | I | S | I | I | R | I | S |
| 31 | Cheese | S | S | S | S | S | S | S | S |
| 32 | Cheese | S | S | S | S | S | S | S | S |
| 33 | Tuma (cheese) | R | I | S | S | S | R | S | S |
| 34 | Pecorino (cheese) | S | S | S | S | S | S | S | S |
| 35 | Food preparation | S | S | S | S | S | S | S | S |
| Number of Strains | Sensitive to All Antibiotics (%) | Single Resistance (%) | Double Resistance (%) | Multiple (≥3) Resistance (%) | Intermediate Sensitivity (%) | |
|---|---|---|---|---|---|---|
| Humans | 14 | 1 (7.1) | 2 (14.3) | 0 (0) | 11 (78.6) | 0 (0) |
| Animals | 21 | 6 (28.5) | 1 (4.8) | 10 (47.6) | 1 (4.8) | 3 (14.3) |
| Internal ID | Sample | Virulence Genes | Biofilm-related Genes | ||
|---|---|---|---|---|---|
| sea, sec, see | seg-i, sej, sep | tsst-1, eta, etb, mecA | agr, bap, ica, sasC | ||
| 1 | Nail injury | ND | seg, sei | ND | ND |
| 2 | Generic swab | ND | ND | ND | sasC |
| 3 | Endoarticular liquid | ND | sei, seg, sej | mecA | ica, sasC |
| 4 | Urine culture | ND | seg, sei | ND | sasC |
| 5 | Sore | ND | seg, sei | mecA | ND |
| 6 | Sputum | ND | ND | ND | sasC |
| 7 | Sputum | ND | seg, sei | ND | ND |
| 8 | Pharyngeal swab | ND | ND | ND | sasC |
| 9 | Pharyngeal swab | ND | seg, sei | mecA | ND |
| 10 | Pharyngeal swab | ND | ND | tsst-1 | agr, ica, sasC |
| 11 | Pharyngeal swab | ND | seh | mecA | ND |
| 12 | Pharyngeal swab | ND | seh | mecA | sasC |
| 13 | Pharyngeal swab | see | seg, sei | ND | sasC |
| 14 | Pharyngeal swab | ND | seg, sei | eta, etb | ND |
| 15 | Cow milk | see | ND | ND | ica |
| 16 | Cow milk | ND | ND | ND | ica |
| 17 | Cow milk | see | ND | ND | ica, sasC |
| 18 | Goat milk | sec | ND | tsst-1 | ica |
| 19 | Goat milk | ND | ND | etb | ica, sasC |
| 20 | Sheep milk | ND | ND | ND | ica |
| 21 | Sheep milk | ND | ND | ND | ica |
| 22 | Sheep milk | ND | ND | ND | ND |
| 23 | Sheep milk | ND | ND | ND | bap |
| 24 | Sheep milk | ND | ND | ND | ica, sasC |
| 25 | Sheep milk | ND | ND | tsst-1 | ND |
| 26 | Sheep milk | ND | ND | ND | ND |
| 27 | Cheese | ND | ND | ND | ND |
| 28 | Cheese | sea | ND | eta | ica, sasC |
| 29 | Cheese | ND | ND | ND | ica, sasC |
| 30 | Cheese | sea | ND | ND | ica, sasC |
| 31 | Cheese | ND | ND | ND | ica |
| 32 | Cheese | ND | ND | etb | ica, sasC |
| 33 | Cheese | ND | ND | tsst-1 | ica |
| 34 | Cheese | ND | ND | tsst-1 | ica |
| 35 | Food preparation | sea | ND | tsst-1 | bap, ica, sasC |
| Sample | n° |
|---|---|
| Cow milk | 3 |
| Cheese | 8 |
| Endoarticular liquid | 1 |
| Food preparation | 1 |
| Generic swab | 1 |
| Goat milk | 2 |
| Nail injury | 1 |
| Pharyngeal swab | 7 |
| Sheep milk | 7 |
| Sore | 1 |
| Sputum | 2 |
| Urine culture | 1 |
| Gene | Primer | Oligonucleotide Sequence | Size of Amplified Product (bp) |
|---|---|---|---|
| sea | GSEAR-1 | GGTTATCAATGTGCGGGTGG | 102 |
| GSEAR-2 | CGGCACTTTTTTCTCTTCGG | ||
| seb | GSEBR-1 | GTATGGTGGTGTAACTGAGC | 164 |
| GSEBR-2 | CCAAATAGTGACGAGTTAGG | ||
| sec | GSECR-1 | AGATGAAGTAGTTGATGTGTATGG | 451 |
| GSECR-2 | CACACTTTTAGAATCAACCG | ||
| sed | GSEDR-1 | CCAATAATAGGAGAAAATAAAAG | 278 |
| GSEDR-2 | ATTGGTATTTTTTTTCGTTC | ||
| see | GSEER-1 | AGGTTTTTTCACAGGTCATCC | 209 |
| GSEER-2 | CTTTTTTTTCTTCGGTCAATC | ||
| mecA | GMECAR-1 | ACTGCTATCCACCCTCAAAC | 163 |
| GMECAR-2 | CTGGTGAAGTTGTAATCTGG | ||
| eta | GETAR-1 | GCAGGTGTTGATTTAGCATT | 93 |
| GETAR-2 | AGATGTCCCTATTTTTGCTG | ||
| etb | GETBR-1 | ACAAGCAAAAGAATACAGCG | 226 |
| GETBR-2 | GTTTTTGGCTGCTTCTCTTG | ||
| tst | GTSSTR-1 | ACCCCTGTTCCCTTATCATC | 326 |
| GTSSTR-2 | TTTTCAGTATTTGTAACGCC | ||
| ica | icaH-1m | TATACCTTTCTTCGATGTCG | 700 |
| icaH-7c | CTTTCGTTATAACAGGCAAG | ||
| bap | sasp-6m | CCCTATATCGAAGGTGTAGAATTGCAC | 1000 |
| sasp-7c | GCTGTTGAAGTTAATACTGTACCTGC | ||
| sasC | CHsasC1for | GCAACGAATCAAGCATTGG | 600 |
| CHsasC1rev | TGACAGCACTTCGTTAGG | ||
| agr | agrB1 | TATGCTCCTGCAGCAACTAA | 1070 |
| agrC2 | CTTGCGCATTTCGTTGTTGA | ||
| femA | GFEMAR-1 | AAAAAAGCACATAACAAGCG | 132 |
| GFEMAR-2 | GATAAAGAAGAAACCAGCAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, M.; Galluzzo, P.; Buffa, P.G.; Carlino, E.; Spezia, O.; Alduina, R. Comparison of Antibiotic Resistance Profile and Biofilm Production of Staphylococcus aureus Isolates Derived from Human Specimens and Animal-Derived Samples. Antibiotics 2019, 8, 97. https://doi.org/10.3390/antibiotics8030097
Vitale M, Galluzzo P, Buffa PG, Carlino E, Spezia O, Alduina R. Comparison of Antibiotic Resistance Profile and Biofilm Production of Staphylococcus aureus Isolates Derived from Human Specimens and Animal-Derived Samples. Antibiotics. 2019; 8(3):97. https://doi.org/10.3390/antibiotics8030097
Chicago/Turabian StyleVitale, Maria, Paola Galluzzo, Patrizia Giuseppina Buffa, Eleonora Carlino, Orazio Spezia, and Rosa Alduina. 2019. "Comparison of Antibiotic Resistance Profile and Biofilm Production of Staphylococcus aureus Isolates Derived from Human Specimens and Animal-Derived Samples" Antibiotics 8, no. 3: 97. https://doi.org/10.3390/antibiotics8030097
APA StyleVitale, M., Galluzzo, P., Buffa, P. G., Carlino, E., Spezia, O., & Alduina, R. (2019). Comparison of Antibiotic Resistance Profile and Biofilm Production of Staphylococcus aureus Isolates Derived from Human Specimens and Animal-Derived Samples. Antibiotics, 8(3), 97. https://doi.org/10.3390/antibiotics8030097







