Regulation of Geldanamycin Biosynthesis by Cluster-Situated Transcription Factors and the Master Regulator PhoP
Abstract
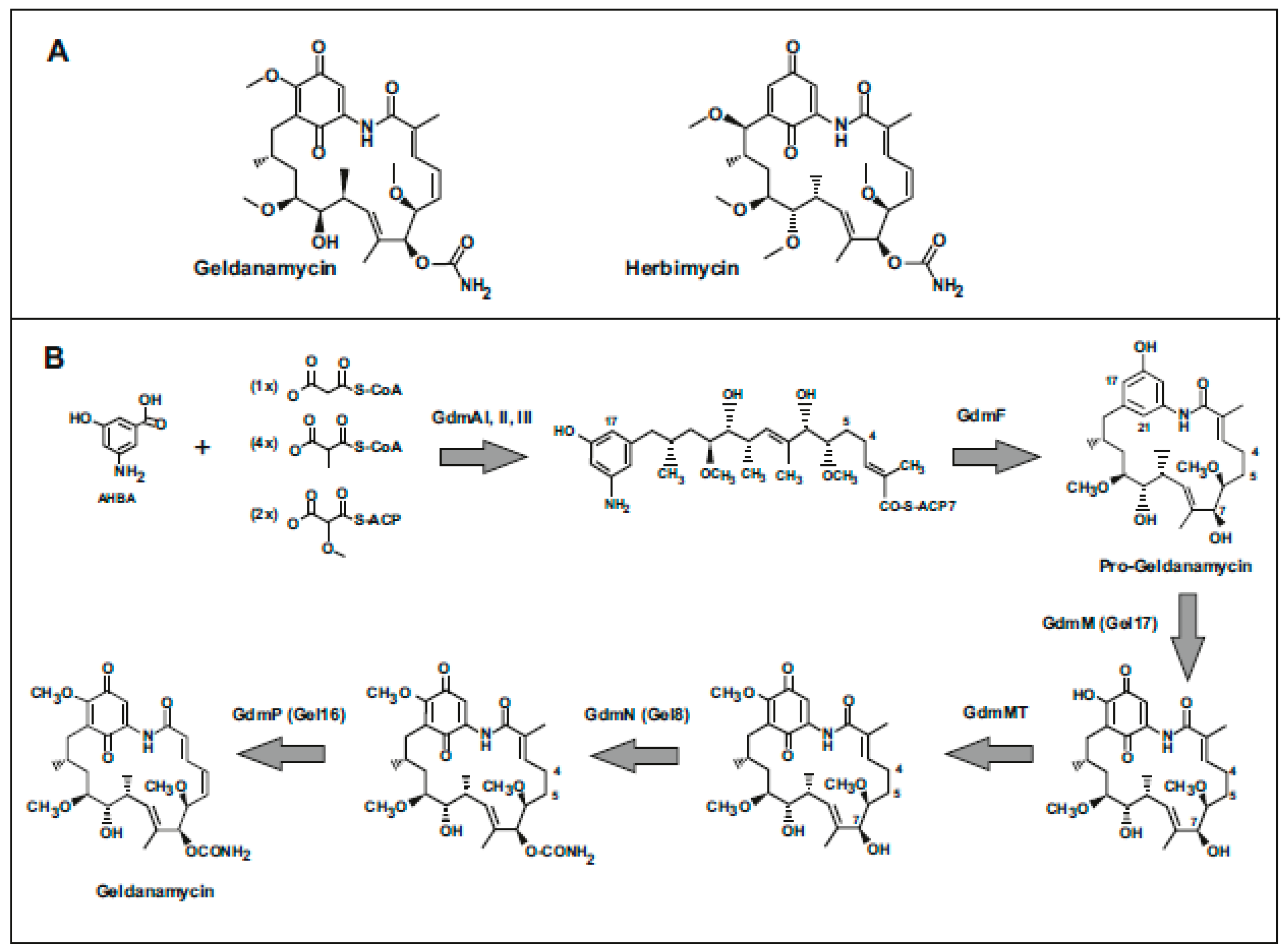
1. Introduction: Antitumor Activity of Geldanamycin and its Derivatives
2. Biosynthesis of Geldanamycins
2.1. Origin and Biosynthesis of the AHBA Unit
2.2. Elongation Steps
2.3. Cyclization of the Lineal Polyketide to Progeldanamycin
2.4. Post-Polyketide Modifications
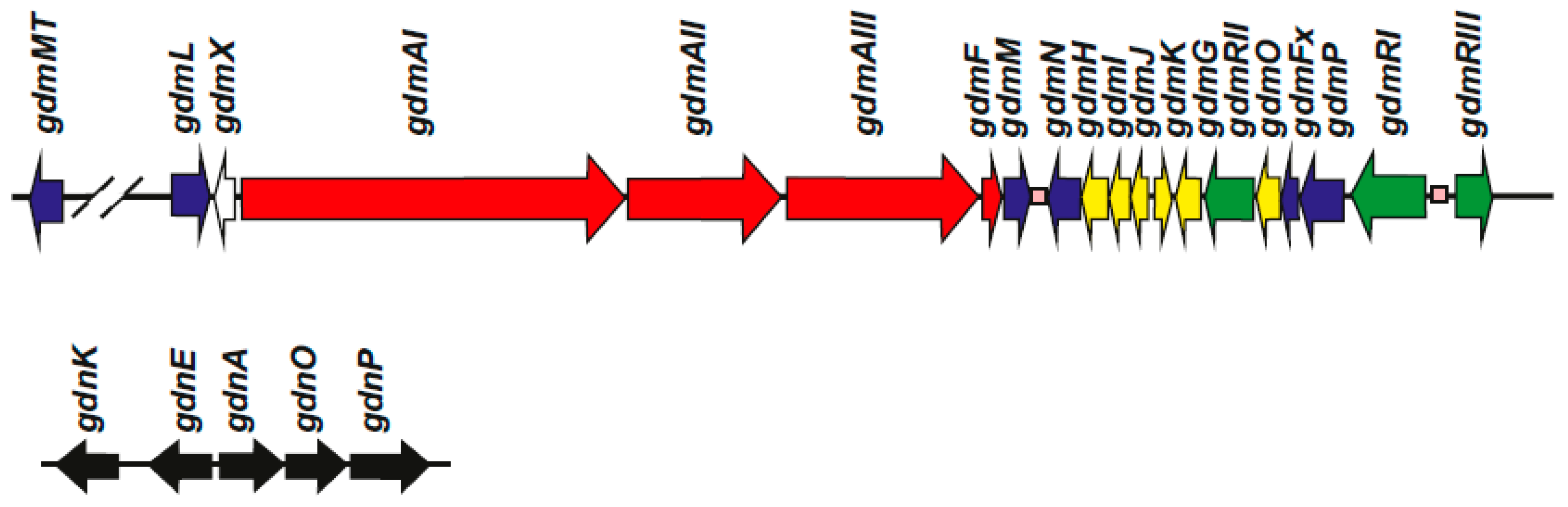
3. Geldanamycin Gene Clusters in Different Streptomyces Species
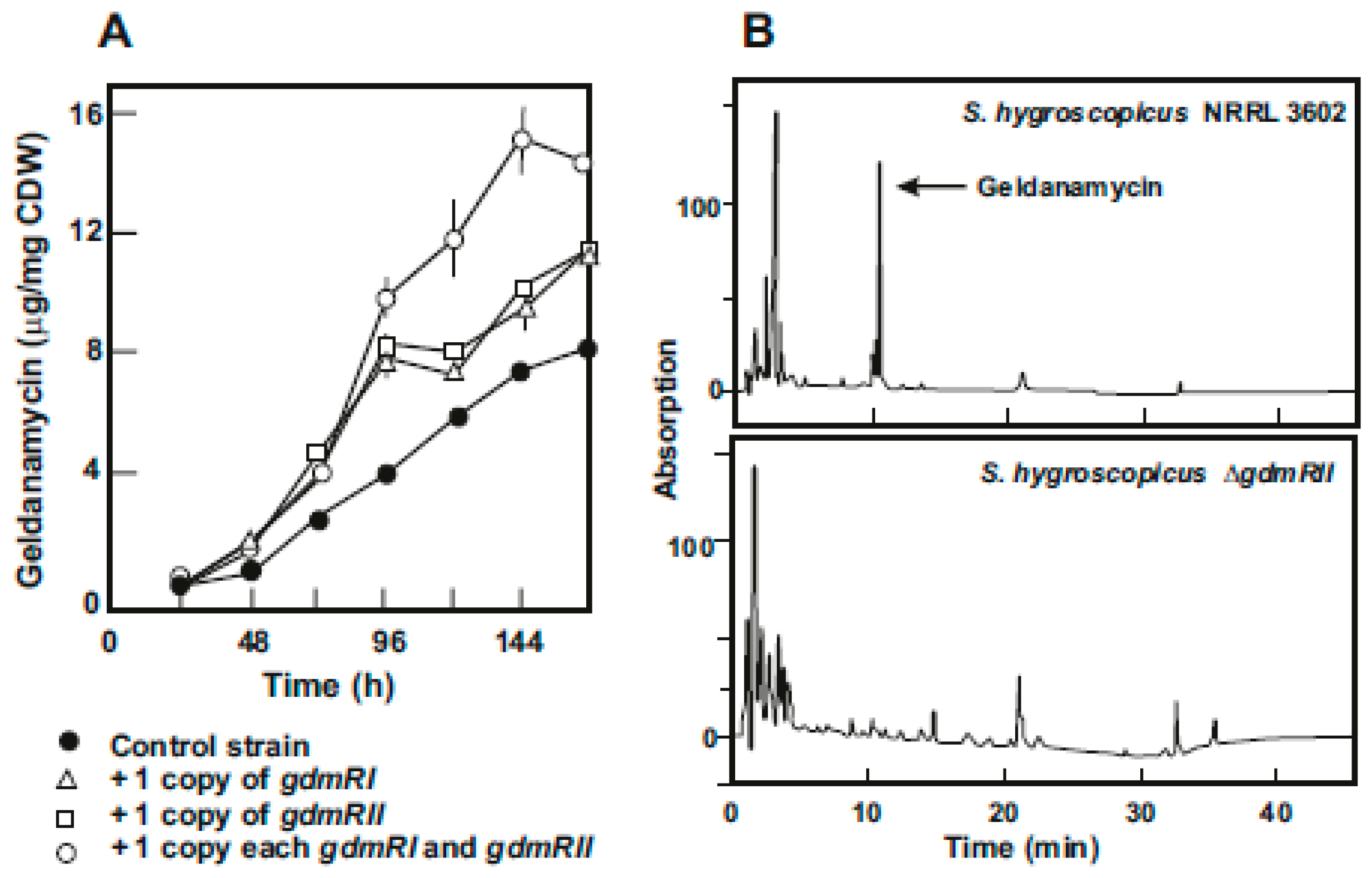
4. Regulatory Genes in the Geldanamycin Gene Cluster
5. Engineering of Polyketide Biosynthesis in Geldanamycin Producing Strains
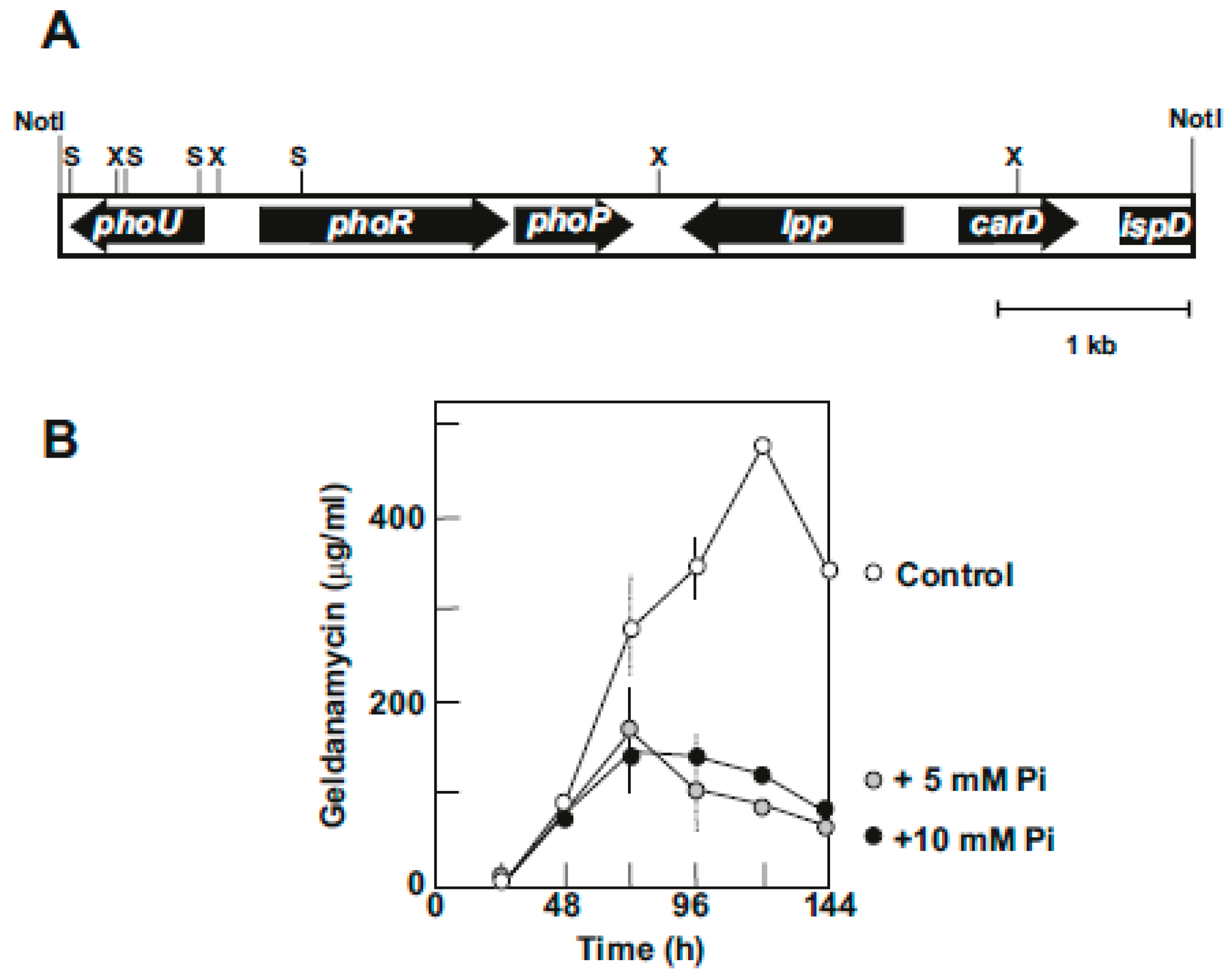
6. Phosphate Control of Geldanamycin Biosynthesis
6.1. Cloning of the phoU- phoRP Gene Cluster of S. hygroscopicus NRRL3602
6.2. Disruption and Characterization of the PhoP Gene: Effect on Growth and Geldanamycin Production
6.3. Identification of PhoP Binding Sequences in S. hygroscopicus Genes
7. Future Outlook
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sasaki, K.; Rinehart, K.L.; Slomp, G.; Grostic, M.F.; Olson, E.C. Geldanamycin. I. Structure assignment. J. Am. Chem. Soc. 1970, 92, 7591–7593. [Google Scholar] [PubMed]
- Fukuyo, Y.; Hunt, C.R.; Horikoshi, N. Geldanamycin and its anti-cancer activities. Cancer Lett. 2010, 290, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Keum, G.; Pae, A.N. Discovery and development of heat shock protein 90 inhibitors as anticancer agents: a review of patented potent geldanamycin derivatives. Expert Opin. Ther. Pat. 2013, 23, 919–943. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, C.; Meulman, P.A.; Wnuk, R.J.; Peterson, D.H. Geldanamycin, a new antibiotic. J. Antibiot. 1970, 23, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Nakagawa, A.; Sadakane, N.; Omura, S.; Oiwa, H.; Matsumoto, S.; Takahashi, M.; Ikai, T.; Ochiai, Y. Herbimycin B, a new benzoquinonoid ansamycin with anti-TMV and herbicidal activities. J. Antibiot. 1980, 33, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Iwai, Y.; Takahashi, Y.; Sadakane, N.; Nakagawa, A.; Oiwa, H.; Hasegawa, Y.; Ikai, T. Herbimycin, a new antibiotic produced by a strain of Streptomyces. J. Antibiot. 1979, 32, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Mimnaugh, E.G.; De Costa, B.; Myers, C.E.; Neckers, L.M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA 1994, 91, 8324–8328. [Google Scholar] [CrossRef] [PubMed]
- Franke, J.; Eichner, S.; Zeilinger, C.; Kirschning, A. Targeting heat-shock-protein 90 (Hsp90) by natural products: geldanamycin, a show case in cancer therapy. Nat. Prod. Rep. 2013, 30, 1299–1323. [Google Scholar] [CrossRef]
- Sasaki, K.; Yasuda, H.; Onodera, K. Growth inhibition of virus transformed cells in vitro and antitumor activity in vivo of geldanamycin and its derivatives. J. Antibiot. 1979, 32, 849–851. [Google Scholar] [CrossRef][Green Version]
- Uehara, Y.; Murakami, Y.; Suzukake-Tsuchiya, K.; Moriya, Y.; Sano, H.; Shibata, K.; Omura, S. Effects of herbimycin, derivatives on src oncogene function in relation to antitumor activity. J. Antibiot. 1988, 41, 831–834. [Google Scholar] [CrossRef]
- Supko, J.G.; Hickman, R.L.; Grever, M.R.; Malspeis, L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother. Pharmacol. 1995, 36, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ryu, J.S.; Jin, Y.; Kim, W.; Kaur, N.; Chung, S.J.; Jeon, Y.J.; Park, J.T.; Bang, J.S.; Lee, H.S.; et al. Synthesis and anticancer activity of geldanamycin derivatives derived from biosynthetically generated metabolites. Org. Biomol. Chem. 2008, 6, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, D.; Hong, S.S.; Na, Z.; Shin, J.C.; Roh, S.H.; Wu, C.Z.; Choi, O.; Lee, K.; Shen, Y.M.; et al. Rational biosynthetic engineering for optimization of geldanamycin analogues. ChemBioChem 2009, 10, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Kitson, R.R.; Chang, C.H.; Xiong, R.; Williams, H.E.; Davis, A.L.; Lewis, W.; Dehn, D.L.; Siegel, D.; Roe, S.M.; Prodromou, C.; et al. Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90. Nat. Chem. 2013, 5, 307–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, M.; Lu, T.; Zhao, L.X.; Chen, Y.; Huang, S.X.; Lohman, J.R.; Xu, L.H.; Jiang, C.L.; Shen, B. The missing C-17 O-methyltransferase in geldanamycin biosynthesis. Org. Lett. 2011, 13, 3726–3729. [Google Scholar] [CrossRef] [PubMed]
- Ivy, P.S.; Schoenfeldt, M. Clinical trials referral resource: current clinical trials of 17-AG and 17-DMAG. Oncology 2004, 18, 610, 615, 619–620. [Google Scholar]
- Schnur, R.C.; Corman, M.L.; Gallaschun, R.J.; Cooper, B.A.; Dee, M.F.; Doty, J.L.; Muzzi, M.L.; Moyer, J.D.; DiOrio, C.I.; Barbacci, E.G.; et al. Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogel danamycin derivatives. J. Med. Chem. 1995, 38, 3806–3812. [Google Scholar] [CrossRef]
- Rascher, A.; Hu, Z.; Viswanathan, N.; Schirmer, A.; Reid, R.; Nierman, W.C.; Lewis, M.; Hutchinson, C.R. Cloning and characterization of a gene cluster for geldanamycin production in Streptomyces hygroscopicus NRRL 3602. FEMS Microbiol. Lett. 2003, 218, 223–230. [Google Scholar] [CrossRef]
- He, W.; Wu, L.; Gao, Q.; Du, Y.; Wang, Y. Identification of AHBA biosynthetic genes related to geldanamycin biosynthesis in Streptomyces hygroscopicus 17997. Curr. Microbiol. 2006, 52, 197–203. [Google Scholar] [CrossRef]
- Shin, J.C.; Na, Z.; Lee, D.H.; Kim, W.C.; Lee, K.; Shen, Y.M.; Paik, S.G.; Hong, Y.S.; Lee, J.J. Characterization of tailoring genes involved in the modification of geldanamycin polyketide in Streptomyces hygroscopicus JCM4427. J. Microbiol. Biotechnol. 2008, 18, 1101–1108. [Google Scholar]
- Wang, X.; Ning, X.; Zhao, Q.; Kang, Q.; Bai, L. Improved PKS Gene Expression With Strong Endogenous Promoter Resulted in Geldanamycin Yield Increase. Biotechnol. J. 2017, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ouyang, Y.; Wang, G.; Li, X. Streptomyces autolyticus JX-47 large-insert bacterial artificial chromosome library construction and identification of clones covering geldanamycin biosynthesis gene cluster. Curr. Microbiol. 2011, 63, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Jiang, M.; Ren, Z.; Dong, Y.; Lu, T. The complete genome sequence of Streptomyces autolyticus CGMCC 0516, the producer of geldanamycin, autolytimycin, reblastatin and elaiophylin. J. Biotechnol. 2017, 252, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Boudjeko, T.; Tchinda, R.A.M.; Mina Zitouni, M.; Nana, J.A.V.T.; Lerat, S.; Beaulieu, C. Streptomyces cameroonensis sp. nov., a Geldanamycin Producer That Promotes Theobroma cacao Growth. Microbes Environ. 2017, 32, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhao, L.; Liu, H.W.; Sherman, D.H. A gene cluster for macrolide antibiotic biosynthesis in Streptomyces venezuelae: architecture of metabolic diversity. Proc. Natl. Acad. Sci. USA 1998, 95, 12111–12116. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; von Bamberg, D.; Hale, V.; Breuer, M.; Hardt, B.; Müller, R.; Floss, H.G.; Reynolds, K.A.; Leistner, E. Biosynthesis of ansatrienin (mycotrienin) and naphthomycin. Identification and analysis of two separate biosynthetic gene clusters in Streptomyces collinus Tü 1892. Eur. J. Biochem. 1999, 261, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.W.; Bai, L.; Clade, D.; Hoffmann, D.; Toelzer, S.; Trinh, K.Q.; Xu, J.; Moss, S.J.; Leistner, E.; Floss, H.G. The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnema pretiosum. Proc. Natl. Acad. Sci. USA 2002, 99, 7968–7973. [Google Scholar] [CrossRef]
- August, P.R.; Tang, L.; Yoon, Y.J.; Ning, S.; Müller, R.; Yu, T.W.; Taylor, M.; Hoffmann, D.; Kim, C.G.; Zhang, X.; et al. Biosynthesis of the ansamycin antibiotic rifamycin: deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem. Biol. 1998, 5, 69–79. [Google Scholar] [CrossRef]
- Admiraal, S.J.; Walsh, C.T.; Khosla, C. The loading module of rifamycin synthetase is an adenylation-thiolation didomain with substrate tolerance for substituted benzoates. Biochemistry 2001, 40, 6116–6123. [Google Scholar] [CrossRef]
- Marahiel, M.A.; Stachelhaus, T.; Mootz, H.D. Modular peptide synthetases involved in non-ribosomal peptide synthesis. Chem. Rev. 1997, 97, 2651–2673. [Google Scholar] [CrossRef]
- Martín, J.F. Alpha-aminoadipyl-cysteinyl-valine synthetases in beta-lactam producing organisms. From Abraham’s discoveries to novel concepts of non-ribosomal peptide synthesis. J. Antibiot. 2000, 53, 1008–1021. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martín, J.F.; Aparicio, J.F. Biosynthesis Enzymology of the Polyenes Pimaricin and Candicidin. Methods Enzymol. 2009, 459, 215–242. [Google Scholar] [PubMed]
- Eichner, S.; Eichner, T.; Floss, H.G.; Fohrer, J.; Hofer, E.; Sasse, F.; Zeilinger, C.; Kirschning, A. Broad substrate specificity of the amide synthase in S. hygroscopicus--new 20-membered macrolactones derived from geldanamycin. J. Am. Chem. Soc. 2012, 134, 1673–1679. [Google Scholar] [CrossRef]
- Li, Y.; He, W.; Wang, Y.; Wang, Y.; Shao, R. A new post-PKS modification process in the carbamoyltransferase gene inactivation strain of Streptomyces hygroscopicus 17997. J. Antibiot. 2008, 61, 347–355. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Lei, J.; Liu, Y.; Wang, Y. The LuxR family members GdmRI and GdmRII are positive regulators of geldanamycin biosynthesis in Streptomyces hygroscopicus 17997. Arch. Microbiol. 2008, 189, 501–510. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.J.; Paik, S.G.; Hong, Y.S. Identification of three positive regulators in the geldanamycin PKS gene cluster of Streptomyces hygroscopicus JCM4427. J. Microbiol. Biotechnol. 2010, 20, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Rimal, H.; Yu, S.C.; Lee, B.; Hong, Y.S.; Oh, T.J. Characterization of Gel16 as a Cytochrome P450 in Geldanamycin Biosynthesis and in-silico Analysis for an Endogenous Electron Transport System. J. Microbiol. Biotechnol. 2019, 29, 44–54. [Google Scholar] [CrossRef]
- Pérez-Redondo, R.; Santamarta, I.; Bovenberg, R.; Martín, J.F.; Liras, P. The enigmatic lack of glucose utilization in Streptomyces clavuligerus is due to inefficient expression of the glucose permease gene. Microbiology 2010, 156, 1527–1537. [Google Scholar] [CrossRef]
- Ramos, J.L.; Martínez-Bueno, M.; Molina-Henares, A.J.; Terán, W.; Watanabe, K.; Zhang, X.; Gallegos, M.T.; Brennan, R.; Tobes, R. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 2005, 69, 326–356. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Nodwell, J.R. The TetR family of regulators. Microbiol. Mol. Biol. Rev. 2013, 77, 440–475. [Google Scholar] [CrossRef]
- Santos-Aberturas, J.; Payero, T.D.; Vicente, C.M.; Guerra, S.M.; Cañibano, C.; Martín, J.F.; Aparicio, J.F. Functional conservation of PAS-LuxR transcriptional regulators in polyene macrolide biosynthesis. Metab. Eng. 2011, 13, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Santos-Aberturas, J.; Vicente, C.M.; Guerra, S.M.; Payero, T.D.; Martín, J.F.; Aparicio, J.F. Molecular control of polyene macrolide biosynthesis: direct binding of the regulator PimM to eight promoters of pimaricin genes and identification of binding boxes. J. Biol. Chem. 2011, 286, 9150–9161. [Google Scholar] [CrossRef] [PubMed]
- Goranovič, D.; Blažič, M.; Magdevska, V.; Horvat, J.; Kuščer, E.; Polak, T.; Santos-Aberturas, J.; Martínez-Castro, M.; Barreiro, C.; Mrak, P.; et al. FK506 biosynthesis is regulated by two positive regulatory elements in Streptomyces tsukubaensis. BMC Microbiol. 2012, 12, 238. [Google Scholar]
- Ordóñez-Robles, M.; Rodríguez-García, A.; Martín, J.F. Target genes of the Streptomyces tsukubaensis FkbN regulator include most of the tacrolimus biosynthesis genes, a phosphopantetheinyl transferase and other PKS genes. Appl. Microbiol. Biotechnol. 2016, 100, 8091–8103. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, X.; Zhou, X.; Bai, L.; He, J.; Jeong, K.J.; Lee, S.Y.; Deng, Z. Organizational and mutational analysis of a complete FR-008/candicidin gene cluster encoding a structurally related polyene complex. Chem. Biol. 2003, 10, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chater, K.F.; Chandra, G.; Niu, G.; Tan, H. Molecular regulation of antibiotic biosynthesis in Streptomyces. Mol. Biol. Rev. 2013, 77, 112–143. [Google Scholar] [CrossRef]
- Jiang, M.; Yin, M.; Wu, S.; Han, X.; Ji, K.; Wen, M.; Lu, T. GdmRIII, a TetR Family Transcriptional Regulator, Controls Geldanamycin and Elaiophylin Biosynthesis in Streptomyces autolyticus CGMCC0516. Sci. Rep. 2017, 7, 4803. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Wang, L.; Zhang, G.; Fan, K.; Tan, H.; Yang, K. A novel role of ‘pseudo’γ-butyrolactone receptors in controlling γ-butyrolactone biosynthesis in Streptomyces. Mol. Microbiol. 2011, 82, 236–250. [Google Scholar] [CrossRef]
- Martínez-Burgo, Y.; Álvarez-Álvarez, R.; Rodríguez-García, A.; Liras, P. The Pathway-Specific Regulator ClaR of Streptomyces clavuligerus Has a Global Effect on the Expression of Genes for Secondary Metabolism and Differentiation. Appl. Environ. Microbiol. 2015, 81, 6637–6648. [Google Scholar] [CrossRef]
- Bentley, S.D.; Chater, K.F.; Cerdeño-Tárraga, A.M.; Challis, G.L.; Thomson, N.R.; James, K.D.; Harris, D.E.; Quail, M.A.; Kieser, H.; Harper, D.; et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 2002, 417, 141–147. [Google Scholar] [CrossRef]
- Omura, S.; Ikeda, H.; Ishikawa, J.; Hanamoto, A.; Takahashi, C.; Shinose, M.; Takahashi, Y.; Horikawa, H.; Nakazawa, H.; Osonoe, T.; et al. Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. USA 2001, 98, 12215–12220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Li, M.; He, W.Q.; Wang, Y.G.; Shao, R.G. Inactivation of putative PKS genes can double geldanamycin yield in Streptomyces hygroscopicus 17997. Genet. Mol. Res. 2013, 12, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Piagentini, M.; Rascher, A.; Tian, Z.Q.; Buchanan, G.O.; Regentin, R.; Hu, Z.; Hutchinson, C.R.; McDaniel, R. Engineered biosynthesis of geldanamycin analogs for Hsp90 inhibition. Chem. Biol. 2004, 11, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Demain, A.L. Control of antibiotic biosynthesis. Microbiol. Rev. 1980, 44, 230–251. [Google Scholar] [PubMed]
- Mendes, M.V.; Tunca, S.; Antón, N.; Recio, E.; Sola-Landa, A.; Aparicio, J.F.; Martín, J.F. The two-component PhoR-PhoP system of Streptomyces natalensis: Inactivation or deletion of phoP reduces the negative phosphate regulation of pimaricin biosynthesis. Metab. Eng. 2007, 9, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Rodríguez-García, A.; Liras, P. The master regulator PhoP coordinates phosphate and nitrogen metabolism, respiration, cell differentiation and antibiotic biosynthesis: comparison in Streptomyces coelicolor and Streptomyces avermitilis. J. Antibiot. 2017, 70, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Robles, M.; Santos-Beneit, F.; Rodríguez-García, A.; Martín, J.F. Analysis of the Pho regulon in Streptomyces tsukubaensis. Microbiol. Res. 2017, 205, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Sola-Landa, A.; Moura, R.S.; Martín, J.F. The two-component PhoR-PhoP system controls both primary metabolism and secondary metabolite biosynthesis in Streptomyces lividans. Proc. Natl. Acad. Sci. USA 2003, 100, 6133–6138. [Google Scholar] [CrossRef]
- Martín, J.F. Phosphate control of the biosynthesis of antibiotics and other secondary metabolites is mediated by the PhoR-PhoP system: An unfinished story. J. Bacteriol. 2004, 186, 5197–5201. [Google Scholar]
- Sola-Landa, A.; Rodríguez-García, A.; Franco-Domínguez, E.; Martín, J.F. Binding of PhoP to promoters of phosphate-regulated genes in Streptomyces coelicolor: identification of PHO boxes. Mol. Microbiol. 2005, 56, 1373–1385. [Google Scholar] [CrossRef]
- Jung, K.; Fried, L.; Behr, S.; Heermann, R. Histidine kinases and response regulators in networks. Curr. Opin. Microbiol. 2012, 15, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castro, M.; Barreiro, C.; Martín, J.F. Analysis and validation of the pho regulon in the tacrolimus-producer strain Streptomyces tsukubaensis: differences with the model organism Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 2018, 102, 7029–7045. [Google Scholar]
- Martín, J.F.; Santos-Beneit, F.; Rodríguez-García, A.; Sola-Landa, A.; Smith, M.C.; Ellingsen, T.E.; Nieselt, K.; Burroughs, N.J.; Wellington, E.M. Transcriptomic studies of phosphate control of primary and secondary metabolism in Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 2012, 95, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Martín-Martín, S.; Rodríguez-García, A.; Santos-Beneit, F.; Franco-Domínguez, E.; Sola-Landa, A.; Martín, J.F. Self-control of the PHO regulon: the PhoP-dependent protein PhoU controls negatively expression of genes of PHO regulon in Streptomyces coelicolor. J. Antibiot. 2017. (In press)
- Allenby, N.E.; Laing, E.; Bucca, G.; Kierzek, A.M.; Smith, C.P. Diverse control of metabolism and other cellular processes in Streptomyces coelicolor by the PhoP transcription factor: genome-wide identification of in vivo targets. Nucleic Acids Res. 2012, 40, 9543–9556. [Google Scholar] [CrossRef] [PubMed]
- Sola-Landa, A.; Rodríguez-García, A.; Amin, R.; Wohlleben, W.; Martín, J.F. Competition between the GlnR and PhoP regulators for the glnA and amtB promoters in Streptomyces coelicolor. Nucleic Acids Res. 2013, 41, 1767–1782. [Google Scholar] [CrossRef]
- Solárová, Z.; Mojžiš, J.; Solár, P. Hsp90 inhibitor as a sensitizer of cancer cells to different therapies. Int. J. Oncol. 2015, 46, 907–926. [Google Scholar]

| Streptomyces Strains | Reference |
|---|---|
| Streptomyces hygroscopicus var geldanus NRRL3602 | [18] |
| Streptomyces hygroscopicus 17997 | [19] |
| Streptomyces hygroscopicus sub. duamyceticus JCM 4427 | [20] |
| Streptomyces hygroscopicus XM 201 | [21] |
| Streptomyces autolyticus JX-47 | [22] |
| Streptomyces autolyticus CGMCC 0516, | [23] |
| Streptomyces cameroonensis sp. | [24] |
| Streptomyces species containing geldanamycin biosynthesis genes identified bioinformatically | |
| Streptomyces violaceusniger Tu 4113 Streptomyces sp. RTd22 Streptomyces lydicus strain 103 Streptomyces rapamycinicus NRRL 5491 Streptomyces iranensis Streptomyces albus DSM 41398 Streptomyces bingchenggensis BCW-1 | Cited in [23] |
| Genes | Function | Reference |
|---|---|---|
| gdmMT | O-methyltransferase | [15] |
| gdmL = gel1 | Flavin-dependent oxygenase | [20] |
| gdmX | Unknown | [18] |
| gdmAI, AII, AIII = gelAI, AII, AIII | Polyketide synthases I, II and III 1 | [18] 2 |
| gdmF | Amide synthase | [33] |
| gdmM = gel7 | Flavin-dependent oxidase | [20] |
| gdmN = gel8 | Carbamoyl transferase | [20,34] |
| gdmH | Methoxymalonyl-ACP biosynthesis | [18] |
| gdmI | Methoxymalonyl-ACP biosynthesis | [18] |
| gdmJ | Methoxymalonyl-ACP biosynthesis | [18] |
| gdmK | Methoxymalonyl-ACP biosynthesis | [18] |
| gdmG | O-methyl transferase for methoxy-malonyl-ACP biosynthesis | [18] |
| gdmRII = gel17 | LAL-type regulator | [35,36] |
| gdmO3 | Amino dehydroquinate synthase | [18] |
| gdmFx | Ferredoxin | [20] |
| gdmP = gel16 | P450 monooxygenase | [20] |
| gdmRI = gel14 | LAL-type regulator | [35,36] |
| gdmRIII = gel 19 | TetR-family positive regulator | [36] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, J.F.; Ramos, A.; Liras, P. Regulation of Geldanamycin Biosynthesis by Cluster-Situated Transcription Factors and the Master Regulator PhoP. Antibiotics 2019, 8, 87. https://doi.org/10.3390/antibiotics8030087
Martín JF, Ramos A, Liras P. Regulation of Geldanamycin Biosynthesis by Cluster-Situated Transcription Factors and the Master Regulator PhoP. Antibiotics. 2019; 8(3):87. https://doi.org/10.3390/antibiotics8030087
Chicago/Turabian StyleMartín, Juan F., Angelina Ramos, and Paloma Liras. 2019. "Regulation of Geldanamycin Biosynthesis by Cluster-Situated Transcription Factors and the Master Regulator PhoP" Antibiotics 8, no. 3: 87. https://doi.org/10.3390/antibiotics8030087
APA StyleMartín, J. F., Ramos, A., & Liras, P. (2019). Regulation of Geldanamycin Biosynthesis by Cluster-Situated Transcription Factors and the Master Regulator PhoP. Antibiotics, 8(3), 87. https://doi.org/10.3390/antibiotics8030087





