Mapping and Analysing Potential Sources and Transmission Routes of Antimicrobial Resistant Organisms in the Environment using Geographic Information Systems—An Exploratory Study
Abstract
1. Introduction
2. Identifying Sources and Transmission Routes
2.1. Sources
2.2. Transmission Routes
3. Materials and Methods
3.1. Materials
3.1.1. Data Review and Selection
3.1.2. Antimicrobial Usage Data
3.2. Methods
3.2.1. Spatial Mapping, Data Analysis, and Geolocation/Geocoding
3.2.2. Data Standardization
3.2.3. Estimating Livestock ‘Intensity’—Generating a Livestock Weighted Index
3.2.4. Cartographic Displays, Spatial Resolutions, and GIS Data Classification
4. Results
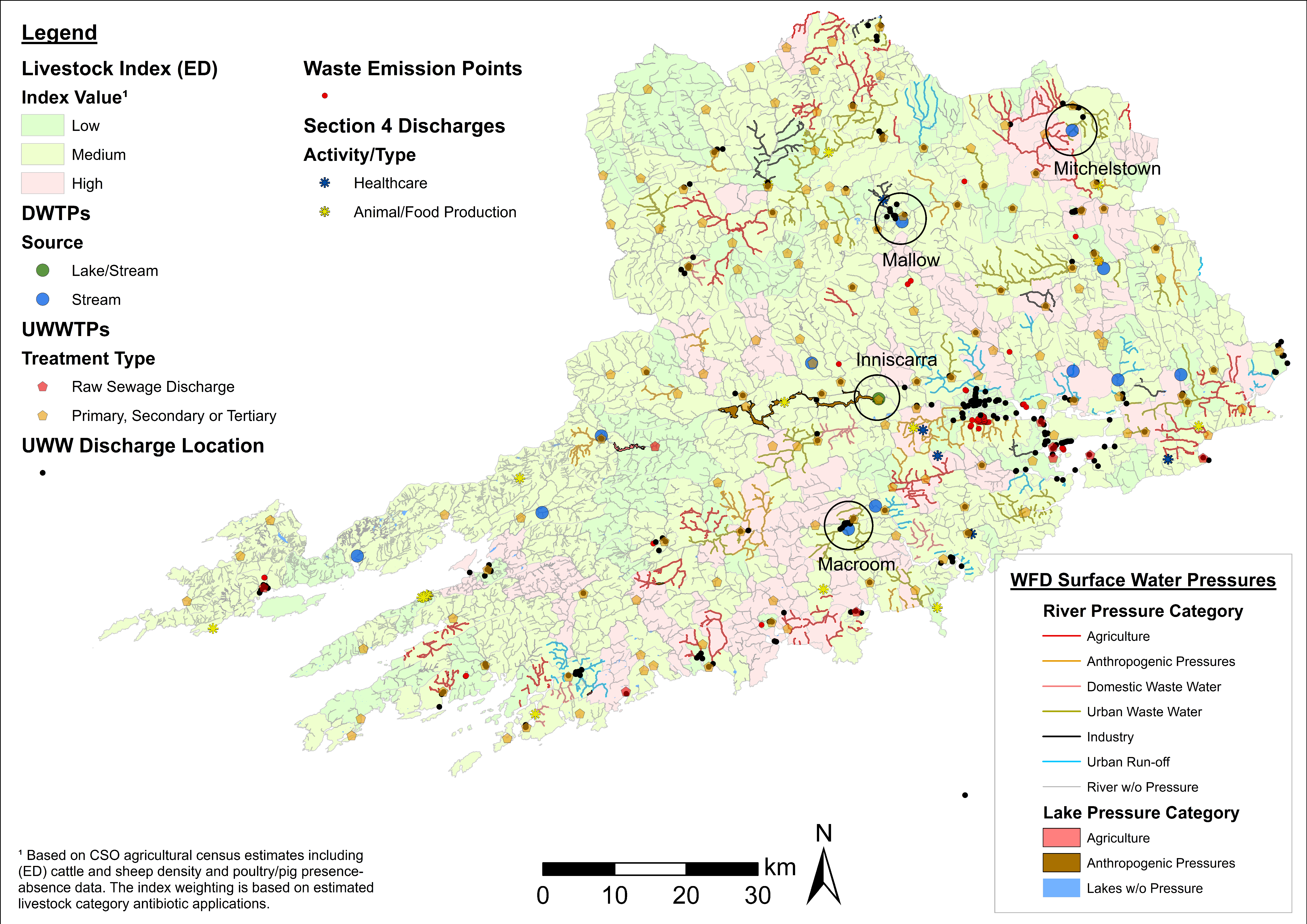
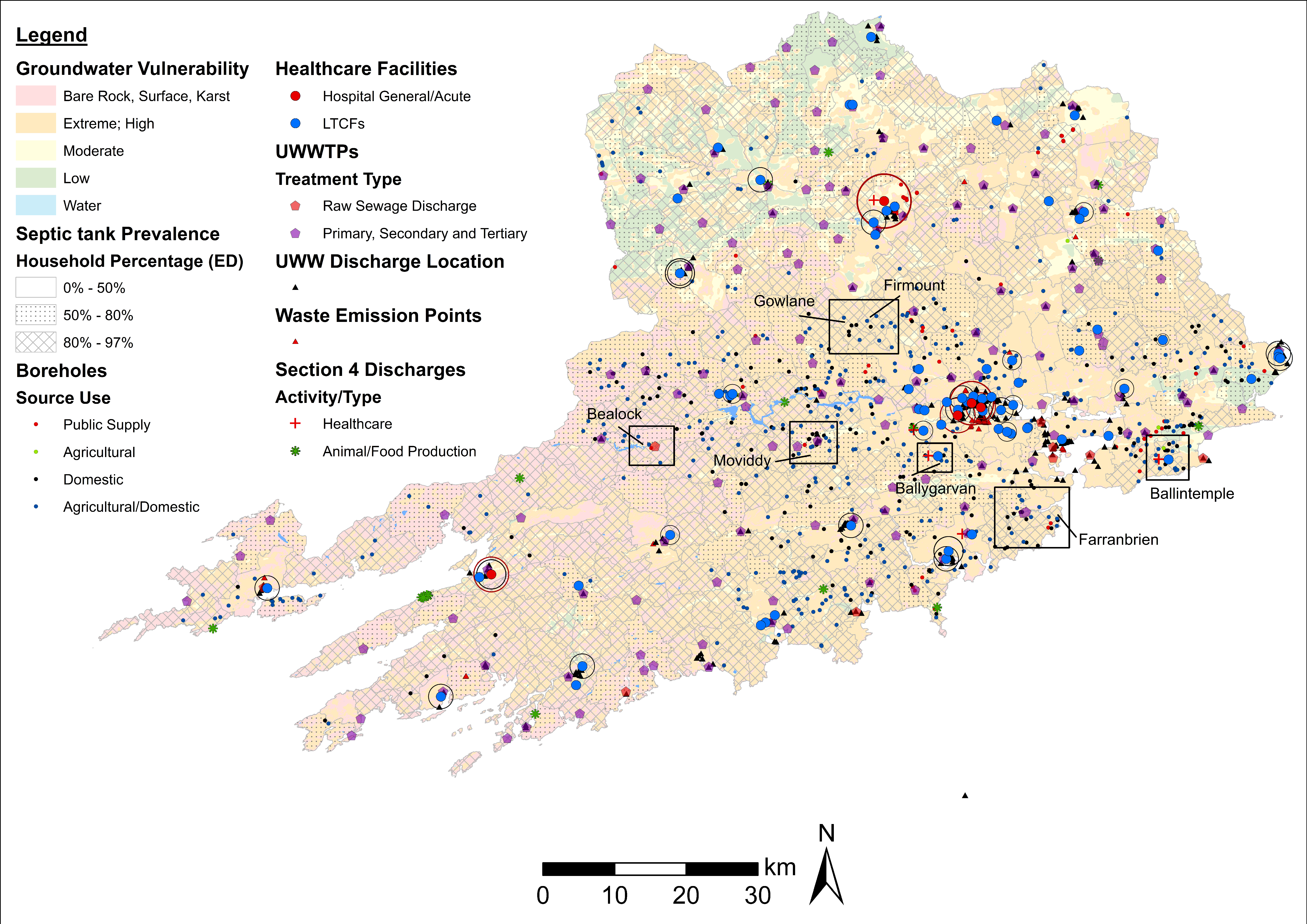
4.1. ARO Sources, Livestock Weighted Index, and LAA Cartographic Displays
4.2. Fine-Scale Cartographic Displays
4.3. Examples of Data Layer Combinations
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Action Plan on Antimicrobial Resistance. 2015. Available online: http://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 (accessed on 7 November 2018).
- Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 7 November 2018).
- Leonard, A.F.C.; Zhang, L.; Balfour, A.J.; Garside, R.; Gaze, W.H. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ. Int. 2015, 82, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.S.; Ward, M.P.; Maldonado, G. Can landscape ecology untangle the complexity of antibiotic resistance? Nat. Rev. Microbiol. 2006, 4, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance: Investigating the Environmental Dimension. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/22263/Frontiers_2017_CH1_EN.pdf?sequence=1&isAllowed=y (accessed on 15 October 2018).
- Morrill, H.J.; Pogue, J.M.; Kaye, K.S.; LaPlante, K.L. Treatment options for carbapenem-resistant enterobacteriaceae infections. Open Forum. Infect. Dis. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Caniaux, I.; van Belkum, A.; Zambardi, G.; Poirel, L.; Gros, M.F. MCR: Modern colistin resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Kwan, M.-P. Introduction: Geospatial health research and GIS. Ann. GIS 2015, 21, 93–95. [Google Scholar] [CrossRef]
- Richardson, D.B.; Volkow, N.D.; Kwan, M.-P.; Kaplan, R.M.; Goodchild, M.F.; Croyle, R.T. Spatial Turn in Health Research. Science 2013, 339, 1390–1392. [Google Scholar] [CrossRef] [PubMed]
- Kiffer, C.R.; Camargo, E.C.; Shimakura, S.E.; Ribeiro, P.J., Jr.; Bailey, T.C.; Pignatari, A.C.; Monteiro, A.M. A spatial approach for the epidemiology of antibiotic use and resistance in community-based studies: The emergence of urban clusters of Escherichia coli quinolone resistance in Sao Paulo. Int. J. Health Geogr. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Bueno, I.; Williams-Nguyen, J.; Hwang, H.; Sargeant, J.M.; Nault, A.J.; Singer, R.S. Systematic review: Impact of point sources on antibiotic-resistant bacteria in the natural environment. Zoonoses Public Health 2018, 65, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qi, R.; Yang, M.; Zhang, Y.; Yu, T. Bacterial community characteristics under long-term antibiotic selection pressures. Water Res. 2011, 45, 6063–6073. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, M.; Hu, J.; Zhang, J.; Liu, R.; Gu, X.; Zhang, Y.; Wang, Z. Antibiotic-resistance profile in environmental bacteria isolated from penicillin production wastewater treatment plant and the receiving river. Environ. Microbiol. 2009, 11, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Sidrach-Cardona, R.; Hijosa-Valsero, M.; Marti, E.; Balcázar, J.L.; Becares, E. Prevalence of antibiotic-resistant fecal bacteria in a river impacted by both an antibiotic production plant and urban treated discharges. Sci. Total Environ. 2014, 488, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ludden, C.; Reuter, S.; Judge, K.; Gouliouris, T.; Blane, B.; Coll, F.; Naydenova, P.; Hunt, M.; Tracey, A.; Hopkins, K.L.; et al. Sharing of carbapenemase-encoding plasmids between Enterobacteriaceae in UK sewage uncovered by MinION sequencing. Microb. Genom. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Point Prevalence Survey of Healthcare-Associated Infections & Antimicrobial Use in Long-Term Care Facilities (HALT)-May 2016. Available online: http://www.hpsc.ie/a-z/microbiologyantimicrobialresistance/infectioncontrolandhai/surveillance/hcaiinlongtermcarefacilities/haltreports/2016report/File,16218,en.pdf (accessed on 15 October 2018).
- Hospital Effluent: Impact on the Microbial Environment and Risk to Human Health. Available online: http://www.epa.ie/pubs/reports/research/health/EPA%20162%20final%20web.pdf (accessed on 8 November 2018).
- Zhou, W.; Wang, Y.; Lin, J. Functional cloning and characterization of antibiotic resistance genes from the chicken gut microbiome. Appl. Environ. Microbiol. 2012, 78, 3028–3032. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Johnson, T.A.; Su, J.-Q.; Qiao, M.; Guo, G.-X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- Food Wise 2025—A 10 Year Vision for the Lrish Agri-Food Industry. Available online: https://www.agriculture.gov.ie/media/migration/foodindustrydevelopmenttrademarkets/agri-foodandtheeconomy/foodwise2025/report/FoodWise2025.pdf (accessed on 8 November 2018).
- WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals. Available online: http://www.who.int/foodsafety/areas_work/antimicrobial-resistance/cia_guidelines/en/ (accessed on 7 November 2018).
- Udikovic-Kolic, N.; Wichmann, F.; Broderick, N.A.; Handelsman, J. Bloom of resident antibiotic-resistant bacteria in soil following manure fertilization. Proc. Natl. Acad. Sci. USA 2014, 111, 15202–15207. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.F.C.; Singer, A.; Ukoumunne, O.C.; Gaze, W.H.; Garside, R. Is it safe to go back into the water? A systematic review and meta-analysis of the risk of acquiring infections from recreational exposure to seawater. Int. J. Epidemiol. 2018, 47, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Urban Waste Water Treatment Report 2017. Available online: http://www.epa.ie/pubs/reports/water/wastewater/Final%20report%20for%20website.pdf (accessed on 14 November 2018).
- Mahon, B.M.; Brehony, C.; McGrath, E.; Killeen, J.; Cormican, M.; Hickey, P.; Keane, S.; Hanahoe, B.; Dolan, A.; Morris, D. Indistinguishable NDM-producing Escherichia coli isolated from recreational waters, sewage, and a clinical specimen in Ireland, 2016 to 2017. Eur. Surveill 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.P.; O’Flaherty, V.; Kramers, G.; Grant, J.; Richards, K.G. Long-term persistence and leaching of Escherichia coli in temperate maritime soils. Appl. Environ. Microbiol. 2010, 76, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Hynds, P.D.; Misstear, B.D.; Gill, L.W. Development of a microbial contamination susceptibility model for private domestic groundwater sources. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Water Quality in 2016. Available online: http://www.epa.ie/pubs/reports/water/waterqua/Water%20Quality%20in%202016%20An%20Indicators%20Report.pdf (accessed on 8 November 2018).
- Census of Agriculture. Available online: http://www.census.cso.ie/censusagriculture (accessed on 10 June 2018).
- Census 2016. Available online: http://www.census.cso.ie/en/databases/ (accessed on 10 June 2018).
- Water Framework Directive 2000/60/EC Establishing a Framework for Community in Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:5c835afb-2ec6-4577-bdf8 756d3d694eeb.0004.02/DOC_1&format=PDF (accessed on 10 June 2018).
- Water Framework Directive Monitoring Programme—Prepared to Meet the Requirements of the EU Water Framework Directive (2000/60/EC) and National Regulations Implementing the Water Framework Directive (S.I. No. 722 of 2003) and National Regulations Implementing the Nitrates Directive (S.I. No. 788 of 2005). Available online: http://www.epa.ie/pubs/reports/water/other/wfd/EPA_water_WFD_monitoring_programme_main_report.pdf (accessed on 10 June 2018).
- A Risk-Based Methodology to Assist in the Regulation of Domestic Waste Water Treatment Systems. Available online: https://www.epa.ie/pubs/reports/water/wastewater/EPA_DWWTS_RiskRanking.pdf (accessed on 10 June 2018).
- Hospital Antimicrobial Consumption Surveillance. Available online: http://www.hpsc.ie/a-z/microbiologyantimicrobialresistance/europeansurveillanceofantimicrobialconsumptionesac/PublicMicroB/SACHC/Report1.html (accessed on 10 June 2018).
- Census 2016 Boundary Files. Available online: https://www.cso.ie/en/census/census2016reports/census2016boundaryfiles/ (accessed on 10 June 2018).
- European Medicines Agency and European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 29 European Countries in 2014. Available online: https://www.ema.europa.eu/documents/report/sixth-esvac-report-sales-veterinary-antimicrobial-agents-29-european-countries-2014_en.pdf (accessed on 10 June 2018).
- Veterinary Medicines Directorate. UK Veterinary Antibiotic Resistance and Sales Surveillance (UK-VARSS) 2014 Report. Available online: https://www.gov.uk/government/publications/veterinary-antimicrobial-resistance-and-sales-surveillance-2014 (accessed on 20 August 2018).
- Stillo, F.; MacDonald, G.J. Exposure to contaminated drinking water and health disparities in North Carolina. Am. J. Public Health 2017, 107, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Water Quality in 2016: An Indicators Report. Available online: https://www.epa.ie/pubs/reports/water/waterqua/Water%20Quality%20in%202016%20An%20Indicators%20Report.pdf (accessed on 10 June 2018).
- Galvin, S.; Bergin, N.; Hennessy, R.; Hanahoe, B.; Murphy, A.W.; Cormican, M.; Vellinga, A. Exploratory spatial mapping of the occurrence of antimicrobial resistance in E. coli in the community. Antibiotics 2013, 2, 328–338. [Google Scholar] [CrossRef] [PubMed]
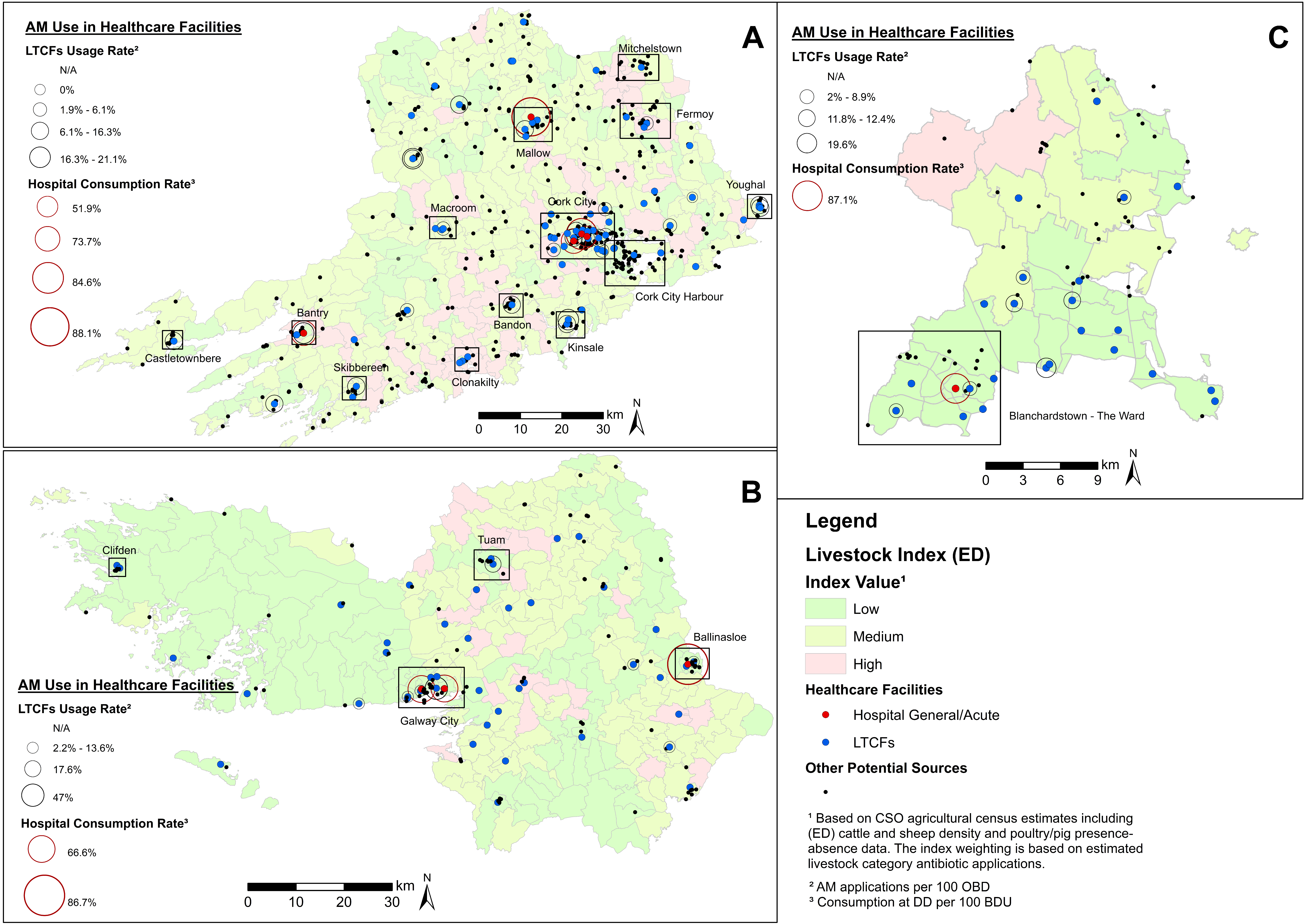
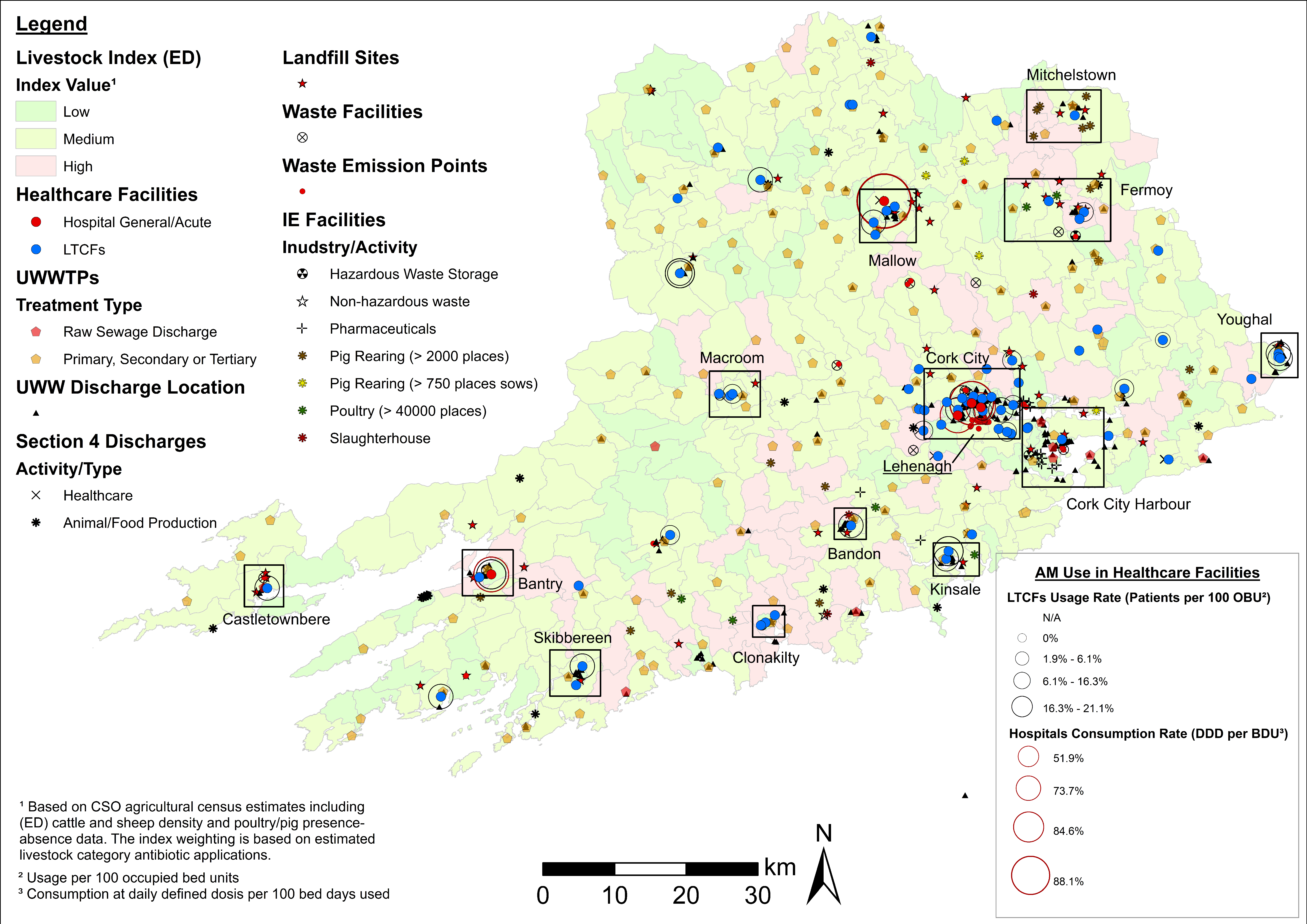
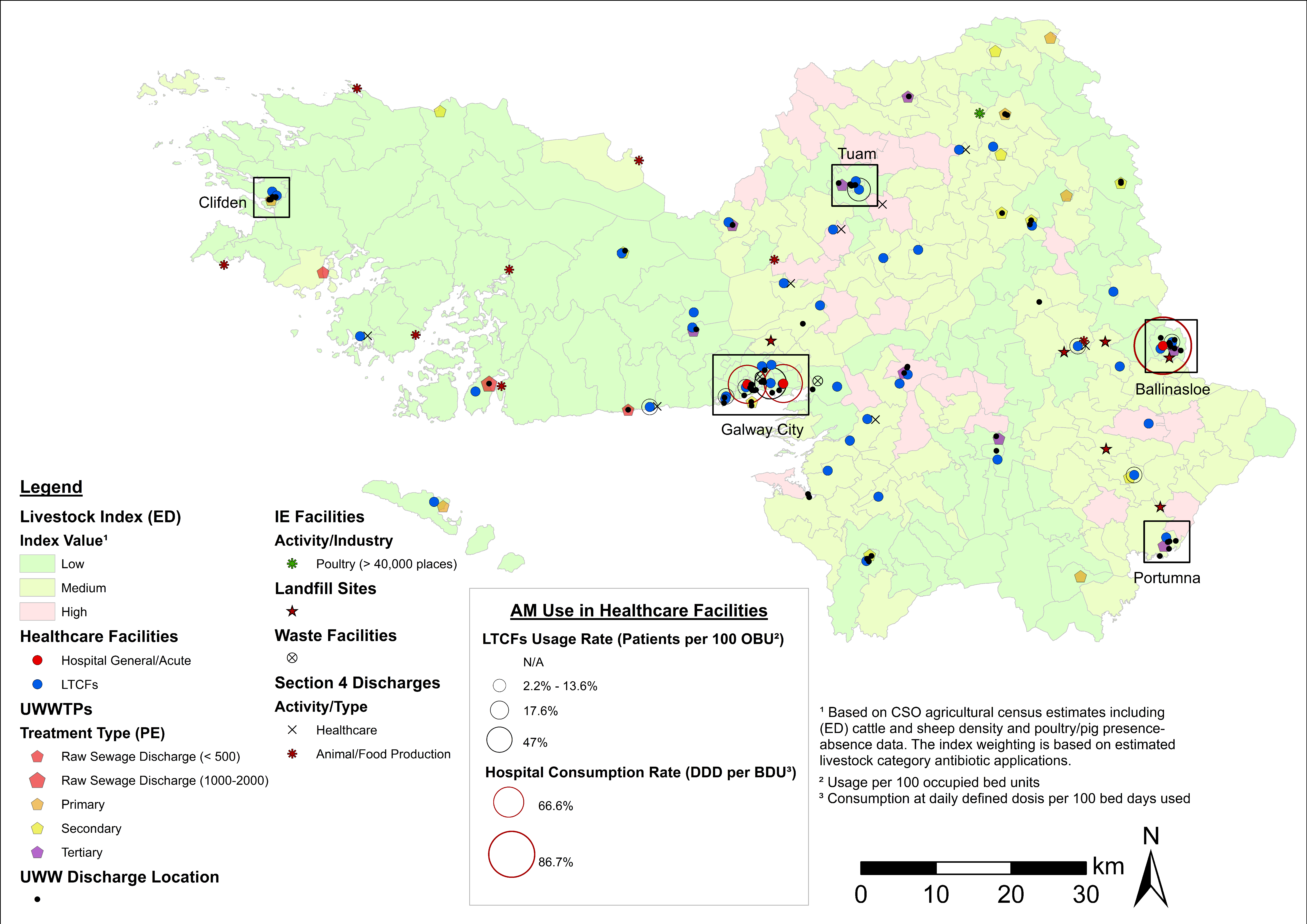
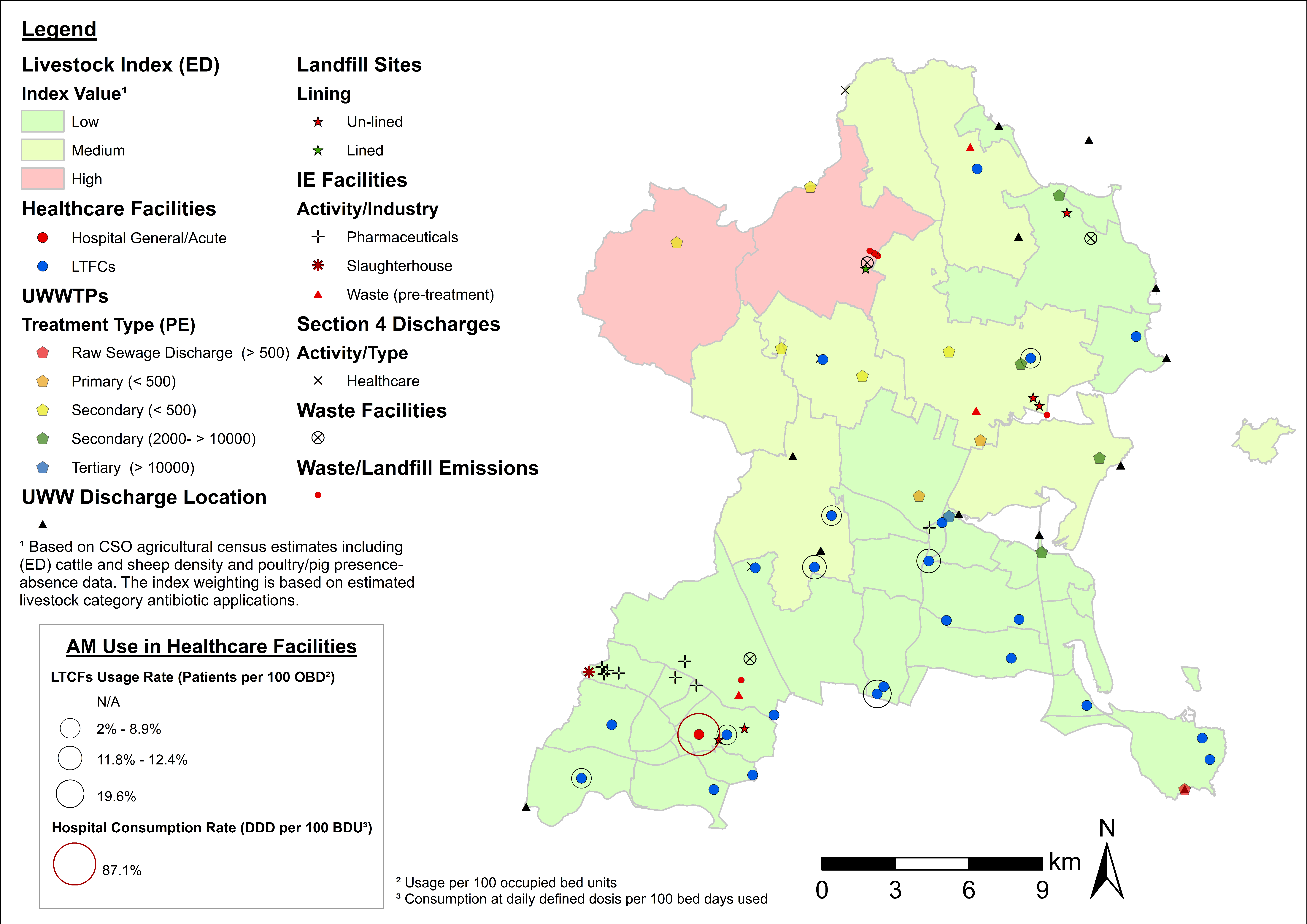
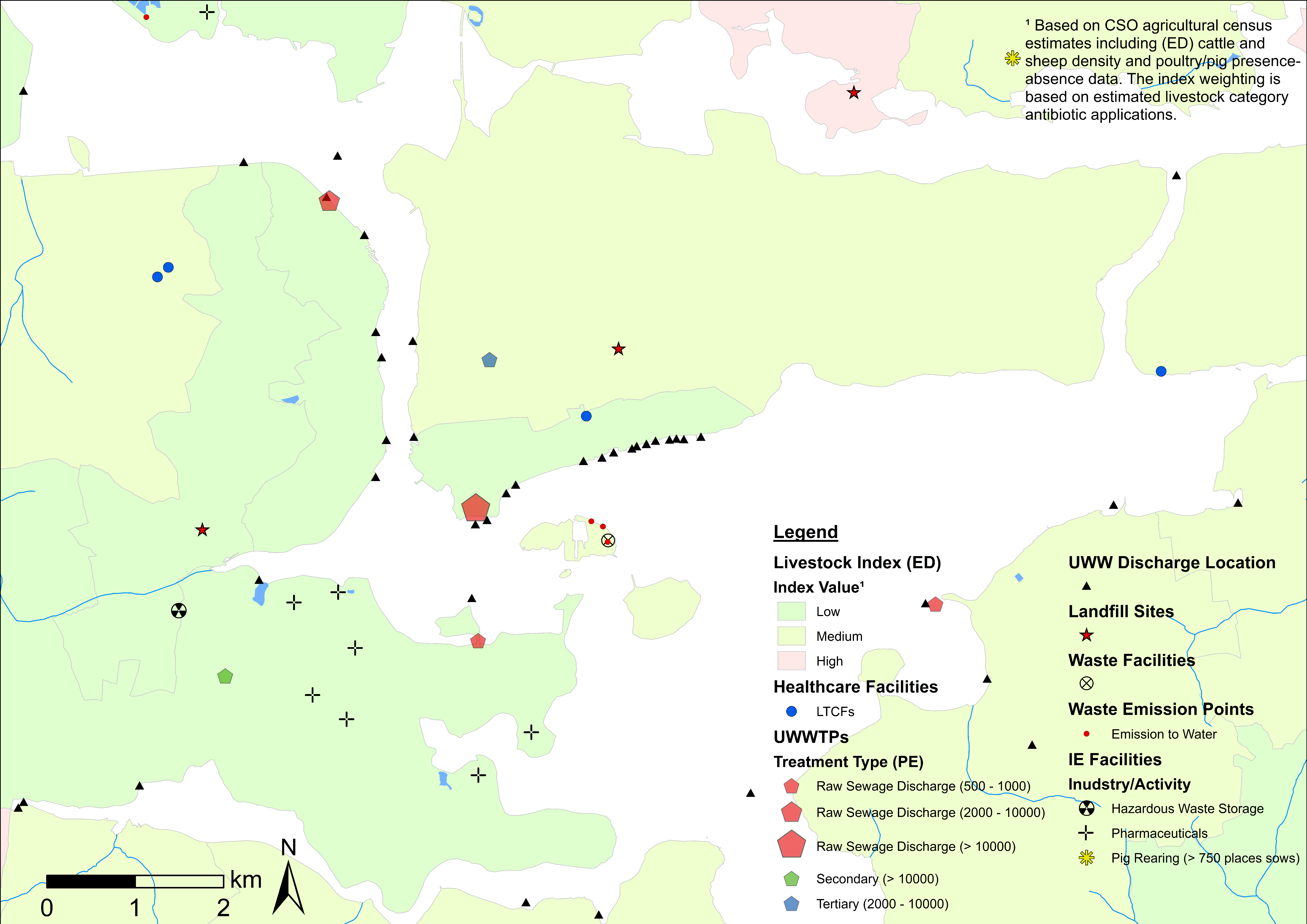
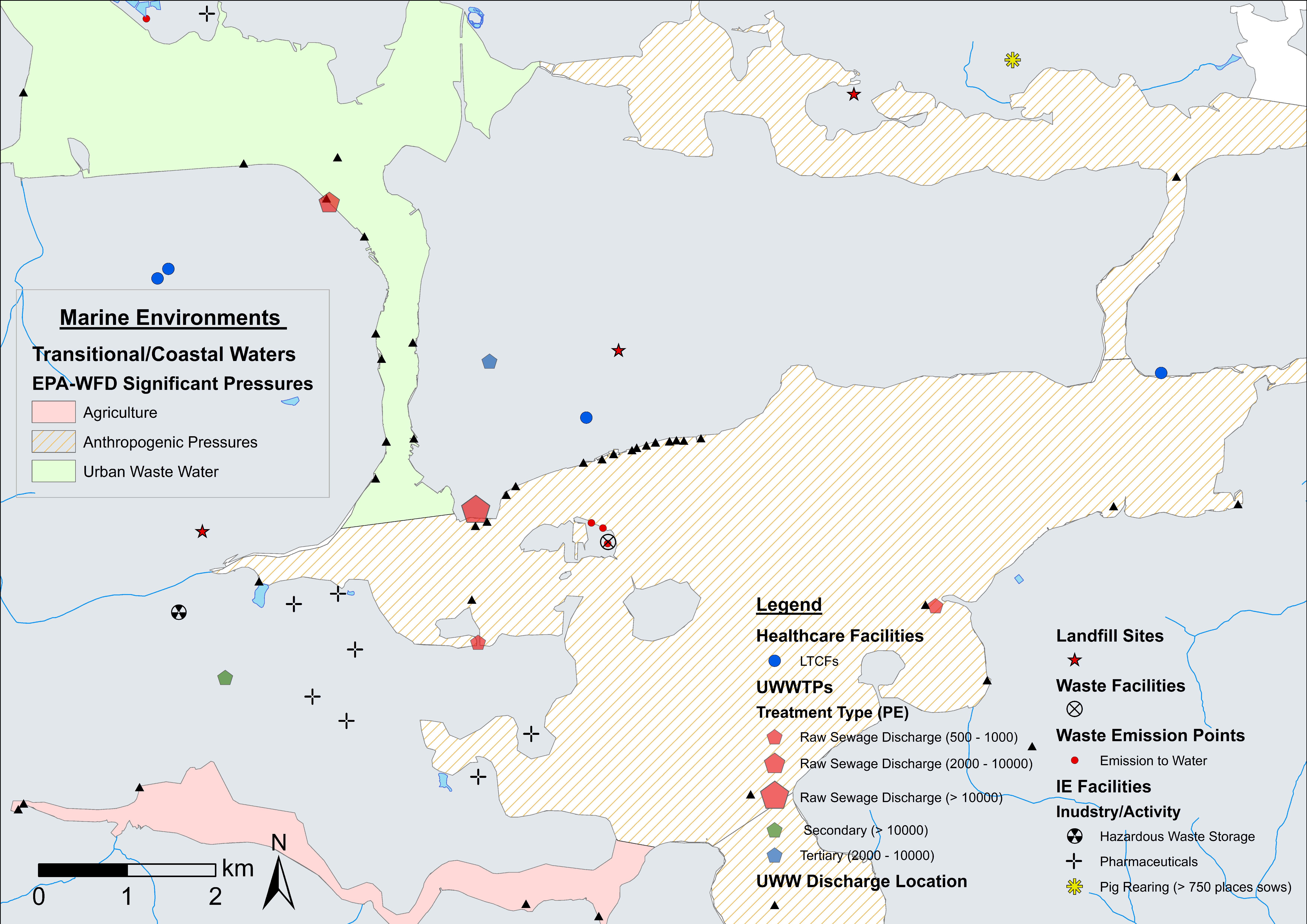
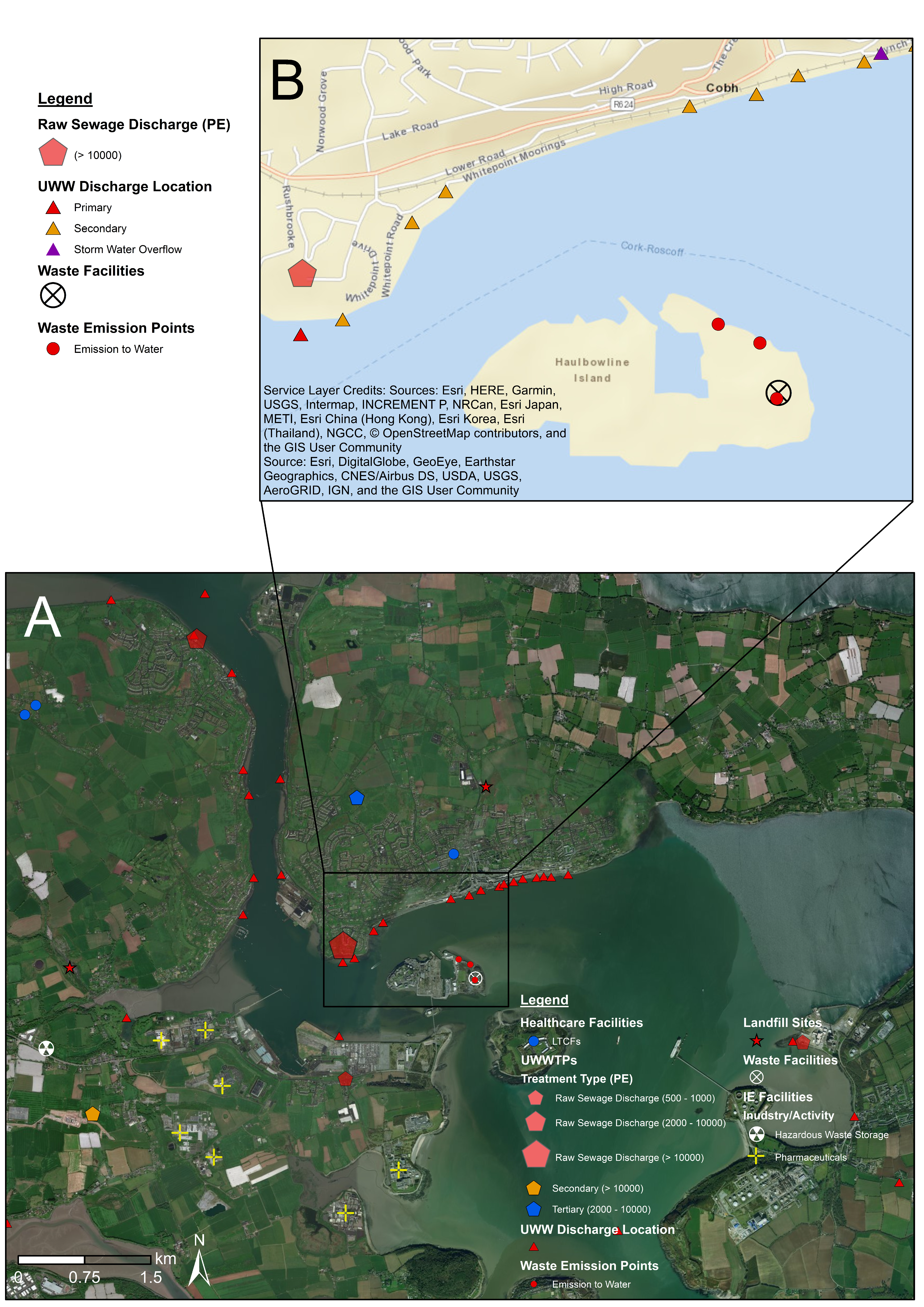
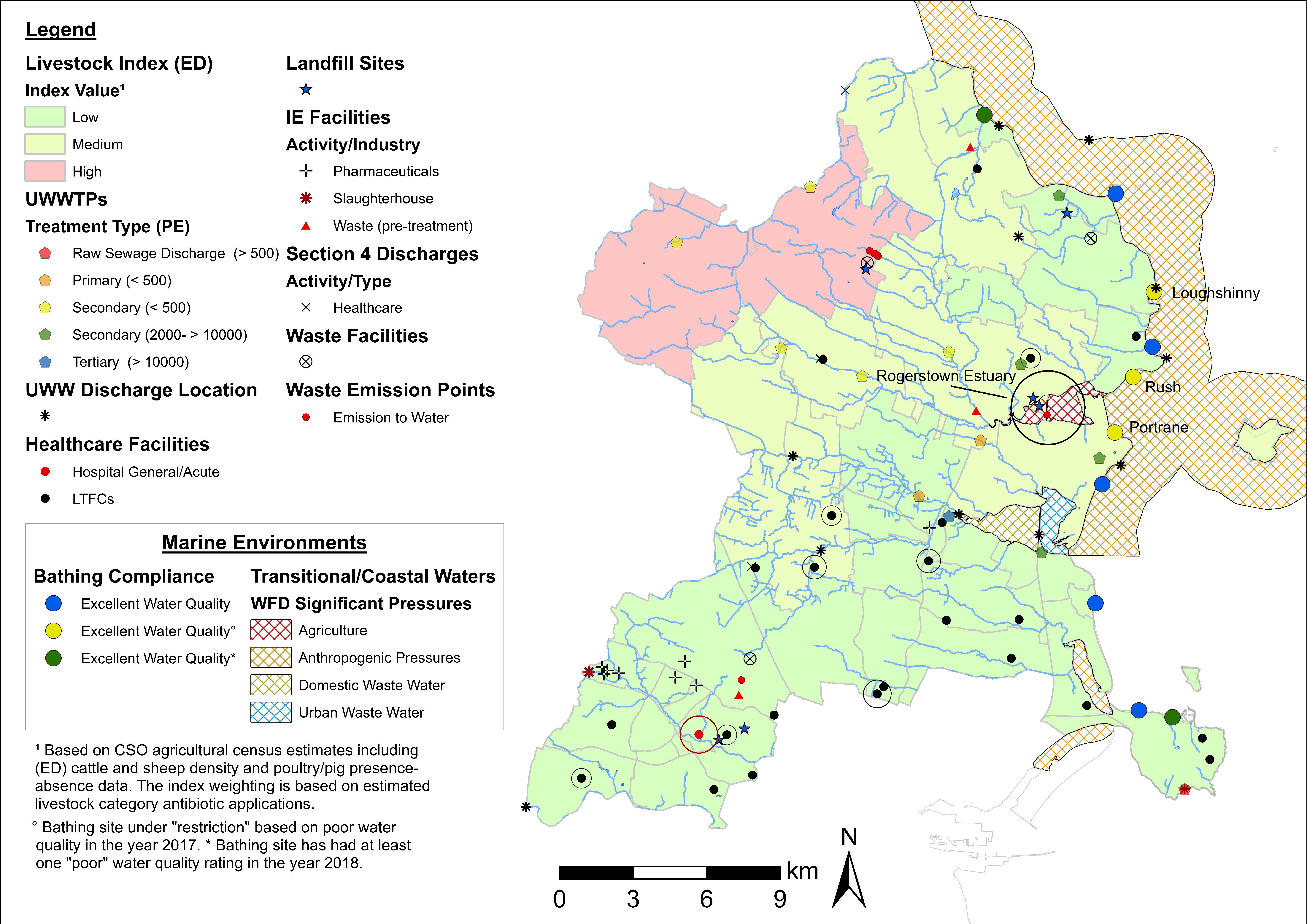
| Theme | Dataset | Source |
|---|---|---|
| Agriculture * | Livestock presence/absence (pig, poultry) *. Livestock numbers (cattle, sheep) *. Livestock density (cattle, sheep) * +. Livestock weighted index * +. | CSO |
| Demographics * | Population numbers *. Population density * +. | CSO |
| Groundwater | Groundwater wells (modified to only include domestic/agricultural boreholes). (EPA) Groundwater monitoring stations (including coliform concentrations). Groundwater vulnerability. (EPA-WFD) Significant human pressures on groundwater bodies. (EPA-NIP) Groundwater susceptibility to microbial pathogen percolation. (EPA-NIP) Groundwater at risk of DWWTS contamination. | EPA, GSI |
| Healthcare | General/acute hospitals and long-term care facilities. Antimicrobial usage rate in long-term care facilities °. Antimicrobial consumption in general/acute hospitals °. | HSE, HPSC |
| Hydrology (or Inland Waters) | Rivers and lakes. (EPA-WFD) Surface water bodies quality and significant human pressures. (EPA-NIP) Surface water bodies at risk of DWWTS contamination. | EPA, OSI |
| Marine Environments | Aquaculture (finfish farms). (EPA-WFD) Coastal/transitional water body quality and significant human pressures. Bathing site compliance (2 year records). Marine dumping sites. | DAFM, EPA |
| Water Supply | Drinking water treatment plants. Household private well prevalence *. | CSO, EPA |
| Wastewater | Combined sewage overflows. Integrated constructed wetlands. Raw sewage discharge points. Household septic tank prevalence *. Urban waste water treatment plants (treatment type and PE). Urban waste water discharge locations. Industrial emissions. Integrated pollution control facilities. Section 4 Discharges. | EPA, CSO |
| Waste Management | Waste and landfill facilities. Waste facility/landfill emissions. | EPA |
| LAAs | EDs | ARO Sources | Livestock Index Estimate | Healthcare Facilities |
|---|---|---|---|---|
| Galway | No Specific ED (off-shore) | 40 | N/A | N/A |
| Ballinasloe Urban | 16 | Low | Hospital (1), LTCFs (2) | |
| Athenry | 11 | High | LTCFs (1) | |
| Gort | 9 | Low | LTCFs (1) | |
| Portumna | 9 | Medium | LTCFs (1) | |
| Ballynakill | 8 | Low | N/A | |
| LAA Total | - | 350 | High (10%), Medium (40%), Low (50%) | Hospitals (3), LTCFs (44) |
| Cork | No Specific ED (off-shore) | 138 | N/A | N/A |
| Lehenagh | 33 | Medium | N/A | |
| Killaconenagh | 16 | Medium | LTCFs (1) | |
| Tramore (C) | 16 | Low | N/A | |
| Mitchelstown | 15 | Medium | LTCFs (1) | |
| Bantry Urban | 14 | Low | Hospital (1), LTCFs (1) | |
| LAA Total | - | 930 | High (16%), Medium (47%), Low (37%) | Hospitals (5), LTCFs (81) |
| Fingal | No Specific ED (off-shore) | 36 | N/A | N/A |
| Lusk | 9 | Medium | LTCFs (1) | |
| The Ward | 9 | Low | LTCFs (1) | |
| Blanchardstown-Abbotstown | 8 | Low | Hospital (1), LTCFs (1) | |
| Hollywood | 7 | High | N/A | |
| Kilsallaghan | 7 | Medium | LTCFs (2) | |
| LAA Total | - | 165 | High (4%), Medium (16%), Low (79%) | Hospitals (1), LTCFs (24) |
| Themes | Data Layers | Application |
|---|---|---|
| Healthcare, Hydrology, Wastewater. | Healthcare facilities (incl. AM use), rivers/lakes, Section 4 Discharges. | Identify water bodies in close proximity to healthcare facilities and/or in which healthcare facilities discharge effluents. |
| Healthcare, Hydrology, Water Supply, Wastewater. | Healthcare facilities (incl. AM use), drinking water treatment plants, rivers/lakes, Section 4 Discharges. | Identify drinking water treatment plants in close proximity to healthcare facilities and/or which are fed by water influenced by healthcare facilities effluents. |
| Healthcare, Groundwater, Water Supply, Wastewater. | Healthcare facilities (incl. AM use), drinking water treatment plants, EPA NIP-WFD groundwater body status *, and wastewater layers (UWW discharges, Section 4 Discharges, UWWTPs). | Identify drinking water treatment plants fed by groundwater at potential risk of contamination from healthcare and wastewater emissions. |
| Healthcare, Groundwater, Wastewater. | Healthcare facilities (including AM use), boreholes, EPA NIP-WFD groundwater body status *, septic tank prevalence, and wastewater layers (UWW discharges, Section 4 Discharges, UWWTPs, waste emission points). | Identify boreholes extracting groundwater at potential risk of contamination from wastewater, healthcare facilities, and septic tank seepage. |
| Hydrology, Groundwater, Wastewater. | Rivers/lakes, EPA-NIP surface water bodies at risk of DWWTPs contamination, EPA-NIP groundwater at risk of DWWTPs contamination, industrial emissions, and wastewater layers (UWW discharge location, UWWTPs, waste emission points). | Identify water bodies (surface and groundwater) at potential risk of contamination from DWWTPs, wastewater, and industrial sources. |
| Groundwater, Wastewater, Water Supply. | Drinking water treatment plants, EPA-NIP groundwater body status *, septic tank prevalence, and wastewater layers (UWW discharge location, UWWTPs, waste emission points). | Identify drinking water treatment plants fed by groundwater at potential risk of contamination from wastewater effluents and septic tank seepage. |
| Agriculture, Water Supply, Groundwater, Wastewater. | Drinking water treatment plants, EPA NIP-WFD groundwater body status *, livestock index, private well prevalence, and wastewater layers (UWW discharge location, UWWTPs, waste emission points). | Identify drinking water treatment plants and EDs with high private well prevalence (high risk of AROs human exposure) fed by groundwater at potential risk of contamination from wastewater effluents and agricultural emissions. |
| Agriculture, Hydrology, Water Supply, Wastewater. | ICWs, livestock index, rivers/lakes, and wastewater layers (UWW discharges, UWWTPs). | Identify ICWs located in areas with high livestock estimates and those receiving effluents from UWW and different types of UWWTPs discharges (including treatment type and PE). |
| Categories → LAAs ↓ | Groundwater Vulnerability | Susceptibility to Microbial Pathogen Percolation | Significant Human Pressures on Groundwater Bodies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rock/Karst | Extreme | High | Moderate | Low | Very High | High | Low | Urban Fabric | Anthropogenic | Agricultural | Domestic Waste Water | Industry | Waste | None | |
| County Galway | 15% (15%) | 25% (39%) | 24% (29%) | 14% (12%) | 19% (4%) | 15% (15%) | 26% (38%) | 58% (42%) | 0.6% (4%) | 38% (15%) | 19% (53%) | 3% (11%) | <0.1% (<0.1%) | <0.1% (N/A) | 40% (17%) |
| County Cork | 21% (9%) | 33% (30%) | 32% (48%) | 8% (10%) | 6% (3%) | 20% (10%) | 31% (28%) | 47% (56%) | 2% (6%) | 48% (82%) | 3% (1%) | 0.2% (2%) | <0.1% (<0.1%) | <0.1% (N/A) | 45% (16%) |
| Fingal County | 5% (5%) | 5% (5%) | 23% (30%) | 17% (N/A) | 39% (60%) | 5% (5%) | 13% (N/A) | 70% (70%) | 12% (25%) | 10% (10%) | N/A | N/A | 0.3% (N/A) | N/A | 89% (90%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chique, C.; Cullinan, J.; Hooban, B.; Morris, D. Mapping and Analysing Potential Sources and Transmission Routes of Antimicrobial Resistant Organisms in the Environment using Geographic Information Systems—An Exploratory Study. Antibiotics 2019, 8, 16. https://doi.org/10.3390/antibiotics8010016
Chique C, Cullinan J, Hooban B, Morris D. Mapping and Analysing Potential Sources and Transmission Routes of Antimicrobial Resistant Organisms in the Environment using Geographic Information Systems—An Exploratory Study. Antibiotics. 2019; 8(1):16. https://doi.org/10.3390/antibiotics8010016
Chicago/Turabian StyleChique, Carlos, John Cullinan, Brigid Hooban, and Dearbhaile Morris. 2019. "Mapping and Analysing Potential Sources and Transmission Routes of Antimicrobial Resistant Organisms in the Environment using Geographic Information Systems—An Exploratory Study" Antibiotics 8, no. 1: 16. https://doi.org/10.3390/antibiotics8010016
APA StyleChique, C., Cullinan, J., Hooban, B., & Morris, D. (2019). Mapping and Analysing Potential Sources and Transmission Routes of Antimicrobial Resistant Organisms in the Environment using Geographic Information Systems—An Exploratory Study. Antibiotics, 8(1), 16. https://doi.org/10.3390/antibiotics8010016





