Streptomyces Differentiation in Liquid Cultures as a Trigger of Secondary Metabolism
Abstract
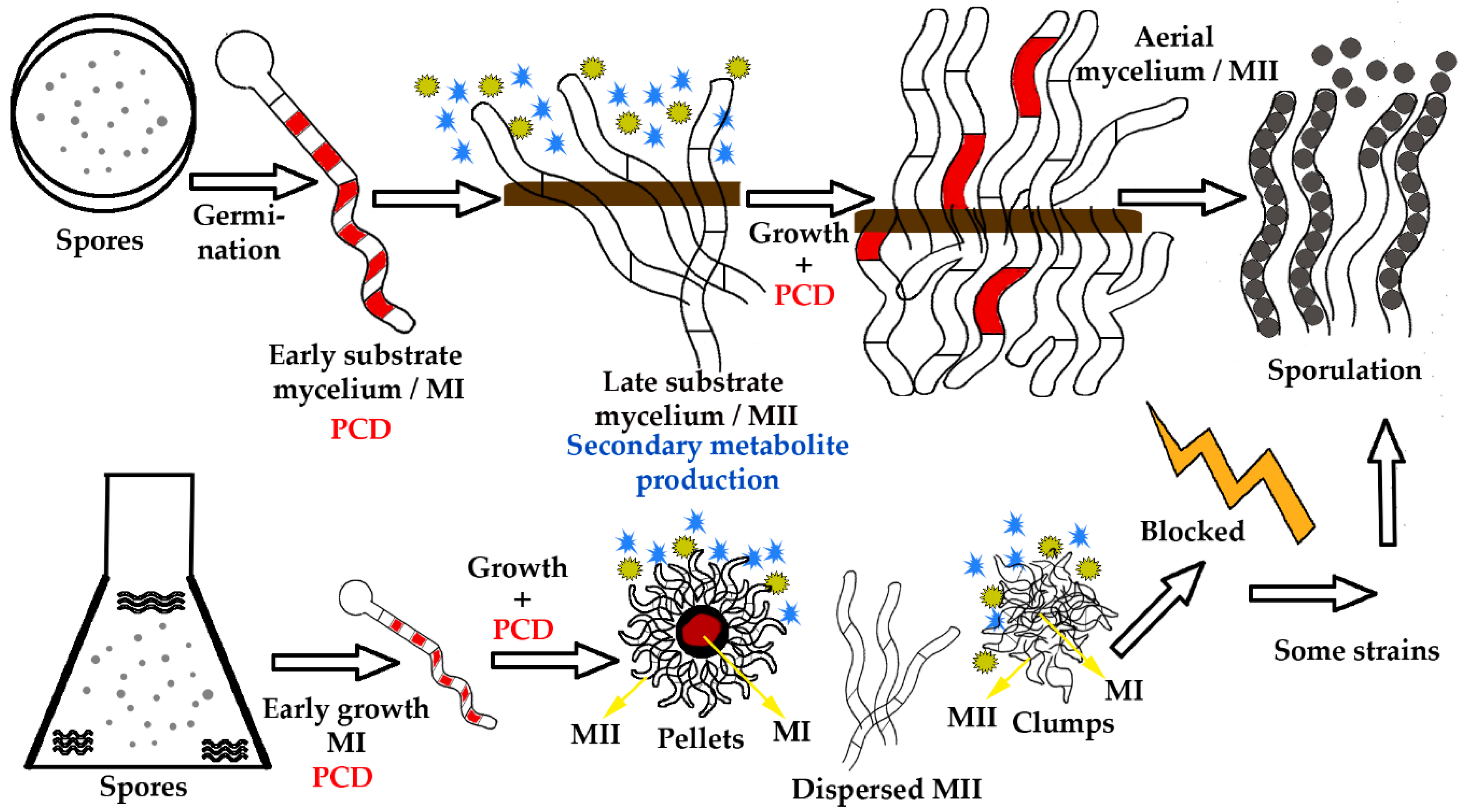
1. Introduction
2. Screening for New Secondary Metabolites from Streptomycetes
2.1. Streptomyces Differentiation Strategies Based on Elicitors
2.2. Differentiation Strategies Based on Macroscopic Morphology
2.2.1. The Genetic Control of Aggregation and Macroscopic Morphology in Liquid Cultures
2.2.2. Monitoring of Streptomyces Macroscopic Morphology and Differentiation in Liquid Cultures
2.2.3. Macroscopic Morphology Conditions, Programmed Cell Death and Second Mycelium Differentiation in Liquid Cultures
2.3. L-Forms
2.4. Other Strategies
3. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Worrall, J.A.; Vijgenboom, E. Copper mining in streptomyces: Enzymes, natural products and development. Nat. Prod. Rep. 2010, 27, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Manteca, A.; Alvarez, R.; Salazar, N.; Yague, P.; Sanchez, J. Mycelium differentiation and antibiotic production in submerged cultures of Streptomyces coelicolor. Appl. Environ. Microbiol. 2008, 74, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Yague, P.; Willemse, J.; Koning, R.I.; Rioseras, B.; Lopez-Garcia, M.T.; Gonzalez-Quinonez, N.; Lopez-Iglesias, C.; Shliaha, P.V.; Rogowska-Wrzesinska, A.; Koster, A.J.; et al. Subcompartmentalization by cross-membranes during early growth of streptomyces hyphae. Nat. Commun. 2016, 7, 12467. [Google Scholar] [CrossRef] [PubMed]
- Manteca, A.; Fernandez, M.; Sanchez, J. A death round affecting a young compartmentalized mycelium precedes aerial mycelium dismantling in confluent surface cultures of streptomyces antibioticus. Microbiology 2005, 151, 3689–3697. [Google Scholar] [CrossRef] [PubMed]
- Flardh, K. Growth polarity and cell division in streptomyces. Curr. Opin. Microbiol. 2003, 6, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Manteca, A.; Jung, H.R.; Schwammle, V.; Jensen, O.N.; Sanchez, J. Quantitative proteome analysis of Streptomyces coelicolor nonsporulating liquid cultures demonstrates a complex differentiation process comparable to that occurring in sporulating solid cultures. J. Proteome Res. 2010, 9, 4801–4811. [Google Scholar] [CrossRef] [PubMed]
- Yague, P.; Rodriguez-Garcia, A.; Lopez-Garcia, M.T.; Rioseras, B.; Martin, J.F.; Sanchez, J.; Manteca, A. Transcriptomic analysis of liquid non-sporulating Streptomyces coelicolor cultures demonstrates the existence of a complex differentiation comparable to that occurring in solid sporulating cultures. PLoS ONE 2014, 9, e86296. [Google Scholar] [CrossRef] [PubMed]
- Giudici, R.; Pamboukian, C.R.; Facciotti, M.C. Morphologically structured model for antitumoral retamycin production during batch and fed-batch cultivations of streptomyces olindensis. Biotechnol. Bioeng. 2004, 86, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Vecht-Lifshitz, S.E.; Sasson, Y.; Braun, S. Nikkomycin production in pellets of streptomyces tendae. J. Appl. Bacteriol. 1992, 72, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Sarra, M.; Casas, C.; Godia, F. Continuous production of a hybrid antibiotic by Streptomyces lividans TK21 pellets in a three-phase fluidized-bed bioreactor. Biotechnol. Bioeng. 1997, 53, 601–610. [Google Scholar] [CrossRef]
- Jonsbu, E.; McIntyre, M.; Nielsen, J. The influence of carbon sources and morphology on nystatin production by streptomyces noursei. J. Biotechnol. 2002, 95, 133–144. [Google Scholar] [CrossRef]
- Park, Y.; Tamura, S.; Koike, Y.; Toriyama, M.; Okabe, M. Mycelial pellet intrastructure visualization and viability prediction in a culture of streptomyces fradiae using confocal scanning laser microscopy. J. Ferment. Bioeng. 1997, 84, 483–486. [Google Scholar] [CrossRef]
- Rioseras, B.; Lopez-Garcia, M.T.; Yague, P.; Sanchez, J.; Manteca, A. Mycelium differentiation and development of Streptomyces coelicolor in lab-scale bioreactors: Programmed cell death, differentiation, and lysis are closely linked to undecylprodigiosin and actinorhodin production. Bioresour. Technol. 2014, 151, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Treppiccione, L.; Ottombrino, A.; Luongo, D.; Maurano, F.; Manteca, A.; Lombó, F.; Rossi, M. Development of gluten with immunomodulatory properties using mTG-active food grade supernatants from Streptomyces mobaraensis cultures. J. Funct. Foods 2017, 34, 390–397. [Google Scholar] [CrossRef]
- Marin, L.; Gutierrez-Del-Rio, I.; Yague, P.; Manteca, A.; Villar, C.J.; Lombo, F. De novo biosynthesis of apigenin, luteolin, and eriodictyol in the actinomycete Streptomyces albus and production improvement by feeding and spore conditioning. Front. Microbiol. 2017, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Han, X.; Du, M.; Zhang, Y.; Feng, Z.; Yi, H.; Zhang, Y. Enhancement of transglutaminase production in Streptomyces mobaraensis as achieved by treatment with excessive MgCl2. Appl. Microbiol. Biotechnol. 2012, 93, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Onaka, H. Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes. J. Antibiot. 2017, 70, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O. The re-emerging role of microbial natural products in antibiotic discovery. Antonie Van Leeuwenhoek 2014, 106, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O. Actinomycetes: Still a source of novel antibiotics. Nat. Prod. Rep. 2017, 34, 1203–1232. [Google Scholar] [CrossRef] [PubMed]
- Craney, A.; Ahmed, S.; Nodwell, J. Towards a new science of secondary metabolism. J. Antibiot. 2013, 66, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Yoon, V.; Nodwell, J.R. Activating secondary metabolism with stress and chemicals. J. Ind. Microbiol. Biotechnol. 2014, 41, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Y.; Chater, K.F.; Ou, H.Y.; Xu, H.H.; Deng, Z.; Tao, M. Large-scale transposition mutagenesis of Streptomyces coelicolor identifies hundreds of genes influencing antibiotic biosynthesis. Appl. Environ. Microbiol. 2017, 83, e02889-16. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, S.; Akhtar, K.; Afzal Ghauri, M.; Iqbal, R.; Mukhtar Khalid, A.; Muddassar, M. Change in colony morphology and kinetics of tylosin production after UV and gamma irradiation mutagenesis of streptomyces fradiae NRRL-2702. Microbiol. Res. 2009, 164, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Korbekandi, H.; Darkhal, P.; Hojati, Z.; Abedi, D.; Hamedi, J.; Pourhosein, M. Overproduction of clavulanic acid by UV mutagenesis of Streptomyces clavuligerus. Iran. J. Pharm. Res. 2010, 9, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Ohnishi-Kameyama, M.; Muramatsu, H.; Murakami, K.; Tsurumi, Y.; Kodani, S.; Yoshida, M.; Fujie, A.; Ochi, K. Antibacterial discovery in actinomycetes strains with mutations in RNA polymerase or ribosomal protein S12. Nat. Biotechnol. 2009, 27, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Craney, A.; Pimentel-Elardo, S.M.; Nodwell, J.R. A synthetic, speciesspecific activator of secondary metabolism and sporulation in Streptomyces coelicolor. Chembiochem 2013, 14, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ece, S.; Lambertz, C.; Fischer, R.; Commandeur, U. Heterologous expression of a Streptomyces cyaneus laccase for biomass modification applications. AMB Express 2017, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Streptomyces and saccharopolyspora hosts for heterologous expression of secondary metabolite gene clusters. J. Ind. Microbiol. Biotechnol. 2010, 37, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Daniel-Ivad, M.; Hameed, N.; Tan, S.; Dhanjal, R.; Socko, D.; Pak, P.; Gverzdys, T.; Elliot, M.A.; Nodwell, J.R. An engineered allele of afsQ1 facilitates the discovery and investigation of cryptic natural products. ACS Chem. Biol. 2017, 12, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Olano, C.; Mendez, C.; Salas, J.A. Post-PKS tailoring steps in natural product-producing actinomycetes from the perspective of combinatorial biosynthesis. Nat. Prod. Rep. 2010, 27, 571–616. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Rodríguez, M.; Braña, A.F.; Méndez, C.; Salas, J.A.; Olano, C. New insights into paulomycin biosynthesis pathway in Streptomyces albus j1074 and generation of novel derivatives by combinatorial biosynthesis. Microb. Cell Fact. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Synthetic biology, genome mining, and combinatorial biosynthesis of NRPS-derived antibiotics: A perspective. J. Ind. Microbiol. Biotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, J.; Li, X.; Wang, J.; Li, S.; Pan, G.; Fan, K.; Yang, K. Angucyclines as signals modulate the behaviors of Streptomyces coelicolor. Proc. Natl. Acad. Sci. USA 2014, 111, 5688–5693. [Google Scholar] [CrossRef] [PubMed]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-cultivation—A powerful emerging tool for enhancing the chemical diversity of microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2004, 21, 519–538. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Q.; Chen, C.; Qi, X. Antifungal activity change of streptomyces rimosus MY02 mediated by confront culture with other microorganism. J. Basic Microbiol. 2017, 57, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.; Munoz-Dorado, J.; Brana, A.F.; Shimkets, L.J.; Sevillano, L.; Santamaria, R.I. Myxococcus Xanthus induces actinorhodin overproduction and aerial mycelium formation by Streptomyces coelicolor. Microb. Biotechnol. 2011, 4, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Zhuang, Y.; Kong, F.; Zhang, C.; Zhu, W. Phenolic polyketides from the co-cultivation of marine-derived Penicillium sp. Wc-29-5 and Streptomyces fradiae 007. Mar. Drugs 2014, 12, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Sung, A.A.; Gromek, S.M.; Balunas, M.J. Upregulation and identification of antibiotic activity of a marine-derived Streptomyces sp. Via co-cultures with human pathogens. Mar. Drugs 2017, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yuan, J.; Gu, S.; Shi, Q. Influence of fungal elicitors on biosynthesis of natamycin by streptomyces natalensis HW-2. Appl. Microbiol. Biotechnol. 2013, 97, 5527–5534. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Jamwal, V.; Singh, V.P.; Wazir, P.; Awasthi, P.; Singh, D.; Vishwakarma, R.A.; Gandhi, S.G.; Chaubey, A. Revelation and cloning of valinomycin synthetase genes in streptomyces lavendulae acr-da1 and their expression analysis under different fermentation and elicitation conditions. J. Biotechnol. 2017, 253, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Gubbens, J.; Janus, M.M.; Florea, B.I.; Overkleeft, H.S.; van Wezel, G.P. Identification of glucose kinase-dependent and -independent pathways for carbon control of primary metabolism, development and antibiotic production in Streptomyces coelicolor by quantitative proteomics. Mol. Microbiol. 2017, 105, 175. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Yang, Y.H.; Song, E.; Kim, E.J.; Kim, W.S.; Sohng, J.K.; Lee, H.C.; Liou, K.K.; Kim, B.G. Mass spectrometric screening of transcriptional regulators involved in antibiotic biosynthesis in Streptomyces coelicolor A3(2). J. Ind. Microbiol. Biotechnol. 2009, 36, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chater, K.F.; Chandra, G.; Niu, G.; Tan, H. Molecular regulation of antibiotic biosynthesis in streptomyces. Microbiol. Mol. Biol. Rev. 2013, 77, 112–143. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Elardo, S.M.; Sorensen, D.; Ho, L.; Ziko, M.; Bueler, S.A.; Lu, S.; Tao, J.; Moser, A.; Lee, R.; Agard, D.; et al. Activity-independent discovery of secondary metabolites using chemical elicitation and cheminformatic inference. ACS Chem. Biol. 2015, 10, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Van Dissel, D.; Claessen, D.; Roth, M.; van Wezel, G.P. A novel locus for mycelial aggregation forms a gateway to improved streptomyces cell factories. Microb. Cell Fact. 2015, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.K.; Petrus, M.L.; Mangiameli, G.; Hough, M.A.; Svistunenko, D.A.; Nicholls, P.; Claessen, D.; Vijgenboom, E.; Worrall, J.A. Glxa is a new structural member of the radical copper oxidase family and is required for glycan deposition at hyphal tips and morphogenesis of Streptomyces lividans. Biochem. J. 2015, 469, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Petrus, M.L.; Vijgenboom, E.; Chaplin, A.K.; Worrall, J.A.; van Wezel, G.P.; Claessen, D. The dyp-type peroxidase dtpa is a tat-substrate required for glxa maturation and morphogenesis in streptomyces. Open Biol. 2016, 6, 150149. [Google Scholar] [CrossRef] [PubMed]
- Petrus, M.L.; Claessen, D. Pivotal roles for streptomyces cell surface polymers in morphological differentiation, attachment and mycelial architecture. Antonie Van Leeuwenhoek 2014, 106, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Susstrunk, U.; Pidoux, J.; Taubert, S.; Ullmann, A.; Thompson, C.J. Pleiotropic effects of camp on germination, antibiotic biosynthesis and morphological development in Streptomyces coelicolor. Mol. Microbiol. 1998, 30, 33–46. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.; Manteca, A.; Sanchez, J.; Bucca, G.; Smith, C.P.; Dijkhuizen, L.; Claessen, D.; Wosten, H.A. Nepa is a structural cell wall protein involved in maintenance of spore dormancy in Streptomyces coelicolor. Mol. Microbiol. 2009, 71, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Bobek, J.; Strakova, E.; Zikova, A.; Vohradsky, J. Changes in activity of metabolic and regulatory pathways during germination of S. coelicolor. BMC Genomics 2014, 15, 1173. [Google Scholar] [CrossRef] [PubMed]
- Rioseras, B.; Yague, P.; Lopez-Garcia, M.T.; Gonzalez-Quinonez, N.; Binda, E.; Marinelli, F.; Manteca, A. Characterization of SCO4439, a d-alanyl-d-alanine carboxypeptidase involved in spore cell wall maturation, resistance, and germination in Streptomyces coelicolor. Sci. Rep. 2016, 6, 21659. [Google Scholar] [CrossRef] [PubMed]
- Sexton, D.L.; St-Onge, R.J.; Haiser, H.J.; Yousef, M.R.; Brady, L.; Gao, C.; Leonard, J.; Elliot, M.A. Resuscitation-promoting factors are Cell wall-lytic enzymes with important roles in the germination and growth of Streptomyces coelicolor. J. Bacteriol. 2015, 197, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Noens, E.E.; Mersinias, V.; Willemse, J.; Traag, B.A.; Laing, E.; Chater, K.F.; Smith, C.P.; Koerten, H.K.; van Wezel, G.P. Loss of the controlled localization of growth stage-specific cell-wall synthesis pleiotropically affects developmental gene expression in an ssga mutant of Streptomyces coelicolor. Mol. Microbiol. 2007, 64, 1244–1259. [Google Scholar] [CrossRef] [PubMed]
- Zacchetti, B.; Willemse, J.; Recter, B.; van Dissel, D.; van Wezel, G.P.; Wosten, H.A.; Claessen, D. Aggregation of germlings is a major contributing factor towards mycelial heterogeneity of streptomyces. Sci. Rep. 2016, 6, 27045. [Google Scholar] [CrossRef] [PubMed]
- Girard, G.; Traag, B.A.; Sangal, V.; Mascini, N.; Hoskisson, P.A.; Goodfellow, M.; van Wezel, G.P. A novel taxonomic marker that discriminates between morphologically complex actinomycetes. Open Biol. 2013, 3, 130073. [Google Scholar] [CrossRef] [PubMed]
- Ronnest, N.P.; Stocks, S.M.; Lantz, A.E.; Gernaey, K.V. Comparison of laser diffraction and image analysis for measurement of Streptomyces coelicolor cell clumps and pellets. Biotechnol. Lett. 2012, 34, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Van Veluw, G.J.; Petrus, M.L.; Gubbens, J.; de Graaf, R.; de Jong, I.P.; van Wezel, G.P.; Wosten, H.A.; Claessen, D. Analysis of two distinct mycelial populations in liquid-grown streptomyces cultures using a flow cytometry-based proteomics approach. Appl. Microbiol. Biotechnol. 2012, 96, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Petrus, M.L.; van Veluw, G.J.; Wosten, H.A.; Claessen, D. Sorting of streptomyces cell pellets using a complex object parametric analyzer and sorter. J. Vis. Exp. 2014, e51178. [Google Scholar] [CrossRef] [PubMed]
- Willemse, J.; Buke, F.; van Dissel, D.; Grevink, S.; Claessen, D.; van Wezel, G.P. Sparticle, an algorithm for the analysis of filamentous microorganisms in submerged cultures. Antonie Van Leeuwenhoek 2017, 171–182. [Google Scholar] [CrossRef]
- Celler, K.; Cristian, P.; van Loosdrecht, M.C.; van Wezel, G.P. Structured morphological modeling as a framework for rational strain design of streptomyces species. Antonie Van Leeuwenhoek 2012, 102, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, L.; Webb, S.; Smith, M.C.; Hoskisson, P.A. A flexible mathematical model platform for studying branching networks: Experimentally validated using the model actinomycete, Streptomyces coelicolor. PLoS ONE 2013, 8, e54316. [Google Scholar] [CrossRef] [PubMed]
- Van Dissel, D.; Claessen, D.; van Wezel, G.P. Morphogenesis of streptomyces in submerged cultures. Adv. Appl. Microbiol. 2014, 89, 1–45. [Google Scholar] [PubMed]
- Ha, S.; Lee, K.J.; Lee, S.I.; Gwak, H.J.; Lee, J.H.; Kim, T.W.; Choi, H.J.; Jang, J.Y.; Choi, J.S.; Kim, C.J.; et al. Optimization of herbicidin a production in submerged culture of streptomyces scopuliridis m40. J. Microbiol. Biotechnol. 2017, 27, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Van Dissel, D.; van Wezel, G.P. Morphology-driven downscaling of streptomyces lividans to micro-cultivation. Antonie Van Leeuwenhoek 2017, 457–469. [Google Scholar] [CrossRef]
- Rigali, S.; Nothaft, H.; Noens, E.E.; Schlicht, M.; Colson, S.; Müller, M.; Joris, B.; Koerten, H.K.; Hopwood, D.A.; Titgemeyer, F.; van Wezel, G.P. The sugar phosphotransferase system of Streptomyces coelicolor is regulated by the gntr-family regulator dasr and links n-acetylglucosamine metabolism to the control of development. Mol. Microbiol. 2006, 61, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Rigali, S.; Titgemeyer, F.; Barends, S.; Mulder, S.; Thomae, A.W.; Hopwood, D.A.; van Wezel, G.P. Feast or famine: The global regulator dasr links nutrient stress to antibiotic production by streptomyces. EMBO Rep. 2008, 9, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Yague, P.; Manteca, A.; Simon, A.; Diaz-Garcia, M.E.; Sanchez, J. New method for monitoring programmed cell death and differentiation in submerged streptomyces cultures. Appl. Environ. Microbiol. 2010, 76, 3401–3404. [Google Scholar] [CrossRef] [PubMed]
- Yague, P.; Lopez-Garcia, M.T.; Rioseras, B.; Sanchez, J.; Manteca, A. Pre-sporulation stages of streptomyces differentiation: State-of-the-art and future perspectives. FEMS Microbiol. Lett. 2013, 342, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Innes, C.M.; Allan, E.J. Induction, growth and antibiotic production of streptomyces viridifaciens l-form bacteria. J. Appl. Microbiol. 2001, 90, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kealey, C.; Creaven, C.A.; Murphy, C.D.; Brady, C.B. New approaches to antibiotic discovery. Biotechnol. Lett. 2017, 39, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, C.; Guo, L.; Piao, C.; Li, Z.; Li, J.; Jia, F.; Wang, X.; Xiang, W. Streptomyces formicae sp. Nov., a novel actinomycete isolated from the head of Camponotus japonicus mayr. Antonie Van Leeuwenhoek 2016, 109, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Vizcaino, A.; Brana, A.F.; Gonzalez, V.; Nava, H.; Molina, A.; Llera, E.; Fiedler, H.P.; Rico, J.M.; Garcia-Florez, L.; Acuna, J.L.; et al. Atmospheric dispersal of bioactive Streptomyces albidoflavus strains among terrestrial and marine environments. Microb. Ecol. 2016, 71, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Vizcaino, A.; Gonzalez, V.; Brana, A.F.; Palacios, J.J.; Otero, L.; Fernandez, J.; Molina, A.; Kulik, A.; Vazquez, F.; Acuna, J.L.; et al. Pharmacological potential of phylogenetically diverse actinobacteria isolated from deep-sea coral ecosystems of the submarine aviles canyon in the cantabrian sea. Microb. Ecol. 2017, 73, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Schniete, J.K.; Cruz-Morales, P.; Selem-Mojica, N.; Fernandez-Martinez, L.T.; Hunter, I.S.; Barona-Gomez, F.; Hoskisson, P.A. Expanding primary metabolism helps generate the metabolic robustness to facilitate antibiotic biosynthesis in streptomyces. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Cihak, M.; Kamenik, Z.; Smidova, K.; Bergman, N.; Benada, O.; Kofronova, O.; Petrickova, K.; Bobek, J. Secondary metabolites produced during the germination of Streptomyces coelicolor. Front. Microbiol. 2017, 8, 2495. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jia, X.; Wen, J.; Lu, W.; Wang, G.; Caiyin, Q.; Chen, Y. Strain improvement of streptomyces roseosporus for daptomycin production by rational screening of he-ne laser and NTG induced mutants and kinetic modeling. Appl. Biochem. Biotechnol. 2011, 163, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Jakeman, D.L.; Graham, C.L.; Young, W.; Vining, L.C. Culture conditions improving the production of jadomycin b. J. Ind. Microbiol. Biotechnol. 2006, 33, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Hofs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chembiochem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Bursy, J.; Kuhlmann, A.U.; Pittelkow, M.; Hartmann, H.; Jebbar, M.; Pierik, A.J.; Bremer, E. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl. Environ. Microbiol. 2008, 74, 7286–7296. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Hobbs, G.; Smith, C.P.; Oliver, S.G.; Butler, P.R. Environmental signals triggering methylenomycin production by Streptomyces coelicolor A3(2). J. Bacteriol. 1997, 179, 5511–5515. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Alam, M.; Breitling, R.; Takano, E. The future of industrial antibiotic production: From random mutagenesis to synthetic biology. Bioeng. Bugs. 2011, 230–233. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Huang, J.; Qiang, H.; Lin, W.L.; Demain, A.L. Mutagenesis of the rapamycin producer streptomyces hygroscopicus FC904. J. Antibiot. 2001, 54, 967–972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, G.; Hosaka, T.; Ochi, K. Dramatic activation of antibiotic production in Streptomyces coelicolor by cumulative drug resistance mutations. Appl. Environ. Microbiol. 2008, 74, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, N.L.; Thaker, M.; Koteva, K.; Hughes, D.W.; Wright, G.D.; Nodwell, J.R. Induction of antimicrobial activities in heterologous streptomycetes using alleles of the Streptomyces coelicolor gene absa1. J. Antibiot. 2010, 63, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Foley, T.L.; Young, B.S.; Burkart, M.D. Phosphopantetheinyl transferase inhibition and secondary metabolism. FEBS J. 2009, 276, 7134–7145. [Google Scholar] [CrossRef] [PubMed]
- Onaka, H. Biosynthesis of indolocarbazole and goadsporin, two different heterocyclic antibiotics produced by actinomycetes. Biosci. Biotechnol. Biochem. 2009, 73, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Recio, E.; Colinas, A.; Rumbero, A.; Aparicio, J.F.; Martin, J.F. Pi factor, a novel type quorum-sensing inducer elicits pimaricin production in streptomyces natalensis. J. Biol. Chem. 2004, 279, 41586–41593. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Ban, Y.H.; Park, J.W.; Yoo, Y.J.; Yoon, Y.J. Enhanced FK506 production in Streptomyces clavuligerus ckd1119 by engineering the supply of methylmalonyl-coa precursor. J. Ind. Microbiol. Biotechnol. 2009, 36, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, M.; Qian, J.; Zhuang, Y.; Zhang, S. Disruption of ZWF2 gene to improve oxytetraclyline biosynthesis in streptomyces rimosus M4018. Wei Sheng Wu Xue Bao 2008, 48, 21–25. [Google Scholar] [PubMed]
- Yagüe, P.; Manteca, A. Optimization of the antibiotic producction in different Streptomyces strains by conditioning the MII differentiation. 2018. Unpublished results. [Google Scholar]
- Malla, S.; Niraula, N.P.; Liou, K.; Sohng, J.K. Self-resistance mechanism in streptomyces peucetius: Overexpression of drra, drrb and drrc for doxorubicin enhancement. Microbiol. Res. 2010, 165, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhuo, Y.; Zhu, D.; Zhou, X.; Zhang, L.; Bai, L.; Deng, Z. Overexpression of the abc transporter avtab increases avermectin production in streptomyces avermitilis. Appl. Microbiol. Biotechnol. 2011, 92, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Willems, A.; Au-Yeung, C.; Tahlan, K.; Nodwell, J.R. A two-step mechanism for the activation of actinorhodin export and resistance in Streptomyces coelicolor. MBio 2012, 3, e00191-12. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, N.L.; Nodwell, J.R. Phosphorylated absa2 negatively regulates antibiotic production in Streptomyces coelicolor through interactions with pathway-specific regulatory gene promoters. J. Bacteriol. 2007, 189, 5284–5292. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, J.; Li, L.; Chen, Z.; Wen, Y. Li, J. The pathway-specific regulator aver from Streptomyces avermitilis positively regulates avermectin production while it negatively affects oligomycin biosynthesis. Mol. Genet. Genomics 2010, 283, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Retzlaff, L.; Distler, J. The regulator of streptomycin gene expression, strr, of streptomyces griseus is a DNA binding activator protein with multiple recognition sites. Mol. Microbiol. 1995, 18, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Laureti, L.; Song, L.; Huang, S.; Corre, C.; Leblond, P.; Challis, G.L.; Aigle, B. Identification of a bioactive 51-membered macrolide complex by activation of a silent polyketide synthase in Streptomyces ambofaciens. Proc. Natl. Acad. Sci. USA 2011, 108, 6258–6626. [Google Scholar] [CrossRef] [PubMed]
- Menendez, N.; Brana, A.F.; Salas, J.A.; Mendez, C. Involvement of a chromomycin abc transporter system in secretion of a deacetylated precursor during chromomycin biosynthesis. Microbiology 2007, 153, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Bunet, R.; Song, L.; Mendes, M.V.; Corre, C.; Hotel, L.; Rouhier, N.; Framboisier, X.; Leblond, P.; Challis, G.L.; Aigle, B. Characterization and manipulation of the pathway-specific late regulator alpw reveals Streptomyces ambofaciens as a new producer of kinamycins. J. Bacteriol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escribano, J.P.; Bibb, M.J. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb. Biotechnol. 2011, 4, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Uchiyama, T.; Omura, S.; Cane, D.E.; Ikeda, H. Genome-minimized streptomyces host for the heterologous expression of secondary metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Novakova, R.; Nunez, L.E.; Homerova, D.; Knirschova, R.; Feckova, L.; Rezuchova, B.; Sevcikova, B.; Menendez, N.; Moris, F.; Cortes, J.; et al. Increased heterologous production of the antitumoral polyketide mithramycin a by engineered streptomyces lividans TK24 strains. Appl. Microbiol. Biotechnol. 2018, 102, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Izumikawa, M.; Kozone, I.; Hashimoto, J.; Kagaya, N.; Koiwai, H.; Komatsu, M.; Fujie, M.; Sato, N.; Ikeda, H.; et al. Neothioviridamide, a polythioamide compound produced by heterologous expression of a Streptomyces sp. Cryptic ripp biosynthetic gene cluster. J. Nat. Prod. 2018, 264–269. [Google Scholar] [CrossRef] [PubMed]
| Methods | Microorganism | Product | Effect | Ref. |
|---|---|---|---|---|
| Media manipulation | S. roseosporus | Daptomycin | Enhance | [78] |
| Stress Response | S. venezuelae | Jadomycin B | Enhance | [79] |
| S. hygroscopicus | Validamycin A | Enhance | [21] | |
| S. parvulus | Manumycin family | Cryptic | [80] | |
| S. coelicolor | Ectoine, 5-hydroxyectoine | Enhance | [81] | |
| S. coelicolor | Methylenomycin | Enhance | [82] | |
| One Strain Many Compounds (OSMAC) | S. parvulus | 20 cryptic compounds | Cryptic | [80] |
| Random Mutagenesis | S. clavuligerus | Clavulanic acid | Enhance | [83] |
| S. hygroscopicus | Rapamycin | Enhance | [84] | |
| S. coelicolor | Actinorhodin, Undecylprodigiosin | Enhance | [22] | |
| Ribosomal Engineering | S. coelicolor | Actinorhodin | Enhance | [85] |
| Engineering Global Regulation | S. coelicolor | Actinorhodin, Prodigiosin, Calcium-Dependent Antibiotic | Enhance | [86] |
| S. griseus | Streptomycin | Enhance | [86] | |
| S. griseochromogenes | Blasticidin S | Enhance | [86] | |
| Elicitors | S. coelicolor | Actinorhodin | Enhance | [87] |
| S. pristinaespiralis | Desferrioxamine B/E | Enhance | [20] | |
| S. peucetius | Doxorubicin, Baumycin | Enhance | [20] | |
| S. coelicolor | Actinorhodin, Undecylprodigiosin | Enhance | [68] | |
| S. lividans | Prodiginine | Enhance | [88] | |
| S. griseus | Streptomycin | Enhance | [21] | |
| S. natalensis | Pimaricin | Enhance | [89] | |
| 29 strains | Cryptic compounds | Cryptic | [45] | |
| Metabolic Engineering | S. clavuligerus | FK606 | Enhance | [90] |
| S. coelicolor | Actinorhodin | Enhance | [90] | |
| S. rimosus | Oxytetracycline | Enhance | [91] | |
| Co-cultures | S. rimosus MY02 | Antifungal activity | Enhance | [36] |
| S. coelicolor | Actinorhodin | Enhance | [37] | |
| S. fradiae 007 | Phenolic polyketides | Enhance | [38] | |
| Marine streptomycetes | See tables in reference | Cryptic | [39] | |
| Conditioning Morphology (PCD + MII) | S. cattleya | Tienamycin | Enhance | [92] |
| S. cinereoruber | Rodomycin | Enhance | ||
| Saccharopolyspora erythraea | Erithromycin | Enhance | ||
| S. coelicolor | Actinorhodin | Enhance |
| Methods | Microorganism | Product | Effect | Ref. |
|---|---|---|---|---|
| Engineering Self-Resistance | S. peucetius | Doxorubicin, Daunorubicin | Enhance | [93] |
| S. avermitilis | Avermectin, | Enhance | [94] | |
| S. coelicolor | Actinorhodin | Enhance | [95] | |
| Regulatory Engineering | ||||
| Delete repressor AbsA2~P | S. coelicolor | Actinorhodin, Undecylprodigiosin, Calcium-dependent antibiotic | Enhance | [96] |
| Overexpress AverR/StrR | S. avermitilis | Avermectin | Enhance | [97] |
| Overexpress AverR/StrR | S. griseous | Streptomycin | Enhance | [98] |
| Overexpress SamR0484 | S. ambofaciens | Stambomicin A-D | Cryptic | [99] |
| Delete repressor cmmRII | S. griseus | Chromomycin | Enhance | [100] |
| Delete repressor AlpW | S. ambofaciens | Alpomycin | Enhance | [101] |
| Heterologous Expression | S. avermitilis | Streptomycin | Enhance | [102] |
| S. coelicolor | Chloramphenicol | Enhance | [103] | |
| S. coelicolor | Congocidine | Enhance | [103] | |
| S. cyaneus | CECT 3335 laccase | Enhance | [27] | |
| S. lividans TK24 | Mithramycin A | Enhance | [104] | |
| Streptomyces sp. | Neothioviridamide | Cryptic | [105] | |
| Several wild-type | Siamycin-I | Cryptic | [29] | |
| Combinatorial Biosynthesis | S. albus J1074 | Novel paulomycin | Cryptic | [31] |
| See table 1 in ref. | [30] | |||
| Conditioning Morphology (PCD + MII) | S. albus | Apigenin, Luteolin | Enhance | [15] |
| S. mobarensis | Microbial transglutaminase | Enhance | [14] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manteca, Á.; Yagüe, P. Streptomyces Differentiation in Liquid Cultures as a Trigger of Secondary Metabolism. Antibiotics 2018, 7, 41. https://doi.org/10.3390/antibiotics7020041
Manteca Á, Yagüe P. Streptomyces Differentiation in Liquid Cultures as a Trigger of Secondary Metabolism. Antibiotics. 2018; 7(2):41. https://doi.org/10.3390/antibiotics7020041
Chicago/Turabian StyleManteca, Ángel, and Paula Yagüe. 2018. "Streptomyces Differentiation in Liquid Cultures as a Trigger of Secondary Metabolism" Antibiotics 7, no. 2: 41. https://doi.org/10.3390/antibiotics7020041
APA StyleManteca, Á., & Yagüe, P. (2018). Streptomyces Differentiation in Liquid Cultures as a Trigger of Secondary Metabolism. Antibiotics, 7(2), 41. https://doi.org/10.3390/antibiotics7020041





