Antimicrobial and Cytoprotective Effects of Tea Extracts Against Escherichia coli-Producing Colibactin Toxin Infections
Abstract
1. Introduction
2. Results
2.1. Phytochemical Compounds in Tea Extracts
2.2. Antibacterial Activity by Agar Well Diffusion
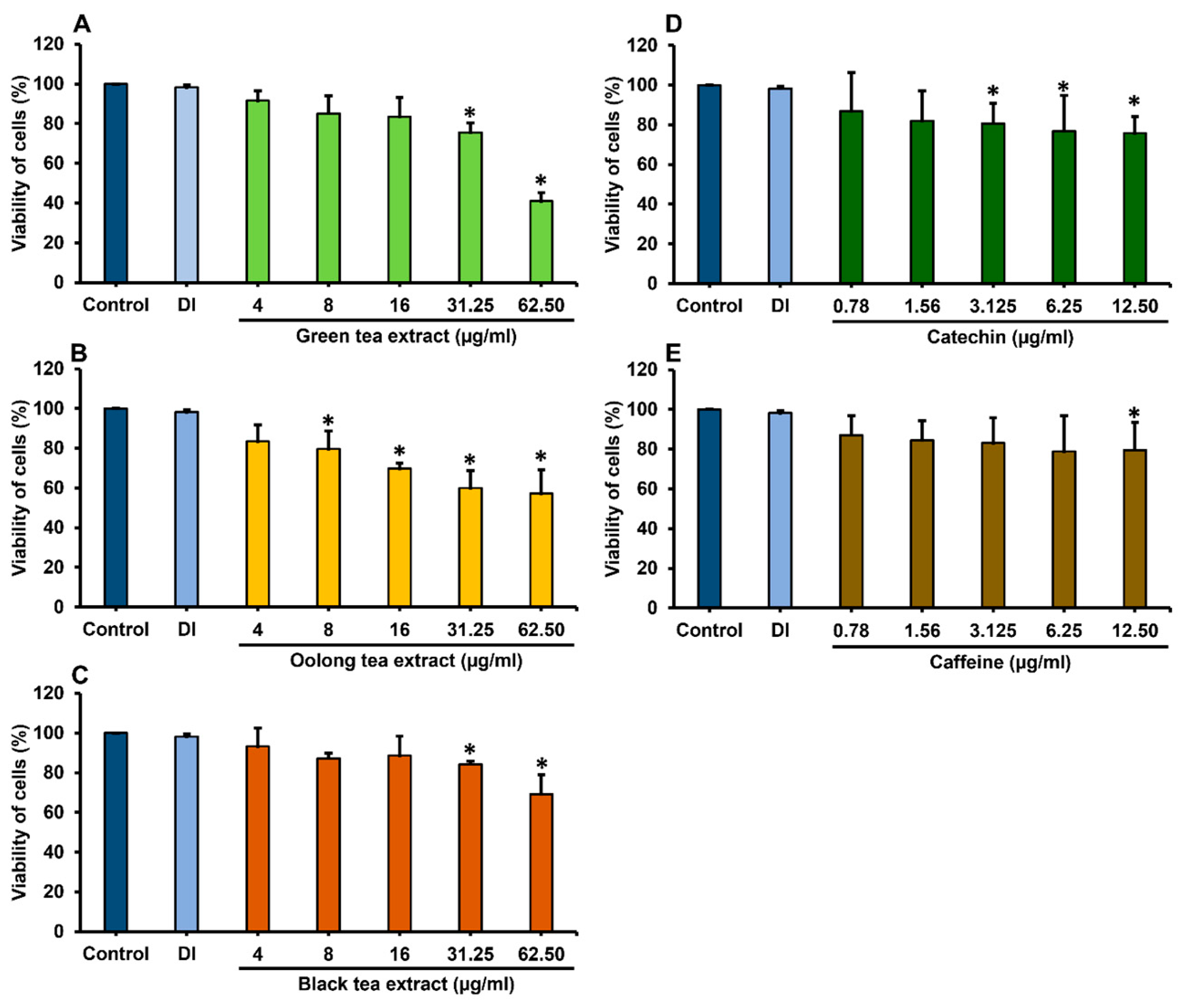
2.3. Cytotoxicity of Tea Extracts and Phytochemical Compounds on Caco-2 Cells
2.4. Inhibitory Effects of Tea Extracts and Phytochemical Compounds on Transient Infection of Caco-2 Cells by E. coli ATCC 25922
2.5. Inhibition of Colibactin-Induced DNA Damage in Eukaryotic Cells by Treatment with Tea Extracts and Phytochemical Compounds

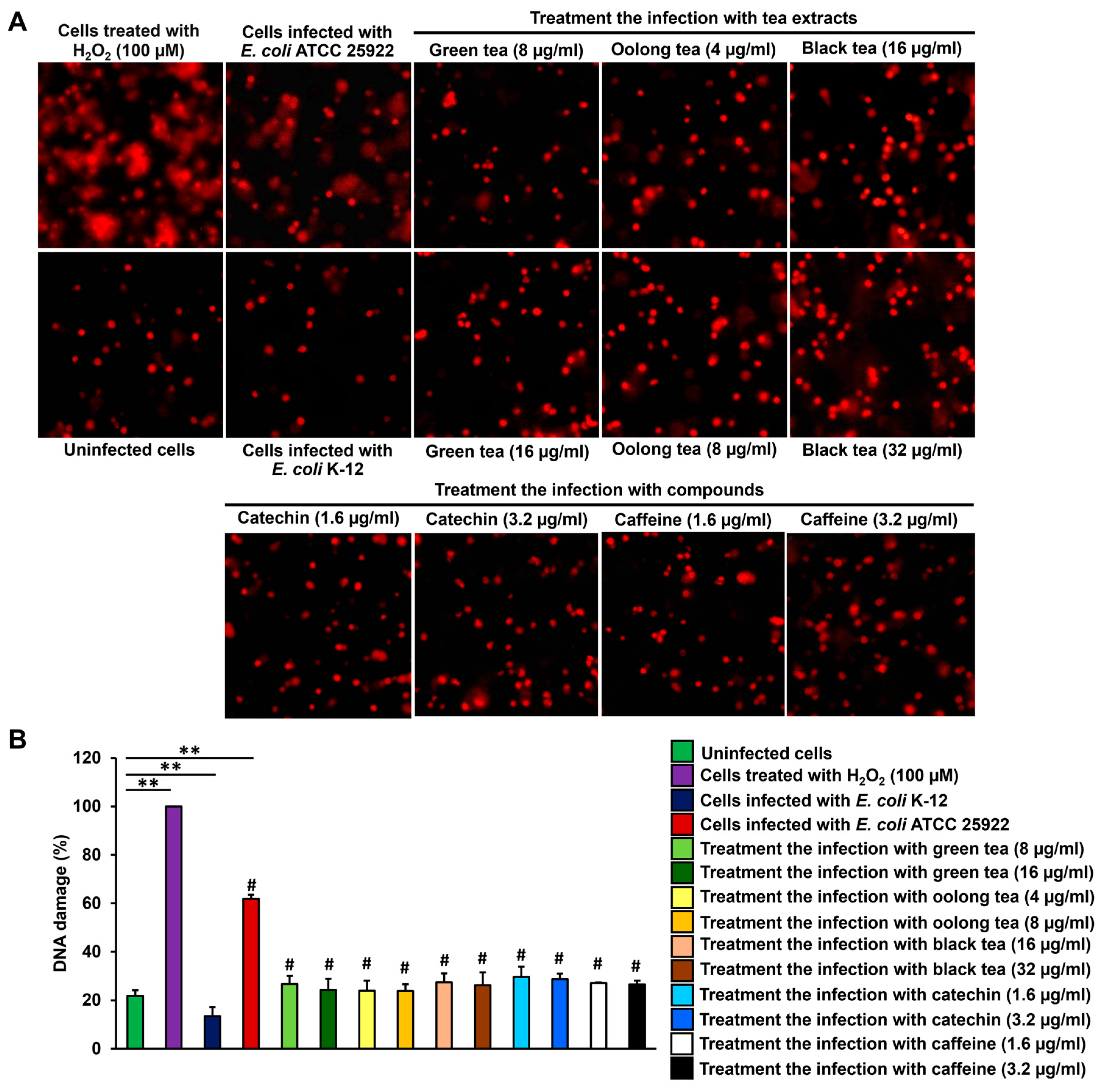
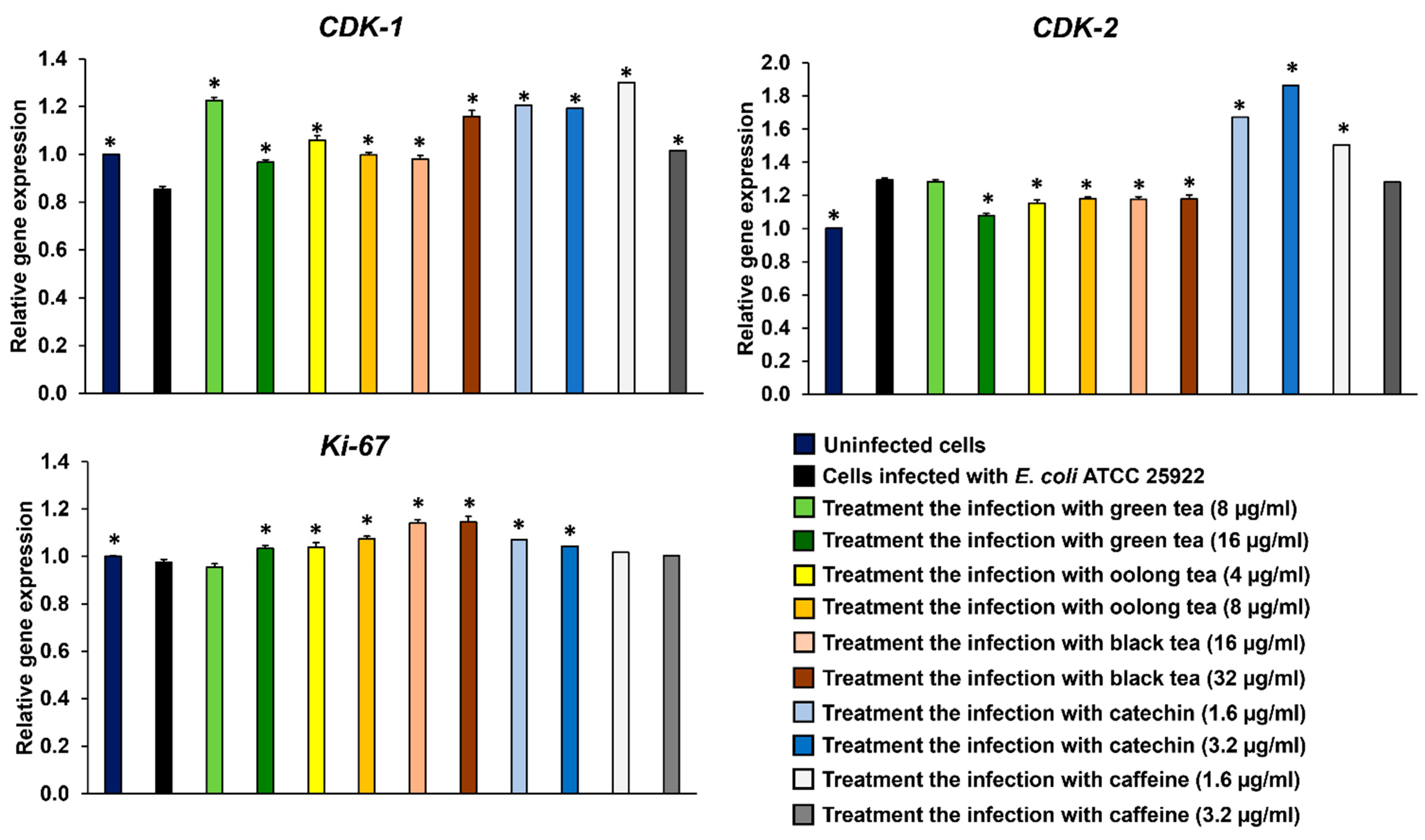
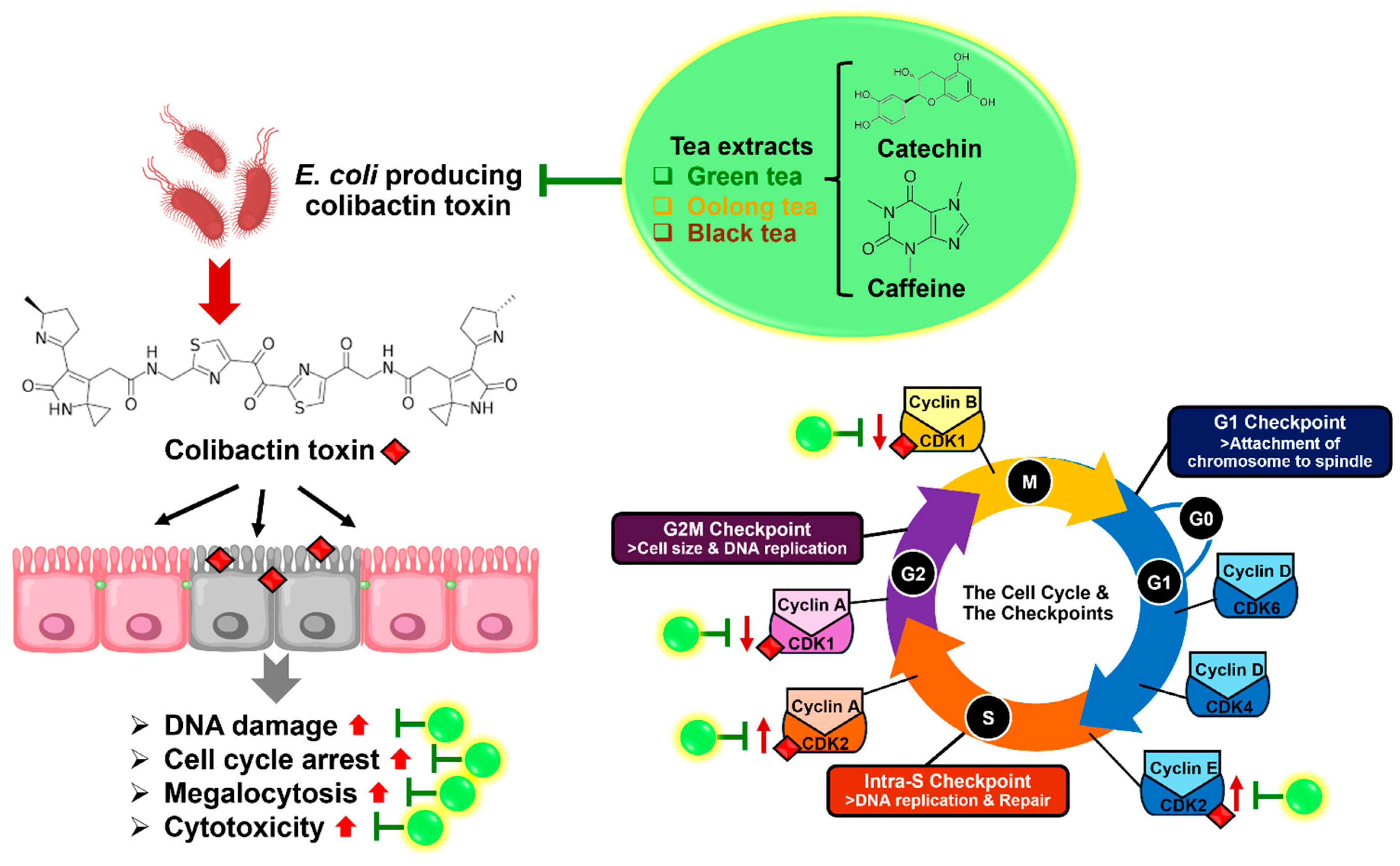
2.6. Effect of Colon Cell Cycle After Infection with E. coli-Producing Colibactin Toxin by Treatment with Tea Extracts and Phytochemical Compounds
3. Discussion
4. Materials and Methods
4.1. Tea Extraction
4.2. High Performance Liquid Chromatography (HPLC)
4.3. Bacterial Strains
4.4. Agar Well Diffusion Assay
4.5. Cytotoxicity Assay
4.6. Transient Infection and Treatment
4.7. DNA Damage by Alkaline Comet Assay
4.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Pekal, A.; Drozdz, P.; Biesaga, M.; Pyrzynska, K. Evaluation of the antioxidant properties of fruit and flavoured black teas. Eur. J. Nutr. 2011, 50, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Koch, W. Handbook of Dietary Phytochemicals; Springer: Singapore, 2020; pp. 1–29. [Google Scholar]
- Jiang, H.; Yu, F.; Qin, L.; Zhang, N.; Cao, Q.; Schwab, W.; Li, D.; Song, C. Dynamic change in amino acids, catechins, alkaloids, and gallic acid in six types of tea processed from the same batch of fresh tea (Camellia sinensis L.) leaves. J. Food Compos. Anal. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Dou, J.; Lee, V.S.; Tzen, J.T.; Lee, M.R. Identification and comparison of phenolic compounds in the preparation of oolong tea manufactured by semifermentation and drying processes. J. Agric. Food Chem. 2007, 55, 7462–7468. [Google Scholar] [CrossRef]
- Salman, S.; Yılmaz, C.; Gökmen, V.; Özdemir, F. Effects of fermentation time and shooting period on amino acid derivatives and free amino acid profiles of tea. LWT-Food Sci. Technol. 2021, 137, 110481. [Google Scholar] [CrossRef]
- McKay, D.; Blumberg, J. The role of tea in human health: An update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, C.T. Polyphenolic chemistry of tea and coffee: A century of progress. J. Agric. Food Chem. 2009, 57, 8109–8114. [Google Scholar] [CrossRef]
- Sabu, M.C.; Kuttan, R. Antidiabetic activity of medicinal plants and its relationship with antioxidant property. J. Ethnopharmacol. 2002, 81, 155–160. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Cancer and metastasis: Prevention and treatment by green tea. Cancer Metastasis Rev. 2010, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyoshi, N.; Isemura, M. Health-promoting effects of green tea. Proc. Jpn. Acad. Ser. B 2012, 88, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.A.; Avramovich-Tirosh, Y.; Reznichenko, L.; Zheng, H.; Weinreb, O.; Amit, T.; Youdim, M.B. Multifunctional activities of green tea catechins in neuroprotection. Neurosignals 2005, 14, 46–60. [Google Scholar] [CrossRef]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsuji, I.; Tsubono, Y. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. J. Am. Med. Assoc. 2006, 296, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Thavanesan, N. The putative effects of green tea on body fat: An evaluation of the evidence and a review of the potential mechanisms. Br. J. Nutr. 2011, 106, 1297–1309. [Google Scholar] [CrossRef]
- Shareef, S.H.; Ibrahim, I.A.A.; Alzahrani, A.R.; Al-Medhtiy, M.H.; Abdulla, M.A. Hepatoprotective effects of methanolic extract of green tea against thioacetamide-induced liver injury in Sprague Dawley rats. Saudi J. Biol. Sci. 2022, 29, 564–573. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, Q.S.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Antiviral effects of green tea EGCG and its potential application against COVID-19. Molecules 2021, 26, 3962. [Google Scholar] [CrossRef]
- Engleberg, N.C.; DiRita, V.J.; Dermody, T.S. Mechanisms of Microbial Disease, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1–864. [Google Scholar]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylotyping method revisited: Improvement of specificity and detection of new phylogroups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Smith, J.L.; Fratamico, P.M.; Gunther, N.W. Extraintestinal pathogenic Escherichia coli. Foodborne Pathog. Dis. 2007, 4, 134–163. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Toh, H.; Ogura, Y.; Sasamoto, H.; Morita, H.; Park, S.H.; Ooka, T.; Iyoda, S.; Taylor, T.D.; Hayashi, T.; et al. Complete genome sequence and comparative analysis of the wildtype commensal Escherichia coli strain SE11 isolated from a healthy adult. DNA Res. 2008, 15, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Samudio, V.; Pimentel-Peralta, G.; De La Cruz, A.; Landires, I. Multidrug-resistant phenotypes of genetically diverse Escherichia coli isolates from healthy domestic cats. Sci. Rep. 2024, 14, 11260. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Páramo, P.; Grenet, K.; Le Menac’h, A.; Rode, L.; Salgado, E.; Amorin, C.; Gouriou, S.; Picard, B.; Rahimy, M.C.; Andremont, A.; et al. Large-scale population structure of human commensal Escherichia coli isolates. Appl. Environ. Microbiol. 2004, 70, 5698–5700. [Google Scholar] [CrossRef]
- Nowrouzian, F.L.; Wold, A.E.; Adlerberth, I. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. J. Infect. Dis. 2005, 191, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Nougayrède, J.P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef]
- Cuevas-Ramos, G.; Petit, C.R.; Marcq, I.; Boury, M.; Oswald, E.; Nougayrède, J.P. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11537–11542. [Google Scholar] [CrossRef]
- Sakurikar, N.; Eastman, A. Critical reanalysis of the methods that discriminate the activity of CDK2 from CDK1. Cell Cycle 2016, 15, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Markelova, N.N.; Semenova, E.F.; Sineva, O.N.; Sadykova, V.S. The role of cyclomodulins and some microbial metabolites in bacterial microecology and macroorganism carcinogenesis. Int. J. Mol. Sci. 2022, 23, 11706. [Google Scholar] [CrossRef]
- Tomkovich, S.; Yang, Y.; Winglee, K.; Gauthier, J.; Mühlbauer, M.; Sun, X.; Mohamadzadeh, M.; Liu, X.; Martin, P.; Wang, G.P.; et al. Locoregional effects of microbiota in a preclinical model of colon carcinogenesis. Cancer Res. 2017, 77, 2620–2632. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef]
- Farhoosh, R.; Golmovahhed, G.A.; Khodaparast, M.H.H. Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.). Food Chem 2007, 100, 231–236. [Google Scholar] [CrossRef]
- Piran, F.; Khoshkhoo, Z.; Hosseini, S.E.; Azizi, M.H. Controlling the antioxidant activity of green tea extract through encapsulation in chitosan-citrate nanogel. J. Food Qual. 2020, 2020, 7935420. [Google Scholar] [CrossRef]
- Johnson, J.R.; Johnston, B.; Kuskowski, M.A.; Nougayrede, J.P.; Oswald, E. Molecular epidemiology and phylogenetic distribution of the Escherichia coli pks genomic island. J. Clin. Microbiol. 2008, 46, 3906–3911. [Google Scholar] [CrossRef]
- Kaewkod, T.; Tobe, R.; Tragoolpua, Y.; Mihara, H. Medicinal plant extracts protect epithelial cells from infection and DNA damage caused by colibactin-producing Escherichia coli, and inhibit the growth of bacteria. J. Appl. Microbiol. 2021, 130, 769–785. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Paul, D.; Kim, D.H.; Chun, S. Bactericidal activity of green tea extracts: The importance of catechin containing nanoparticles. Sci. Rep. 2016, 6, 19710. [Google Scholar] [CrossRef] [PubMed]
- Maeyama, R.; Kwon, I.K.; Mizunoe, Y.; Anderson, J.M.; Tanaka, M.; Matsuda, T. Novel bactericidal surface: Catechin-loaded surface-erodible polymer prevents biofilm formation. J. Biomed. Mater. Res. 2005, 75 Pt A, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Matsumoto, M.; Tanaka, T.; Maeda, M.; Nakai, M.; Hamada, S.; Ooshima, T. Antibacterial activity of polyphenol components in oolong tea extract against Streptococcus mutans. Caries Res. 2004, 38, 2–8. [Google Scholar] [CrossRef]
- Kiran, S.; Ratho, R.K.; Sharma, P.; Harjai, K.; Capalash, N.; Tiwari, R.P. Effect of black tea (Camellia sinensis) on virulence traits of clinical isolates of Shigella dysenteriae and Escherichia coli EPEC P2 1265 strain. Eur. Food Res. Technol. 2010, 231, 763–770. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Bajaj, P.R.; Singhal, R.S. Tea polyphenols as nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2008, 7, 229–254. [Google Scholar] [CrossRef]
- Rathi, B.; Gupta, S.; Kumar, P.; Kesarwani, V.; Dhanda, R.S.; Kushwaha, S.K.; Yadav, M. Anti-biofilm activity of caffeine against uropathogenic E. coli is mediated by curli biogenesis. Sci. Rep. 2022, 12, 18903. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Derbyshire, E. Tea compounds and the gut microbiome: Findings from trials and mechanistic studies. Nutrients 2019, 11, 2364. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Canani, R.B.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef]
- Açar, Y.; Ağagündüz, D.; De Cicco, P.; Capasso, R. Flavonoids: Their putative neurologic roles, epigenetic changes, and gut microbiota alterations in Parkinson’s disease. Biomed. Pharmacother. 2023, 168, 115788. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, L.; You, Y.; Gao, R.; Xiang, J.; Wang, G.; Yu, W. Quantitative analysis of bioactive compounds in commercial teas: Profiling catechin alkaloids, phenolic acids, and flavonols using targeted statistical approaches. Foods 2023, 12, 3098. [Google Scholar] [CrossRef]
- Fernandez, P.L.; Fernando, P.; Martin, J.; Gonzalez, A.G. Study of catechin and xanthine tea profiles as geographical tracers. J. Agric. Food Chem. 2002, 50, 1833–1839. [Google Scholar] [CrossRef]
- Minogue, T.D.; Daligault, H.A.; Davenport, K.W.; Bishop Lilly, K.A.; Broomall, S.M.; Bruce, D.C.; Chain, P.S.; Chertkov, O.; Coyne, S.R.; Freitas, T.; et al. Complete genome assembly of Escherichia coli ATCC 25922, a serotype O6 reference strain. Genome Announc. 2014, 2, 5. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.; Wang, J.; Chen, Q.; Zeng, J.; Liu, A.; Chen, Z.; Lu, X. Oral administration of green tea polyphenols (TP) improves ileal injury and intestinal flora disorder in mice with Salmonella typhimurium infection via resisting inflammation, enhancing antioxidant action and preserving tight junction. J. Funct. Foods 2020, 64, 103654. [Google Scholar] [CrossRef]
- Hsu, S. Green tea and the skin. J. Am. Acad. Dermatol. 2005, 52, 1049–1059. [Google Scholar] [CrossRef]
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Okaji, Y.; Yazawa, K.; Asakage, M.; Hori, N.; Watanabe, T.; Takahashi, K.; Nagawa, H. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J. Allergy Clin. Immunol. 2003, 112, 951–957. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Zhang, Y. Studies on the effects of tea catechins against hepatitis B virus infection. Chin. J. Prev. Med. 2001, 35, 404–407. [Google Scholar]
- Apostolides, Z.; Selematsela, M. Anti-Retroviral Agent in Combination with Tea Polyphenol for the Treatment of Viral Infections. PCT International Application WO 2003002126 A1, 9 January 2003. [Google Scholar]
- Storozhuk, M.; Lee, S.; Lee, J.I.; Park, J. Green tea consumption and the COVID-19 omicron pandemic era: Pharmacology and epidemiology. Life 2023, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.F.; Fisher, L.J.; Hara, Y.; Harris, T.; Mak, W.B.; Melton, L.D.; Packer, J.E. Green tea catechins partially protect DNA from ·OH radical-induced strand breaks and base damage through fast chemical repair of DNA radicals. Carcinogenesis 2001, 22, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jeong, W.S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Mercer, J.R.; Gray, K.; Figg, N.; Kumar, S.; Bennett, M.R. The methyl xanthine caffeine inhibits DNA damage signaling and reactive species and reduces atherosclerosis in ApoE−/− mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2461–2467. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H. Pleiotropic effects of caffeine leading to chromosome instability and cytotoxicity in eukaryotic microorganisms. J. Microbiol. Biotechnol. 2021, 31, 171. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Torii, Y.; Uchida, K.; Nakamura, Y.; Hara, Y.; Osawa, T. Black tea polyphenols, theaflavins, prevent cellular DNA damage by inhibiting oxidative stress and suppressing cytochrome P450 1A1 in cell cultures. J. Agric. Food Chem. 2002, 50, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kager, N.; Ferk, F.; Kundi, M.; Wagner, K.H.; Mišík, M.; Knasmüller, S. Prevention of oxidative DNA damage in inner organs and lymphocytes of rats by green tea extract. Eur. J. Nutr. 2010, 49, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Hou, J.; Wu, Z.; Zhuang, Y.; Lu, M.; Zhang, Y.; Zhou, X.; Li, Z.; Xiao, W.; et al. CDK1 interplays with Oct4 to repress differentiation of embryonic stem cells into trophectoderm. FEBS Lett. 2012, 586, 4100–4107. [Google Scholar] [CrossRef]
- Neganova, I.; Tilgner, K.; Buskin, A.; Paraskevopoulou, I.; Atkinson, S.P.; Peberdy, D.; Passos, J.F.; Lako, M. CDK1 plays an important role in the maintenance of pluripotency and genomic stability in human pluripotent stem cells. Cell Death Dis. 2014, 5, e1508. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zhang, X.J.; Liu, H.X.; Chen, J.; Ren, Y.H.; Huang, D.G.; Zou, X.H.; Xiao, W. Cdk1 is required for self-renewal of mouse embryonic stem cells. J. Cell. Biochem. 2011, 112, 942–948. [Google Scholar] [CrossRef]
- Zardavas, D.; Pondé, N.; Tryfonidis, K. CDK4/6 blockade in breast cancer: Current experience and future perspectives. Expert Opin. Investig. Drugs 2017, 26, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, A.; Santamaría, D.; Martínez-Pastor, B.; Cuadrado, M.; Fernández-Capetillo, O.; Barbacid, M. Overall CDK activity modulates the DNA damage response in mammalian cells. J. Cell Biol. 2009, 187, 773–780. [Google Scholar] [CrossRef]
- Wang, J.; Yang, T.; Xu, G.; Liu, H.; Ren, C.; Xie, W.; Wang, M. Cyclin-dependent kinase 2 promotes tumor proliferation and induces radio resistance in glioblastoma. Transl. Oncol. 2016, 9, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R.; Battista, J.R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013, 8, e56964. [Google Scholar] [CrossRef] [PubMed]
- Prorok-Hamon, M.; Friswell, M.K.; Alswied, A.; Roberts, C.L.; Song, F.; Flanagan, P.K.; Knight, P.; Codling, C.; Marchesi, J.R.; Winstanley, C.; et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 2014, 63, 761–770. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Eric, W.C.; Eu, Y.S.; Pei, P.T.; Yon, P.L. Antioxidant and antibacterial properties of green, black and herbal teas of Camellia sinensis. J. Pharm. Pharmacogn. Res. 2011, 3, 266–272. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Umthong, S.; Phuwapraisirisan, P.; Puthong, S.; Chanchao, C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement. Altern. Med. 2011, 11, 37. [Google Scholar] [CrossRef]
- Bossuet-Greif, N.; Belloy, M.; Boury, M.; Oswald, E.; Nougayrede, J. Protocol for HeLa cells infection with Escherichia coli strains producing colibactin and quantification of the induced DNA-damage. Bio-protocol 2017, 7, e2325. [Google Scholar] [CrossRef]
- Kothandapani, A.; Sawant, A.; Dangeti, V.S.; Sobol, R.W.; Patrick, S.M. Epistatic role of base excision repair and mismatch repair pathways in mediating cisplatin cytotoxicity. Nucleic Acids Res. 2013, 41, 7332–7343. [Google Scholar] [CrossRef]
- Sawant, A.; Floyd, A.M.; Dangeti, M.; Lei, W.; Sobol, R.W.; Patrick, S.M. Differential role of base excision repair proteins in mediating cisplatin cytotoxicity. DNA Repair 2017, 51, 46–59. [Google Scholar] [CrossRef]
- Chang, H.Y.; Shih, M.H.; Huang, H.C.; Tsai, S.R.; Juan, H.F.; Lee, S.C. Middle infrared radiation induces G2/M cell cycle arrest in A549 lung cancer cells. PLoS ONE 2013, 8, e54117. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.; Anshabo, A.T.; Portman, N.; Lim, E.; Tilley, W.; Caldon, C.E.; Wang, S. Targeting CDK2 in cancer: Challenges and opportunities for therapy. Drug Discov. Today 2020, 25, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Chierico, L.; Rizzello, L.; Guan, L.; Joseph, A.S.; Lewis, A.; Battaglia, G. The role of the two splice variants and extranuclear pathway on Ki-67 regulation in non-cancer and cancer cells. PLoS ONE 2017, 12, e0171815. [Google Scholar] [CrossRef] [PubMed]
| Tea Extracts | Chemical Compound Contents (mg/mL) | |||
|---|---|---|---|---|
| Catechin | EGCG | Caffeine | Theaflavin | |
| Green tea | 12.680 ± 0.019 a | 37.622 ± 0.163 a | 56.909 ± 0.444 a | ND a |
| Oolong tea | 233.475 ± 13.320 b | 251.839 ± 8.751 b | 2.658 ± 0.053 b | 2.192 ± 0.297 b |
| Black tea | ND c | 269.714 ± 7.860 c | ND c | ND c |
| Samples | Concentration (mg/mL) | Zone of Inhibition (mm) * | |
|---|---|---|---|
| E. coli ATCC 25922 | E. coli K-12 | ||
| Tea extracts | |||
| Green tea | 500 | 16.3 ± 4.5 | 15.0 ± 1.7 |
| Oolong tea | 500 | 18.3 ± 0.5 | 16.0 ± 1.0 |
| Black tea | 500 | 13.7 ± 1.3 | 14.0 ± 1.0 |
| Phytochemical compounds | |||
| Catechin | 5.0 | 16.3 ± 0.6 | 15.7 ± 0.6 |
| Caffeine | 5.0 | 19.3 ± 0.6 | 19.0 ± 0.0 |
| Gentamycin | 1.0 | 23.8 ± 0.5 | 26.3 ± 0.6 |
| Samples | E. coli ATCC 25922 Infection with Caco-2 Cells | E. coli K-12 Infection with Caco-2 Cells | ||
|---|---|---|---|---|
| Concentration (µg/mL) | Viability of Cells (%) * | Concentration (µg/mL) | Viability of Cells (%) * | |
| Tea extracts | ||||
| Green tea | 8 | 86.15 ± 7.03 b | 8 | 80.58 ± 16.05 a |
| 16 | 83.13 ± 1.99 b | 16 | 86.47 ± 12.59 a | |
| Oolong tea | 4 | 87.93 ± 14.22 a | 4 | 88.01 ± 8.03 a |
| 8 | 90.78 ± 2.76 b | 8 | 82.31 ± 11.49 a | |
| Black tea | 16 | 81.42 ± 9.96 a | 16 | 86.98 ± 11.88 a |
| 32 | 87.58 ± 9.74 b | 32 | 88.28 ± 11.25 a | |
| Phytochemical compounds | ||||
| Catechin | 1.6 | 81.77 ± 9.47 b | 1.6 | 80.97 ± 7.04 a |
| 3.2 | 85.59 ± 9.74 b | 3.2 | 84.49 ± 7.13 a | |
| Caffeine | 1.6 | 76.43 ± 13.64 a | 1.6 | 83.58 ± 11.42 a |
| 3.2 | 79.81 ± 6.46 a | 3.2 | 85.29 ± 9.19 a | |
| No treatment | - | 69.74 ± 4.18 a | - | 78.92 ± 0.62 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teppabut, W.; Tragoolpua, Y.; Kaewkod, T. Antimicrobial and Cytoprotective Effects of Tea Extracts Against Escherichia coli-Producing Colibactin Toxin Infections. Antibiotics 2025, 14, 886. https://doi.org/10.3390/antibiotics14090886
Teppabut W, Tragoolpua Y, Kaewkod T. Antimicrobial and Cytoprotective Effects of Tea Extracts Against Escherichia coli-Producing Colibactin Toxin Infections. Antibiotics. 2025; 14(9):886. https://doi.org/10.3390/antibiotics14090886
Chicago/Turabian StyleTeppabut, Wipawadee, Yingmanee Tragoolpua, and Thida Kaewkod. 2025. "Antimicrobial and Cytoprotective Effects of Tea Extracts Against Escherichia coli-Producing Colibactin Toxin Infections" Antibiotics 14, no. 9: 886. https://doi.org/10.3390/antibiotics14090886
APA StyleTeppabut, W., Tragoolpua, Y., & Kaewkod, T. (2025). Antimicrobial and Cytoprotective Effects of Tea Extracts Against Escherichia coli-Producing Colibactin Toxin Infections. Antibiotics, 14(9), 886. https://doi.org/10.3390/antibiotics14090886








