Utilization of a Multi-Tissue Extracellular Matrix in Complex Wound Care in Gaza: A Case Series
Abstract
1. Introduction
2. Results
2.1. Case A (Upper Extremity Reconstruction)
2.2. Case B (Pediatric Multi-System Injury)
2.3. Case C (Traumatic Amputation and Limb Salvage)
2.4. Case D (Complex Foot Injury with Exposed Bone and Tendon)
2.5. Case E (Infected Lower Limb Wound with Exposed Implant)
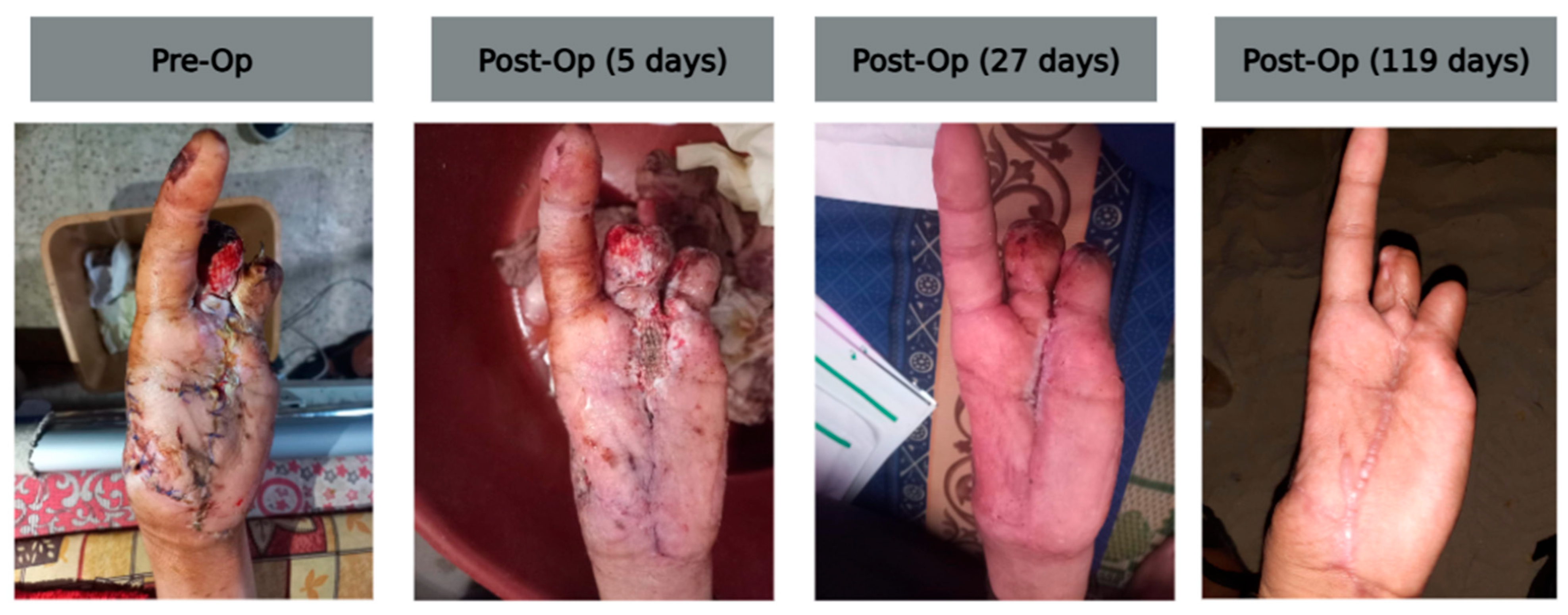
2.6. Case F (Explosive Thumb Injury with Skeletal Fixation)
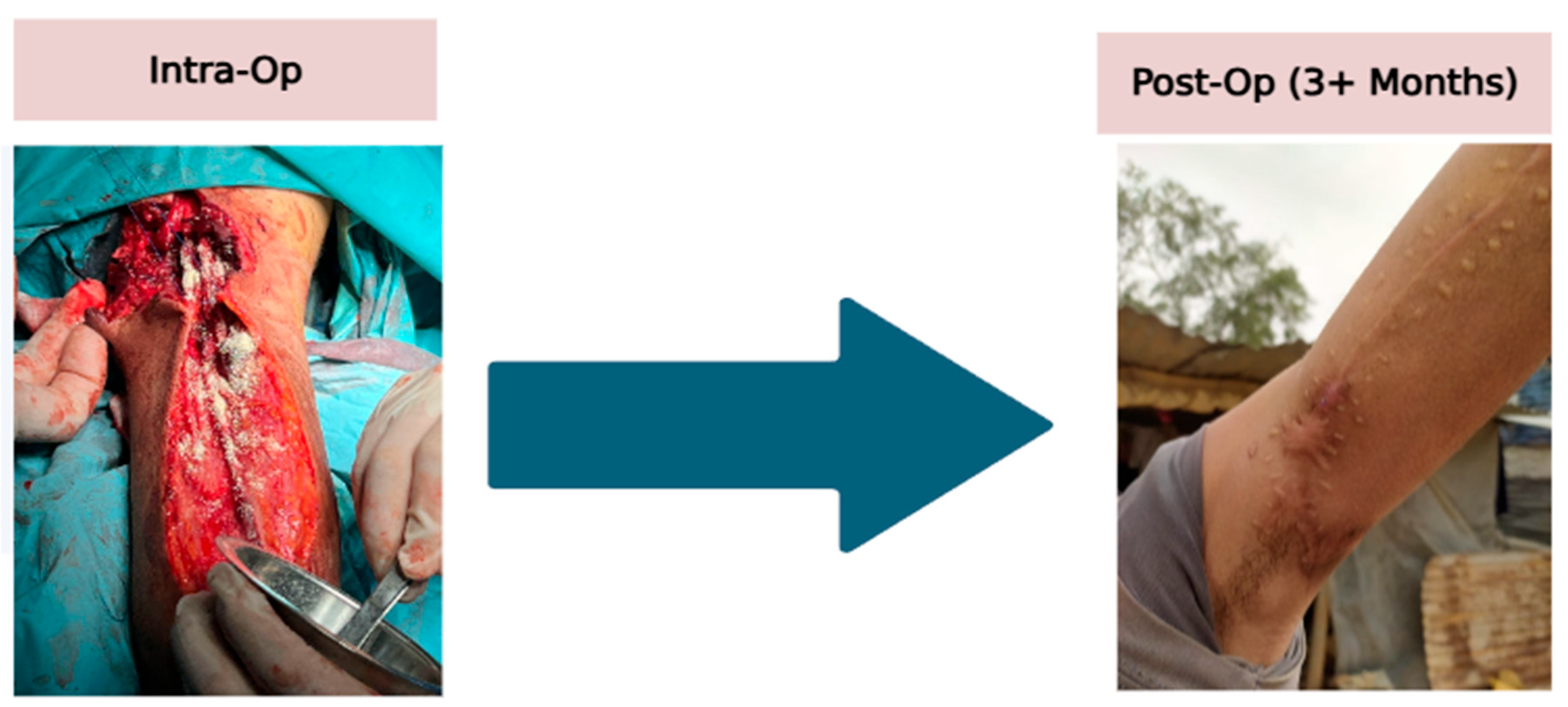
2.7. Case G (Complex Upper Arm Injury with Nerve and Vascular Repair)
3. Discussion
4. Methods
4.1. Study Design and Setting
4.2. Selection of Cases
4.3. Intervention Protocol
4.4. Outcome Measures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shingleton, S.; Folwell, J.; Jones, I.; Gleason, M.; Williams, A. Burn Wound Care Strategies for the Battlefield and Austere Settings. Eur. Burn. J. 2024, 5, 49–65. [Google Scholar] [CrossRef]
- Irfan, B.; Lulu, I.; Hamawy, A.; Abu Shammala, A.; Kullab, S.; Fawaz, M.; Sammour, A.A.; Khawaja, H.; Alshaer, N.; Abu-Sittah, G.; et al. Combating infections under siege: Healthcare challenges amidst the military assault in Gaza. World Med. Health Policy 2025, 17, 188–213. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Johnson, O.N.; Nelson, M.; Estabrooke, I.; Sopko, N.; Swanson, E.W. Successful Treatment of War Zone Traumatic Lower Extremity Wound with Exposed Tendons Using an Autologous Homologous Skin Construct. Cureus 2020, 12, e7952. [Google Scholar] [CrossRef] [PubMed]
- Jevtić, M.; Petrović, M.; Ignjatović, D.; Ilijevski, N.; Misovic, S.; Kronja, G.; Stankovic, N. Treatment of wounded in the combat zone. J. Trauma. 1996, 40, S173–S176. [Google Scholar] [CrossRef]
- Danks, R.R.; Lairet, K. Innovations in caring for a large burn in the Iraq war zone. J. Burn. Care Res. 2010, 31, 665–669. [Google Scholar] [CrossRef]
- Riddez, L. Wounds of war in the civilian sector: Principles of treatment and pitfalls to avoid. Eur. J. Trauma. Emerg. Surg. 2014, 40, 461–468. [Google Scholar] [CrossRef]
- Cao, X.; Wu, X.; Zhang, Y.; Qian, X.; Sun, W.; Zhao, Y. Emerging biomedical technologies for scarless wound healing. Bioact. Mater. 2024, 42, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. 2024, 15, 1395479. [Google Scholar] [CrossRef] [PubMed]
- Preetam, S.; Ghosh, A.; Mishra, R.; Pandey, A.; Roy, D.S.; Rustagi, S.; Malik, S. Electrical stimulation: A novel therapeutic strategy to heal biological wounds. RSC Adv. 2024, 14, 32142–32173. [Google Scholar] [CrossRef]
- Jonidi Shariatzadeh, F.; Currie, S.; Logsetty, S.; Spiwak, R.; Liu, S. Enhancing wound healing and minimizing scarring: A comprehensive review of nanofiber technology in wound dressings. Progress. Mater. Sci. 2025, 147, 101350. [Google Scholar] [CrossRef]
- Fertala, J.; Wang, M.L.; Rivlin, M.; Beredjiklian, P.K.; Abboud, J.; Arnold, W.V.; Fertala, A. Extracellular Targets to Reduce Excessive Scarring in Response to Tissue Injury. Biomolecules 2023, 13, 758. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Martinez-Rodriguez, S.; Md Fadilah, N.I.; Hao, D.L.Q.; Markey, G.; Shukla, P.; Fauzi, M.B.; Panetsos, F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers 2024, 16, 1280. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.; Soker, S.; Murphy, S.V. Regenerative Medicine Approaches for Skin Wound Healing: From Allografts to Engineered Skin Substitutes. Curr. Transpl. Rep. 2024, 11, 207–221. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Hachim, D.; LoPresti, S.T.; Yates, C.C.; Brown, B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 2017, 112, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Petrie, K.; Cox, C.T.; Becker, B.C.; MacKay, B.J. Clinical applications of acellular dermal matrices: A review. Scars Burn. Heal. 2022, 8, 20595131211038313. [Google Scholar] [CrossRef]
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burn. Trauma. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Moiemen, N.S.; Yarrow, J.; Kamel, D.; Kearns, D.; Mendonca, D. Topical negative pressure therapy: Does it accelerate neovascularisation within the dermal regeneration template, Integra? A prospective histological in vivo study. Burns 2010, 36, 764–768. [Google Scholar] [CrossRef]
- Liang, R.; Pan, R.; He, L.; Dai, Y.; Jiang, Y.; He, S.; Li, B.; Li, Y. Decellularized Extracellular Matrices for Skin Wound Treatment. Materials 2025, 18, 2752. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, A.E.; Dowling, S.G.; Kosyk, M.S.; Bosque, B.A. Surgical reconstruction of pilonidal sinus disease with concomitant extracellular matrix graft placement: A case series. J. Wound Care 2021, 30, S28–S34. [Google Scholar] [CrossRef] [PubMed]
- Sasse, K.C.; Brandt, J.; Lim, D.C.; Ackerman, E. Accelerated healing of complex open pilonidal wounds using MatriStem extracellular matrix xenograft: Nine cases. J. Surg. Case Rep. 2013, 2013, rjt025. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, A.E.; Buckley, M.-C. Extracellular matrix graft for the surgical management of Hurley stage III hidradenitis suppurativa: A pilot case series. J. Wound Care 2020, 29, 624–630. [Google Scholar] [CrossRef]
- Bohn, G.A.; Gass, K. Leg ulcer treatment outcomes with new ovine collagen extracellular matrix dressing: A retrospective case series. Adv. Skin. Wound Care 2014, 27, 448–454. [Google Scholar] [CrossRef]
- de Fátima Cordeiro Petz, F.; Meier, M.J.; Roehrs, H.; Pott, F.S. Effectiveness of extracellular matrix dressings and topical agents in the treatment of leg ulcers: A systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 1153–1160. [Google Scholar] [CrossRef]
- Valerio, I.L.; Campbell, P.; Sabino, J.; Dearth, C.L.; Fleming, M. The use of urinary bladder matrix in the treatment of trauma and combat casualty wound care. Regen. Med. 2015, 10, 611–622. [Google Scholar] [CrossRef]
- AbouIssa, A.; Mari, W.; Simman, R. Clinical Usage of an Extracellular, Collagen-rich Matrix: A Case Series. Wounds 2015, 27, 313–318. [Google Scholar]
- Bohn, G.A.; Chaffin, A.E. Extracellular matrix graft for reconstruction over exposed structures: A pilot case series. J. Wound Care 2020, 29, 742–749. [Google Scholar] [CrossRef]
- Cheung, T.; Laidley, Z.; Jones, J.; Wu, S. Outcomes of an Esterified Hyaluronic Acid Matrix in the Treatment of Chronic Lower Extremity Wounds: A Case Series. Wounds 2018, 30, 367–371. [Google Scholar]
- Desvigne, M.N.; Bauer, K.; Holifield, K.; Day, K.; Gilmore, D.; Wardman, A.L. Case Report: Surgical Closure of Chronic Soft Tissue Defects Using Extracellular Matrix Graft Augmented Tissue Flaps. Front. Surg. 2020, 7, 559450. [Google Scholar] [CrossRef]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef] [PubMed]
- Lintzeris, D.; Vernon, K.; Percise, H.; Strickland, A.; Yarrow, K.; White, A.; Gurganus, M.; Sherrod, S.; Vergin, K.; Johnson, L. Effect of a New Purified Collagen Matrix with Polyhexamethylene Biguanide on Recalcitrant Wounds of Various Etiologies: A Case Series. Wounds 2018, 30, 72–78. [Google Scholar]
- Simman, R.; Mari, W.; Younes, S.; Wilson, M. Use of Hyaluronic Acid-Based Biological Bilaminar Matrix in Wound Bed Preparation: A Case Series. Eplasty 2018, 18, e10. [Google Scholar]
- Shin, J.; Park, G.; Lee, J.; Bae, H. The Effect of Polydeoxyribonucleotide on Chronic Non-healing Wound of an Amputee: A Case Report. Ann. Rehabil. Med. 2018, 42, 630–633. [Google Scholar] [CrossRef]
- Collins, R.A.; Zhu, C.; Daniel, H.; Puckett, Y.; Ronaghan, C.A. Crush injury with significant soft tissue loss managed utilising biological and dynamic tissue systems: A case study. J. Wound Care 2023, 32, S17–S19. [Google Scholar] [CrossRef]
- Swinehart, I.T.; Badylak, S.F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dyn. 2016, 245, 351–360. [Google Scholar] [CrossRef]
- Novelli, G.; Daleffe, F.; Birra, G.; Canzi, G.; Mazzoleni, F.; Boni, P.; Maino, C.; Giussani, C.; Sozzi, D.; Bozzetti, A. Negative pressure wound therapy in complex cranio-maxillofacial and cervical wounds. Int. Wound J. 2017, 15, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Kahn, K.; Karmy-Jones, R. Use of Negative Pressure Wound Therapy with Automated, Volumetric Instillation for the Treatment of Extremity and Trunk Wounds: Clinical Outcomes and Potential Cost-Effectiveness. Eplasty 2014, 14, e41. [Google Scholar] [PubMed]
- Norman, G.; Shi, C.; Goh, E.L.; Murphy, E.M.; Reid, A.; Chiverton, L.; Stankiewicz, M.; Dumville, J.C.; Cochrane Wounds Group. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst. Rev. 2022, 2022, CD009261. [Google Scholar] [CrossRef]
- Peinemann, F.; Sauerland, S. Negative-Pressure Wound Therapy. Dtsch. Arztebl. Int. 2011, 108, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.; Khan, W.S.; Palmer, J. The Evidence-Based Principles of Negative Pressure Wound Therapy in Trauma & Orthopedics. Open Orthop. J. 2014, 8, 168–177. [Google Scholar] [CrossRef]
- Dowsett, C.; Davis, L.; Henderson, V.; Searle, R. The economic benefits of negative pressure wound therapy in community-based wound care in the NHS. Int. Wound J. 2012, 9, 544–552. [Google Scholar] [CrossRef]
- Slavkovic, M.; Zivanovic, D.; Dučić, S.; Lasić, V.; Bukvić, N.; Nikolić, H.; Martinović, V. Comparison of Negative Pressure Wound Therapy (NPWT) and Classical Wet to Moist Dressing (WtM) in the Treatment of Complicated Extremity Wounds in Children. Children 2023, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Elhage, K.G.; Awad, M.E.; Irfan, F.B.; Lumbley, J.; Mostafa, G.; Saleh, K.J. Closed-incision negative pressure therapy at −125 mmHg significantly reduces surgical site complications following total hip and knee arthroplasties: A stratified meta-analysis of randomized controlled trials. Health Sci. Rep. 2022, 5, e425. [Google Scholar] [CrossRef]
- Keenan, C.; Obaidi, N.; Neelon, J.; Yau, I.; Carlsson, A.H.; Nuutila, K. Negative Pressure Wound Therapy: Challenges, Novel Techniques, and Future Perspectives. Adv. Wound Care 2025, 14, 33–47. [Google Scholar] [CrossRef]
- Nicolai, M.; Safi, S.S.S.; Casera, M.; Dekhili, D.; Hook, C.; Gaudron, C.; Cilliers, A.E.; Baidjoe, A.Y. War wounds caused by explosive weapons in Gaza: Data from a 2024 study by Médecins Sans Frontières. Lancet 2025, 406, 687–688. [Google Scholar] [CrossRef]
- Irfan, B.; Shammala, A.A.; Saleh, K. Will there be a future for newborns in Gaza? Lancet 2024, 404, 1725–1726. [Google Scholar] [CrossRef] [PubMed]
- Paris, L.; Al-Jadba, G.; Zeidan, W.; Spiegel, P.; Elkhatib, Z.; Habash, R.; Al Najjar, S.; Khammash, H.; Albeik, S.; Shaer, T.; et al. Deterioration of health outcomes in Gaza: 19 months of protracted conflict. Lancet 2025, 405, 2041–2044. [Google Scholar] [CrossRef]
- Irfan, B.; Abu Shammala, A.; Saleh, K.J. Prevention of births in Gaza: Where lies the future? Int. J. Gynaecol. Obs. 2024, 169, 841–842. [Google Scholar] [CrossRef]
- Abuzerr, S.; Hamdan, H.; Charafeddine, J. Paediatric meningitis outbreak in Gaza amid health system collapse. Lancet 2025, 406, 689–690. [Google Scholar] [CrossRef]
- Boye, M.; Py, N.; Paris, R.; Prunet, B.; Sarda, A.; Lamblin, A.; Chiron, P.; Martin, E. Trauma care of Gazan civilians: Key lessons from the French military humanitarian mission. J. Trauma. Acute Care Surg. 2025, 99, S99–S105. [Google Scholar] [CrossRef]
- Fogel, I.; Balziano, S.; Tunik, M.; Prat, D.M.; Barzilay, R.; Greenstein, N. Efficient evacuation–enhanced survival: Insights from Gaza conflict trauma care. J. Trauma. Acute Care Surg. 2025, 98, 798–805. [Google Scholar] [CrossRef]
- Albelbeisi, A.; Zinszer, K.; El Bilbeisi, A.H.; Abuzerr, S. The burden of acute malnutrition among children under five in conflict-afflicted Gaza strip: Prevalence and associated factors. Front. Nutr. 2024, 11, 1478485. [Google Scholar] [CrossRef] [PubMed]
- Abukarsh, I.; Abu Motlaq, A.; Atallah, A.; Irfan, B.; Khan, S.; Kattan, A.; Hamawy, A. Utilising high-volume spinal anaesthesia as an alternative to general anaesthesia: Lessons from Gaza. Br. J. Anaesth. 2025, 134, 1801–1803. [Google Scholar] [CrossRef] [PubMed]
- Smadi, Z.; Ghali, A.; Rizek, J.; Al-Zubi, A.; Irfan, B.; Awad, D.; Wentz, C.; Koujah, D.; Muhammad, A. Pediatric Emergency Nursing in Gaza: Challenges, Adaptations, and Lessons from a Conflict Zone. SAGE Open Nurs. 2025, 11, 23779608251349070. [Google Scholar] [CrossRef] [PubMed]
- Aftab, S.; Yaqoob, E.; Khan, S.A.; Javed, S. Addressing emergency trauma care needs in the Gaza Strip. East. Mediterr. Health J. 2025, 31, 226–227. [Google Scholar] [CrossRef]
- Abuzerr, S.; Al-Jawaldeh, A.; Ashour, Y.; Zinszer, K.; El Bilbeisi, A.H. The silent crisis: Effect of malnutrition and dehydration on children in Gaza during the war. Front. Nutr. 2024, 11, 1395903. [Google Scholar] [CrossRef]
- Bahour, N.; Anabtawi, O.; Muhareb, R.; Wispelwey, B.; Asi, Y.; Hammoudeh, W.; Bassett, M.T.; Mills, D.; Tanous, O. Food insecurity, starvation and malnutrition in the Gaza Strip. East. Mediterr. Health J. 2025, 31, 281–284. [Google Scholar] [CrossRef]
- Osendarp, S.; Haddad, L.; Fabrizio, C.; Andridge, C.; E Black, R.; E Brown, M.; Bryan, E.; Campbell, B.M.; D’ALimonte, M.; Fanzo, J.; et al. The famines in Gaza and other conflict areas are a moral failure. Lancet 2025, 406, 572–573. [Google Scholar] [CrossRef]
- Abuzerr, S.; Zinszer, K.; Mahmoud, H. Healthcare collapse and disease spread: A qualitative study of challenges in Gaza strip. BMC Public Health 2025, 25, 589. [Google Scholar] [CrossRef]
- Souilla, L.; Shaheen, A.; Mostafa, A.N.; Abuzerr, S. The escalating health crisis in Gaza amidst armed conflict and heatwaves. Glob. Health Action. 2025, 18, 2513856. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Irfan, B. Cross-Border Health Governance in Collapse: The Case for Buffer Health Corridors in the Gaza Strip. Int. J. Health Plann. Manag. 2025. [Google Scholar] [CrossRef]
- Qian, L.-W.; Fourcaudot, A.B.; Yamane, K.; You, T.; Chan, R.K.; Leung, K.P. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair. Regen. 2016, 24, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Amani, H.; Alipour, M.; Shahriari, E.; Taboas, J.M. Immunomodulatory Biomaterials: Tailoring Surface Properties to Mitigate Foreign Body Reaction and Enhance Tissue Regeneration. Adv. Healthc. Mater. 2024, 13, 2401253. [Google Scholar] [CrossRef]
- Diaz-Valadez, F.; Griffin, K.; Sasse, K. Case Reports of Clinical and Histologic Wound Healing Response with Multi-Tissue Extracellular Matrix with Cost Analysis Compared to Negative Pressure Wound Therapy. Clin. Case Rep. J. 2023, 4, 1–7. [Google Scholar]
- Nasser, E.; Alshaer, N.; Wajahath, M.; Irfan, B.; Tahir, M.; Nasser, M.; Saleh, K.J. Management of Fracture-Related Infection in Conflict Zones: Lessons Learned from Medical Missions to Gaza. Antibiotics 2024, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Alser, K.; Mallah, S.I.; El-Oun, Y.R.A.; Ghayada, M.; Sammour, A.A.-K.; Gilbert, M.; Fitzgerald, S.; Shaikh, Z.; Alser, O. Trauma care supported through a global telemedicine initiative during the 2023–2024 military assault on the Gaza Strip, occupied Palestinian territory: A case series. Lancet 2024, 404, 874–886. [Google Scholar] [CrossRef]
- Irfan, B.; Sultan, M.J.; Khawaja, H.; Wajahath, M.; Nasser, E.; Hasan, A.; Fawaz, M.; Nasser, M.; Saleh, K. Infection control in conflict zones: Practical insights from recent medical missions to Gaza. J. Hosp. Infect. 2024, 152, 177–179. [Google Scholar] [CrossRef]
- Wajahath, M.; Nasser, E.; Nayfeh, T.; Irfan, B.; Balasundaram, R.; Nasser, M.; Saleh, K.J. Trauma by the Numbers: A Cross-Sectional Analysis and Categorization of Trauma Cases in the Gaza War. Int. J. Public Health 2025, 70, 1607877. [Google Scholar] [CrossRef]
- Harghandiwal, B. Impact of the humanitarian crisis in Gaza on children’s health: Evidence and recommendations for mitigation. Glob. Public Health 2025, 20, 2495326. [Google Scholar] [CrossRef]
- Boussaa, S.; Dardona, Z.; Amane, M. Challenges and risk factors for infectious diseases in Gaza due to the current conflict. East. Mediterr. Health J. 2025, 31, 127–133. [Google Scholar] [CrossRef]
- Zayed, D.; Banat, M.; Al-Tammemi, A.B. Infectious diseases within a war-torn health system: The re-emergence of polio in Gaza. New Microbes New Infect. 2024, 62, 101483. [Google Scholar] [CrossRef]
- Hussein, S.; Ahmed, S.K.; Qurbani, K.; Fareeq, A.; Essa, R.A. Infectious diseases threat amidst the war in Gaza. J. Med. Surg. Public Health 2024, 2, 100067. [Google Scholar] [CrossRef]
- Alser, O.; Tahboub, H.; Al-Slaibi, I.; Abuowda, Y.; Elshami, M.; Albarqouni, L. Surgical site infections following gastrointestinal surgery in Palestine: A multicentre, prospective cohort study. Lancet 2019, 393, S12. [Google Scholar] [CrossRef][Green Version]
- Jiménez-Gastélum, G.R.; Aguilar-Medina, E.M.; Soto-Sainz, E.; Ramos-Payán, R.; Silva-Benítez, E.L. Antimicrobial Properties of Extracellular Matrix Scaffolds for Tissue Engineering. Biomed. Res. Int. 2019, 2019, 9641456. [Google Scholar] [CrossRef] [PubMed]
- Moghnieh, R.; Bizri, N.; Abdallah, D.; Sayegh, M.H. Antimicrobial resistance surveillance and trends in armed conflict, fragile, and non-conflict countries of the Eastern Mediterranean Region. Infect. Dis. Poverty 2025, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.; Shortall, C.; Dewachi, O.; Naim, C.; Green, A.; Hussain, S.; Abbara, A. Conflict-associated wounds and burns infected with GLASS pathogens in the Eastern Mediterranean Region: A systematic review. BMC Infect. Dis. 2025, 25, 187. [Google Scholar] [CrossRef]
- Kumar, R.; Tanous, O.; Mills, D.; Wispelwey, B.; Asi, Y.; Hammoudeh, W.; Dewachi, O.; Ishaq, S.L. Antimicrobial resistance in a protracted war setting: A review of the literature from Palestine. mSystems 2025, 10, e0167924. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Petersen, E.; Capone, A.; Donati, D.; Andriolo, B.; Gross, M.; Cicalini, S.; Petrosillo, N. The impact of armed conflict on the development and global spread of antibiotic resistance: A systematic review. Clin. Microbiol. Infect. 2024, 30, 858–865. [Google Scholar] [CrossRef]
- Dalal, H.A.; Irfan, B.; Elmanama, A. Multidrug-resistant bacteria amid health-system collapse in Gaza. Lancet Infect. Dis. 2025. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Shahabudin, N.A.; Mohd Razif, R.A.; Sanyal, A.; Ghosh, A.; Baharin, K.I.; Ahmad, H.; Maarof, M.; Motta, A.; Fauzi, M.B. Discovery of bioactive peptides as therapeutic agents for skin wound repair. J. Tissue Eng. 2024, 15, 20417314241280359. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Hawksworth, J.S.; Sheppard, F.R.; Tadaki, D.K.; Elster, E. Inflammatory response is associated with critical colonization in combat wounds. Surg. Infect. 2011, 12, 351–357. [Google Scholar] [CrossRef]
- White, E.S.; Mantovani, A.R. Inflammation, wound repair, and fibrosis: Reassessing the spectrum of tissue injury and resolution. J. Pathol. 2013, 229, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Irfan, B.; Abu Shammala, A.; Alshaer, N.; Nasser, E.; Wajahath, M.; Hamawy, A.; Tahir, M.; Khawaja, H.; Khan, O.; Fikry, K.; et al. There are dementia patients in Gaza too. J. Alzheimer’s Dis. 2024, 102, 317–320. [Google Scholar] [CrossRef]
- Ghali, A.; Hafeez, H.; Alamrain, A.A.; Smadi, Z.; Qasim, T.; Irfan, B.; Perlmutter, M.; Elaydi, A. Challenges in orthopaedic data collection in Gaza Strip: Observational findings and bibliometric analysis. Int. Orthop. (SICOT) 2025, 49, 1293–1301. [Google Scholar] [CrossRef]
- Irfan, B.; Al-Hasan, M.; Abu Alamrain, A.; Aljamal, A.; Al-Khaldi, M.; Shaikhkhalil, H.; Abu Shammala, A.; Sammour, A.A.-K.; Alshaer, N.; Braika, F.; et al. Challenges in ENT Data Collection in Conflict Zones: Insights from Gaza. Ear Nose Throat J. 2024, 01455613241309785. [Google Scholar] [CrossRef]
- Ghali, A.; Nasser, E.; Nasser, T.; Elaydi, A.; Irfan, B.; Nasser, M.; Saleh, K. Don’t shoot the messenger: The role of journalists in Gaza’s health sector. East. Mediterr. Health J. 2025, 31, 99–100. [Google Scholar] [CrossRef]
- Irfan, B.; Alamrain, A.A.; Shammala, A.A. War-related trauma and displacement in Gaza: The impact on the health and longevity of older adults. Lancet Healthy Longev. 2025, 6, 100713. [Google Scholar] [CrossRef]
- Centeno, J.A.; Rogers, D.A.; van der Voet, G.B.; Fornero, E.; Zhang, L.; Mullick, F.G.; Chapman, G.D.; Olabisi, A.O.; Wagner, D.J.; Stojadinovic, A.; et al. Embedded Fragments from U.S. Military Personnel—Chemical Analysis and Potential Health Implications. Int. J. Environ. Res. Public Health 2014, 11, 1261–1278. [Google Scholar] [CrossRef]
- Almigdad, A. Orthopedic War-Related Injuries in Gaza: In-Depth Insights from within the Strip. Eur. J. Med. Health Sci. 2025, 7, 15–21. [Google Scholar] [CrossRef]
- Almigdad, A.; Bani Salameh, A.; Abu Hilaleh, H. When Simple Orthopedic Cases Become Complex: Case Presentations from Gaza. Cureus 2024, 16, e75601. [Google Scholar] [CrossRef] [PubMed]
- Alsaid, B.; Alhimyar, M.; Alnweilaty, A.; Alhasan, E.; Shalhoum, Z.A.A.; Bathich, M.; Ahmad, A.M.; Ahmad, T.; Turkmani, K.; Sara, S. Laparotomy Due to War-Related Penetrating Abdominal Trauma in Civilians: Experience from Syria 2011-2017. Disaster Med. Public Health Prep. 2021, 15, 615–623. [Google Scholar] [CrossRef]
- Manring, M.M.; Hawk, A.; Calhoun, J.H.; Andersen, R.C. Treatment of War Wounds: A Historical Review. Clin. Orthop. Relat. Res. 2009, 467, 2168–2191. [Google Scholar] [CrossRef]
- Sahli, Z.T.; Bizri, A.R.; Abu-Sittah, G.S. Microbiology and risk factors associated with war-related wound infections in the Middle East. Epidemiol. Infect. 2016, 144, 2848–2857. [Google Scholar] [CrossRef]
- Rodero Roldán, M.D.M.; Yuste Benavente, V.; Martínez Álvarez, R.M.; Calleja, A.I.L.; García-Lechuz, J.M. Characterization of wound infections among patients injured during the Ruso-Ukrainian war in a Role 4 hospital. Enferm. Infecc. Microbiol. Clin. 2024, 42, 501–506. [Google Scholar] [CrossRef]
- Coupland, R.M. War wounds of bones and external fixation. Injury 1994, 25, 211–217. [Google Scholar] [CrossRef]
- Sabbatani, S.; Fiorino, S. The treatment of wounds during World War I. Infez. Med. 2017, 25, 184–192. [Google Scholar]
- Schoenfeld, A.J.; Dunn, J.C.; Bader, J.O.; Belmont, P.J. The nature and extent of war injuries sustained by combat specialty personnel killed and wounded in Afghanistan and Iraq, 2003–2011. J. Trauma. Acute Care Surg. 2013, 75, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Kobeissi, E.; Menassa, M.; Honein-AbouHaidar, G.; El Achi, N.; Abdul-Sater, Z.; Farhat, T.; Al Mohtar, D.; Hajjar, M.; Abdul-Khalek, R.A.; Chaya, B.F.; et al. Long-term burden of war injuries among civilians in LMICs: Case of the July 2006 war in Lebanon. Front. Public Health 2023, 11, 1305021. [Google Scholar] [CrossRef] [PubMed]
- Asgedom, A.A.; Etsedingl, A.; Hailemariam, T.T.; Tequare, M.H.; Hailu, T.; Tsegay, A.T.; Hailu, A.G.; Weldebirhan, S.N.; Hailu, M.; Weldesenbet, N.A.; et al. Prevalence, causes and outcomes of war-related civilian injuries in Ethiopia’s war-torn Tigray region: A community-based descriptive study. BMC Res. Notes 2023, 16, 352. [Google Scholar] [CrossRef] [PubMed]
- Irfan, B.; Clarisse, A.; Smith, J.; Tahir, M.; Nasser, E.; Wajahath, M.; Tarab, B.; Lulu, I.; Alshaer, N.; Nasser, M.; et al. Paediatric medical evacuations from Gaza have been obstructed and are increasingly difficult. Med. Confl. Surviv. 2025, 41, 99–107. [Google Scholar] [CrossRef]
- 510(k) Premarket Notification. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K172593 (accessed on 2 August 2025).
- Krause, S.D. US Food & Drug Administration Pre-Market Notification for XCelliStem Wound Powder; US Food & Drug Administration: Silver Spring, MD, USA, 2018.
- Mazher, M. US Food & Drug Administration Pre-Market Notification for ReyaGel; US Food & Drug Administration: Silver Spring, MD, USA, 2024.

| Characteristics | Number of Patients (n = 15) |
|---|---|
| Mechanism of injury: Explosive trauma | 10 |
| Mechanism of injury: Gunshot wound | 5 |
| Exposed bone or tendon at time of ECM application | 12 |
| Required ≥ 2 surgical debridements | 11 |
| Received concurrent vancomycin powder | 13 |
| Evidence of granulation tissue by day 7 | 12 |
| Wound closure via skin graft or flap | 9 |
| Wound closure via secondary intention | 4 |
| Adverse reaction attributable to ECM | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irfan, B.; Hamawy, A.; Musallam, R.; Abudagga, R.; Khan, S.; Alshaer, N.; Tabash, M.; Ghali, A.; Saleh, K.; Tahir, M. Utilization of a Multi-Tissue Extracellular Matrix in Complex Wound Care in Gaza: A Case Series. Antibiotics 2025, 14, 885. https://doi.org/10.3390/antibiotics14090885
Irfan B, Hamawy A, Musallam R, Abudagga R, Khan S, Alshaer N, Tabash M, Ghali A, Saleh K, Tahir M. Utilization of a Multi-Tissue Extracellular Matrix in Complex Wound Care in Gaza: A Case Series. Antibiotics. 2025; 14(9):885. https://doi.org/10.3390/antibiotics14090885
Chicago/Turabian StyleIrfan, Bilal, Adam Hamawy, Ruba Musallam, Rahaf Abudagga, Sameer Khan, Nour Alshaer, Mohammed Tabash, Abdullah Ghali, Khaled Saleh, and Mohammed Tahir. 2025. "Utilization of a Multi-Tissue Extracellular Matrix in Complex Wound Care in Gaza: A Case Series" Antibiotics 14, no. 9: 885. https://doi.org/10.3390/antibiotics14090885
APA StyleIrfan, B., Hamawy, A., Musallam, R., Abudagga, R., Khan, S., Alshaer, N., Tabash, M., Ghali, A., Saleh, K., & Tahir, M. (2025). Utilization of a Multi-Tissue Extracellular Matrix in Complex Wound Care in Gaza: A Case Series. Antibiotics, 14(9), 885. https://doi.org/10.3390/antibiotics14090885







