Prevalence and Characterization of the Antimicrobial Resistance and Virulence Profiles of Staphylococcus aureus in Ready-to-Eat (Meat, Chicken, and Tuna) Pizzas in Mansoura City, Egypt
Abstract
1. Introduction
2. Results
2.1. Prevalence and Count of Staphylococcus aureus in Pizza Samples
2.2. Molecular Characterization of S. aureus Isolates Recovered from Examined Pizza Samples
2.2.1. Identification of Methicillin- and Vancomycin-Resistant S. aureus (MRSA and VRSA) Among S. aureus Isolates
2.2.2. Screening of Different Virulence Genes Among S. aureus Isolates Recovered from Meat, Chicken, and Canned Tuna Pizza
2.3. Antimicrobial Resistance Profile of S. aureus Isolated from Different Types of Pizza
3. Discussion
3.1. Prevalence of Staphylococcus aureus in Pizza Samples in Comparison with RTE Meat Products in Other Studies
3.2. Prevalence of Methicillin- and Vancomycin-Resistant S. aureus (MRSA and VRSA) in Pizza Samples in Comparison with Their Prevalence Rates in Other Studies
3.3. Comparison of the Virulent Genes of S. aureus Isolates Recovered from Meat, Chicken, and Canned Tuna Pizza with Those Detected in Other Studies
3.4. Antimicrobial Resistance Profile of S. aureus Recovered from Pizza in Comparison with the Profile Determined in S. aureus Isolated from RTE Meat Products in Other Studies
4. Materials and Methods
4.1. Collection of Samples
4.2. Isolation and Identification of Staphylococcus aureus
4.3. Molecular Analysis
4.3.1. Preparation of Genomic DNA
4.3.2. Molecular Characterization of S. aureus Isolates
4.4. Antimicrobial Resistance Profile of Staphylococcus aureus Isolates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, R.W.; Monday, S.R. Staphylococcus aureus. In International Handbook of Foodborne Pathogens; Dodd, C.E.R., Aldsworth, T.G., Stein, R.A., Cliver, D.O., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 61–80. [Google Scholar]
- Piessens, V.; Van Coillie, E.; Verbist, B.; Supré, K.; Braem, G.; Van Nuffel, A.; De Vuyst, L.; Heyndrickx, M.; De Vliegher, S. Distribution of coagulase-negative Staphylococcus species from milk and environment of dairy cows differs between herds. J. Dairy Sci. 2011, 94, 2933–2944. [Google Scholar] [CrossRef]
- Puah, S.M.; Chua, K.H.; Tan, J.A.M.A. Virulence factors and antibiotic susceptibility of Staphylococcus aureus isolates in ready-to-eat foods: Detection of S. aureus contamination and a high prevalence of virulence genes. Int. J. Environ. Res. Public Health 2016, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K. Staphylococcus aureus. In Foodborne Microbial Pathogens; Food Science Text Series; Springer: New York, NY, USA, 2018; pp. 209–228. [Google Scholar]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. BioMed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef]
- De Buyser, M.L.; Dufour, B.; Maire, M.; Lafarge, V. Implication of milk and milk products in food-borne diseases in France and in different industrialised countries. Int. J. Food Microbiol. 2001, 67, 1–17. [Google Scholar] [CrossRef]
- Morar, A.; Ban-Cucerzan, A.; Herman, V.; Tîrziu, E.; Sallam, K.I.; Abd-Elghany, S.M.; Imre, K. Multidrug Resistant Coagulase-Positive Staphylococcus aureus and Their Enterotoxins Detection in Traditional Cheeses Marketed in Banat Region, Romania. Antibiotics 2021, 10, 1458. [Google Scholar] [CrossRef]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic resistance and the MRSA problem. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- Paterson, G.K.; Harrison, E.M.; Holmes, M.A. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- NFSA (Egypt’s National Food Safety Authority). Egypt’s National Food Safety Authority Issues New Decision Regulating Microbiological Contaminants; Decision of the Board of Directors No. (1) of the Year 2021 on the Technical Regulations of Microbiological Criteria for Food; Official Gazette: Cairo, Egypt, 2021. [Google Scholar]
- Saifm, M.; Saad, S.M.; Hassanine, F.; Shaltout, F.; Zhagloul, M. Prevalence of methicillin-resistant Staphylococcus aureus in some ready-to-eat meat products. Benha Vet. Med. J. 2019, 37, 12–15. [Google Scholar] [CrossRef]
- Büyükyörük, S.; Beyaz, D.; Göksoy, E.Ö.; Filiz, K.Ö.K.; Koçak, P. Microbiological evaluation of ready-to-eat sandwiches served near hospitals and schools. Ankara Üniv. Vet. Fak. Derg. 2014, 61, 193–198. [Google Scholar][Green Version]
- Tavakoli, H.R.; Riazipour, M. Microbial quality of cooked meat foods in Tehran University’s restaurants. Pak. J. Med. Sci. 2008, 24, 595–599. [Google Scholar][Green Version]
- Chung, M.S.; Kim, C.M.; Ha, S.D. Detection and enumeration of microorganisms in ready-to-eat foods, ready-to-cook foods, and fresh-cut produce in Korea. J. Food Saf. 2010, 30, 480–489. [Google Scholar] [CrossRef]
- Contreras, C.P.Á.; da Silva, L.N.N.; Ferreira, D.C.G.; dos Santos Ferreira, J.; de Castro Almeida, R.C. Prevalence of methicillin-resistant Staphylococcus aureus in raw hamburgers and ready-to-eat sandwiches commercialized in supermarkets and fast food outlets in Brazil. Food Nutr. Sci. 2015, 6, 1324. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Wu, Q.; Zhang, J.; Zhang, F.; Yang, X.; Wu, H.; Zen, H.; Chen, M.; Ding, Y.; et al. Staphylococcus aureus isolated from retail meat and meat products in China: Incidence, antibiotic resistance and genetic diversity. Front. Microbiol. 2018, 9, 2767. [Google Scholar] [CrossRef] [PubMed]
- Mahros, M.A.; Abd-Elghany, S.M.; Sallam, K.I. Multidrug-, methicillin-, and vancomycin-resistant Staphylococcus aureus isolated from ready-to-eat meat sandwiches: An ongoing food and public health concern. Int. J. Food Microbiol. 2021, 346, 109165. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, M.; Ali, S.S.; Khalil, M.A.; Chen, X.; Cui, H.; Lin, L.; El-Sapagh, S. Exploring the potential of zinc oxide nanoparticles against pathogenic multi-drug resistant Staphylococcus aureus from ready-to-eat meat and its proposed mechanism. Food Control 2024, 156, 110117. [Google Scholar] [CrossRef]
- Nagy, N.; Kirrella, G.A.; Moustafa, N.Y.; Abdallah, R. Quality assessment of some imported and local canned tuna sold in Kafrelsheikh, Egypt. J. Adv. Vet. Res. 2023, 13, 377–383. [Google Scholar]
- Wu, X.; Su, Y.C. Growth of Staphylococcus aureus and enterotoxin production in pre-cooked tuna meat. Food Control 2014, 42, 63–70. [Google Scholar] [CrossRef]
- Jang, H.G.; Kim, N.H.; Choi, Y.M.; Rhee, M.S. Microbiological quality and risk factors related to sandwiches served in bakeries, cafés, and sandwich bars in South Korea. J. Food Prot. 2013, 76, 231–238. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Xia, X.; Yang, B.; Xi, M.; Meng, J. Antimicrobial susceptibility and molecular typing of methicillin-resistant Staphylococcus aureus in retail foods in Shaanxi, China. Pathog. Dis. 2014, 11, 281–286. [Google Scholar]
- Abd El-Razik, K.A.; Arafa, A.A.; Ibrahim, E.S.; El-Shater, N.S.; El-Demerdash, G.O.; El-Shinawy, N.M.; Elmahallawy, E.K. Phenotypic and genotypic characterization of tetracycline-resistant Staphylococcus aureus isolated from food of animal origin. Egypt. J. Environ. Res. 2017, 9, 63–90. [Google Scholar]
- Ghanem, M.A.; Idris, A.B.M.; Abu El Roos, N.; Darwish, W.S. Prevalence of multidrug-resistant Staphylococcus aureus in meals served at hospitals. Egypt. J. Vet. Sci. 2025, 56, 1479–1488. [Google Scholar] [CrossRef]
- Wells, M.L.; Juett, B.W. Prevalence of MRSA on meat products in central Kentucky. Bios 2012, 83, 33–38. [Google Scholar] [CrossRef]
- Safarpoor Dehkordi, F.; Gandomi, H.; Basti, A.A.; Misaghi, A.; Rahimi, E. Phenotypic and genotypic characterization of antibiotic resistance of methicillin-resistant Staphylococcus aureus isolated from hospital food. J. Antimicrob. Resist. Infect. Control 2017, 6, 104. [Google Scholar] [CrossRef]
- Moellering, R.C., Jr. MRSA: The first half century. J. Antimicrob. Chemother. 2012, 67, 4–11. [Google Scholar] [CrossRef]
- Asokan, G.V.; Vanitha, A. WHO global priority pathogens list on antibiotic resistance: An urgent need for action to integrate One Health data. Perspect. Public Health 2018, 138, 87–88. [Google Scholar]
- Alonso, V.P.; Queiroz, M.M.; Gualberto, M.L.; Nascimento, M.S. Klebsiella pneumoniae carbapenemase (KPC), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus spp. (VRE) in the food production chain and biofilm formation on abiotic surfaces. Curr. Opin. Food Sci. 2019, 26, 79–86. [Google Scholar] [CrossRef]
- Maharjan, M.; Sah, A.K.; Pyakurel, S.; Thapa, S.; Maharjan, S.; Adhikari, N.; Rijal, K.R.; Ghimire, P.; Shrestha, U.T. Molecular confirmation of vancomycin-resistant Staphylococcus aureus with vanA gene from a hospital in Kathmandu. Int. J. Microbiol. 2021, 2021, 3847347. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin-resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef]
- Saber, T.; Samir, M.; El-Mekkawy, R.M.; Ariny, E.; El-Sayed, S.R.; Enan, G.; Abdelatif, S.H.; Askora, A.; Merwad, A.M.A.; Tartor, Y.H. Methicillin- and vancomycin-resistant Staphylococcus aureus from humans and ready-to-eat meat: Characterization of antimicrobial resistance and biofilm formation ability. Front. Microbiol. 2022, 12, 735494. [Google Scholar] [CrossRef]
- Afshari, A.; Taheri, S.; Hashemi, M.; Norouzy, A.; Nematy, M.; Mohamadi, S. Methicillin- and vancomycin-resistant Staphylococcus aureus and vancomycin-resistant enterococci isolated from hospital foods: Prevalence and antimicrobial resistance patterns. Curr. Microbiol. 2022, 79, 326. [Google Scholar] [CrossRef]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 6 June 2025).
- Ertas Onmaz, N.; Demirezen Yilmaz, D.; Imre, K.; Morar, A.; Gungor, C.; Yilmaz, S.; Gundog, D.A.; Dishan, A.; Herman, V.; Gungor, G. Green Synthesis of Gold Nanoflowers Using Rosmarinus officinalis and Helichrysum italicum Extracts: Comparative Studies of Their Antimicrobial and Antibiofilm Activities. Antibiotics 2022, 11, 1466. [Google Scholar] [CrossRef]
- Chieffi, D.; Fanelli, F.; Cho, G.S.; Schubert, J.; Blaiotta, G.; Franz, C.M.A.P.; Bania, J.; Fusco, V. Novel insights into the enterotoxigenic potential and genomic background of Staphylococcus aureus isolated from raw milk. J. Food Microbiol. 2020, 90, 103482. [Google Scholar] [CrossRef]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2003, 2, 63–76. [Google Scholar]
- Jamshidi, A.; Kazerani, H.R.; Seifi, H.A.; Moghaddas, E. Growth limits of Staphylococcus aureus as a function of temperature, acetic acid, NaCl concentration, and inoculum level. Iran. J. Vet. Res. 2008, 9, 353–359. [Google Scholar]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, F.; Wu, Q.; Zhang, J.; Pang, R.; Zeng, H.; Yang, X.; Chen, M.; Wang, G.; et al. Prevalence and characterization of food-related methicillin-resistant Staphylococcus aureus (MRSA) in China. Front. Microbiol. 2019, 10, 304. [Google Scholar] [CrossRef]
- Ulusoy, B.H.; Sancar, B.C.; Öztürk, M. Prevalence of staphylococcal enterotoxins in ready-to-eat foods sold in Istanbul. J. Food Prot. 2017, 80, 1734–1736. [Google Scholar] [CrossRef]
- Hughes, A.C.; Kirkland, M.; Du, W.; Rasooly, R.; Hernlem, B.; Tam, C.; Zhang, Y.; He, X. Development of thermally stable nanobodies for detection and neutralization of staphylococcal enterotoxin B. Toxins 2023, 15, 400. [Google Scholar] [CrossRef]
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal enterotoxins. Toxins 2010, 2, 2177–2197. [Google Scholar] [CrossRef]
- Baumgartner, A.; Niederhauser, I.; Johler, S. Virulence and resistance gene profiles of Staphylococcus aureus strains isolated from ready-to-eat foods. J. Food Prot. 2014, 77, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahry, S.N.; Mahmoud, I.Y.; Al-Musharafi, S.K.; Sivakumar, N. Staphylococcus aureus contamination during food preparation, processing and handling. Int. J. Chem. Eng. Appl. 2014, 5, 388. [Google Scholar] [CrossRef]
- Wright, J.D.; Holland, K.T. The effect of cell density and specific growth rate on accessory gene regulator and toxic shock syndrome toxin-1 gene expression in Staphylococcus aureus. FEMS Microbiol. Lett. 2003, 218, 377–383. [Google Scholar] [CrossRef]
- Özdemir, H.; Keyvan, E. Isolation and characterization of Staphylococcus aureus strains isolated from beef, sheep and chicken meat. J. Ankara Üniv. Vet. Fak. Derg. 2016, 63, 333–338. [Google Scholar]
- Beshiru, A.; Igbinosa, I.H.; Igbinosa, E.O. Characterization of enterotoxigenic Staphylococcus aureus from ready-to-eat seafood (RTES). LWT 2021, 135, 110042. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modeling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Elshebrawy, H.A.; Kasem, N.G.; Sallam, K.I. Methicillin- and vancomycin-resistant Staphylococcus aureus in chicken carcasses, ready-to-eat chicken meat sandwiches, and buffalo milk. Int. J. Food Microbiol. 2025, 427, 110968. [Google Scholar] [CrossRef]
- Sallam, K.I.; Abd-Elghany, S.M.; Elhadidy, M.; Tamura, T. Molecular characterization and antimicrobial resistance profile of methicillin-resistant Staphylococcus aureus in retail chicken. J. Food Prot. 2015, 78, 1879–1884. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Yu, S.; Wu, Q.; Guo, W.; Huang, J.; Cai, S. Prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus in retail ready-to-eat foods in China. Front. Microbiol. 2016, 7, 816. [Google Scholar] [CrossRef]
- Parisi, A.; Caruso, M.; Normanno, G.; Latorre, L.; Sottili, R.; Miccolupo, A.; Fraccalvieri, R.; Santagada, G. Prevalence, antimicrobial susceptibility and molecular typing of methicillin-resistant Staphylococcus aureus (MRSA) in bulk tank milk from southern Italy. Food Microbiol. 2016, 58, 36–42. [Google Scholar] [CrossRef]
- Childs-Kean, L.M.; Shaeer, K.M.; Varghese Gupta, S.; Cho, J.C. Aminoglycoside allergic reactions. Pharmacy 2019, 7, 124. [Google Scholar] [CrossRef]
- Tang, Y.; Larsen, J.; Kjeldgaard, J.; Andersen, P.S.; Skov, R.; Ingmer, H. Methicillin-resistant and -susceptible Staphylococcus aureus from retail meat in Denmark. Int. J. Food Microbiol. 2017, 249, 72–76. [Google Scholar] [CrossRef]
- Sadiq, A.; Samad, M.; Saddam; Basharat, N.; Ali, S.; Roohullah; Saad, Z.; Khan, A.N.; Ahmed, Y.; Khan, A.; et al. Methicillin-resistant Staphylococcus aureus (MRSA) in slaughterhouses and meat shops in the capital territory of Pakistan during 2018–2019. Front. Microbiol. 2020, 11, 577707. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Davis, J.A.; Barrett, J.B. Prevalence and characterization of methicillin-resistant Staphylococcus aureus isolates from retail meat and humans in Georgia. J. Clin. Microbiol. 2013, 51, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Nalepa, B.; Sierpińska, M.; Łaniewska-Trokenheim, Ł. Retail ready-to-eat food as a potential vehicle for Staphylococcus spp. harboring antibiotic resistance genes. J. Food Prot. 2014, 77, 993–998. [Google Scholar] [CrossRef]
- Mesbah, A.; Mashak, Z.; Abdolmaleki, Z. A survey of prevalence and phenotypic and genotypic assessment of antibiotic resistance in Staphylococcus aureus bacteria isolated from ready-to-eat food samples collected from Tehran Province, Iran. Trop. Med. Health 2021, 49, 81. [Google Scholar] [CrossRef]
- Chieffi, D.; Fanelli, F.; Fusco, V. Antimicrobial and biocide resistance in Staphylococcus aureus: Genomic features, decontamination strategies, and the role of S. aureus complex-related species, with a focus on ready-to-eat food and food-contact surfaces. Front. Food Sci. Technol. 2023, 3, 1165871. [Google Scholar] [CrossRef]
- Lin, Q.; Sun, H.; Yao, K.; Cai, J.; Ren, Y.; Chi, Y. The prevalence, antibiotic resistance and biofilm formation of Staphylococcus aureus in bulk ready-to-eat foods. Biomolecules 2019, 9, 524. [Google Scholar] [CrossRef]
- Beshiru, A.; Isichei-Ukah, B.O.; Uwhuba, K.E.; Igere, B.E.; Igbinosa, E.O. Prevalence, characterization, and implications of methicillin-resistant Staphylococcus aureus (MRSA) in ready-to-eat foods from Delta, Nigeria: A concern for consumer safety. Sustain. Microbiol. 2024, 1, qvae007. [Google Scholar] [CrossRef]
- Adeyemi, F.M.; Oyedara, O.O.; Yusuf-Omoloye, N.A.; Ajigbewu, O.H.; Ndaji, O.L.; Adegbite-Badmus, M.K.; Olumakinde, T.S.; Oluokun, T.E. Guardians of resistance and virulence: Detection of mec, femA, Van, pvl, hlg and spa genes in methicillin- and vancomycin-resistant Staphylococcus aureus from clinical and food samples in Southwestern Nigeria. BMC Microbiol. 2024, 24, 498. [Google Scholar] [CrossRef]
- Leach, K.L.; Brickner, S.J.; Noe, M.C.; Miller, P.F. Linezolid, the first oxazolidinone antibacterial agent. Ann. N. Y. Acad. Sci. 2011, 1222, 49–54. [Google Scholar] [CrossRef]
- Baldoni, D.; Haschke, M.; Rajacic, Z.; Zimmerli, W.; Trampuz, A. Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection. Antimicrob. Agents Chemother. 2009, 53, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Oh, S.K.; Cha, J.O.; Lee, Y.S.; Koo, M. Characterization of antibiotic-resistant Staphylococcus aureus isolates from ready-to-eat foods in Korea. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 387–395. [Google Scholar] [CrossRef]
- ISO 6888-1:2021-08; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Method Using Baird-Parker Agar Medium. International Organization for Standardization: Geneva, Switzerland, 2021.
- Wang, X.; Zeng, Y. Study on the hemolysin phenotype and the genotype distribution of Staphylococcus aureus causing bovine mastitis in Shandong dairy farms. Int. J. Appl. Res. Vet. Med. 2011, 9, 416–421. [Google Scholar]
- Mehrotra, M.; Wang, G.; Johnson, W.M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol. 2000, 38, 1032–1035. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
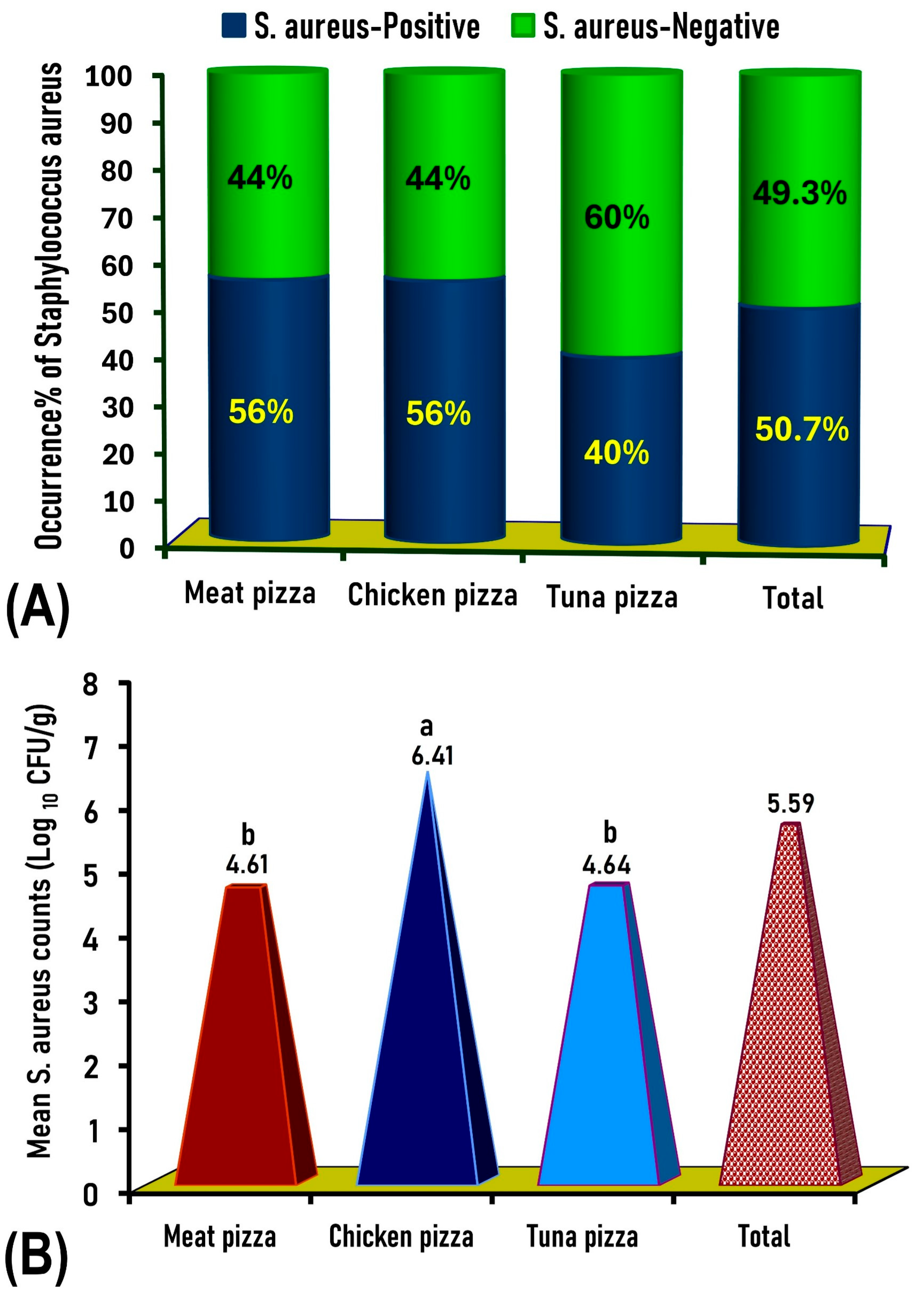




| Type of Pizza | Number of Samples Tested | Number of Coagulase-Positive S. aureus Samples | Coagulase-Positive S. aureus Count (Log10 CFU/g) | No. (%) of Coagulase-Positive S. aureus Exceeding the Maximal Permissible Limits * | |
|---|---|---|---|---|---|
| Minimum | Maximum | ||||
| Chicken pizza | 100 | 56 | 2.9 | 7.7 | 52 (52%) |
| Meat pizza | 100 | 56 | 2.0 | 5.8 | 48 (48%) |
| Canned tuna pizza | 100 | 40 | 4.2 | 5.3 | 40 (40%) |
| Total | 300 | 152 | 2.0 | 7.7 | 140 (46.7%) |
| Antimicrobial | Sensitive | Intermediate | Resistant |
|---|---|---|---|
| Ampicillin (AMP) (25 µg) | 0 (0%) | 0 (0%) | 560 (100%) |
| Oxacillin (OX) (1 µg) | 0 (0%) | 0 (0%) | 560 (100%) |
| Penicillin (P) (10IU) | 0 (0%) | 0 (0%) | 560 (100%) |
| Cefuroxime (CXM) (30 µg) | 73 (13%) | 0 (0%) | 487 (87%) |
| Cefotaxime (CTX) (30 µg) | 129 (23%) | 0 (0%) | 431 (77%) |
| Kanamycin (K) (30 µg) | 134 (24%) | 0 (0%) | 426 (76%) |
| Ciprofloxacin (CIP) (30 µg) | 336 (60%) | 90 (16%) | 134 (24%) |
| Tetracycline (30 µg) | 459 (82%) | 23 (4%) | 78 (14%) |
| Azithromycin (AZM) (30 µg) | 381 (68%) | 134 (24%) | 45 (8%) |
| Clindamycin (CD) (2 µg) | 498 (89%) | 22 (4%) | 40 (7%) |
| Sulfamethoxazole-trimethoprim (COT) (25 µg) | 520 (93%) | 0 (0%) | 40 (7%) |
| Gentamycin (GEN) (30 µg) | 416 (74%) | 120 (22%) | 24 (4%) |
| Nitrofurantoin (F) (300 µg) | 375 (67%) | 163 (29%) | 22 (4%) |
| Vancomycin (VA) (30 µg) | 512 (91.4%) | 0 (0%) | 48 (8.6%) |
| Rifampin (RIF) (30 µg) | 560 (100%) | 0 (0%) | 0 (0%) |
| Linezolid (LZ) (30 µg) | 560 (100%) | 0 (0%) | 0 (0%) |
| Sources and (Number of Isolates) | Number and (%) of Isolates | Antimicrobial Resistance Pattern | Antimicrobial Classes with Resistance | MAR Index | Classification of Strains |
|---|---|---|---|---|---|
| Chicken pizza (6) Meat pizza (6) | 12 (2.1%) | AMP-OX-P-CXM-CTX-K-CIP-TE-AZM-CD-COT | Penicillins, cephalosporins, aminoglycosides, fluoroquinolones, tetracyclines, macrolides, lincomycins, sulfonamides | 0.688 | Multidrug-resistant Staphylococcus aures |
| Meat pizza (34) Canned tuna pizza (10) | 44 (7.8%) | AMP-OX-P-CXM-CTX-K-CIP-TE | Penicillins, cephalosporins, aminoglycosides, fluoroquinolones, tetracyclines | 0.500 | |
| Chicken pizza (78) Meat pizza (12) Canned tuna pizza (10) | 100 (17.9%) | AMP-OX-P-CXM-CTX-K-CIP | Penicillins, cephalosporins, aminoglycosides, fluoroquinolones | 0.438 | |
| Chicken pizza (104) Meat pizza (96) Canned tuna pizza (92) | 292 (52.1%) | AMP-OX-P-CXM-CTX-K | Penicillins, cephalosporins, aminoglycosides | 0.375 | |
| Chicken pizza (22) Meat pizza (12) | 34 (6.1%) | AMP-OX-P-CXM-CTX | Penicillins, cephalosporins | 0.313 | |
| Chicken pizza (32) Canned tuna pizza (24) | 56 (10%) | AMP-OX-P-CXM | Penicillins, cephalosporins | 0.250 | |
| Chicken pizza (22) | 22 (4%) | AMP-OX-P | Penicillins | 0.188 | |
| Sum = 560 | Mean MAR Index for all isolates = 0.375 | ||||
| Target Gene | Primer Direction and Sequence | Amplicon Size (bp) | Reference |
|---|---|---|---|
| nuc | F: 5′-gcgattgatggtgatacggtt-3′ R: 5′-agccaagccttgacgaactaa-3′ | 278 | This study |
| hla | F: 5′-gaagtctggtgaaaaccctga-3 R: 5′-tgaatcctgtcgctaatgcc-3′ | 704 | Xiaohong and Yanjun [71] |
| sea | F: 5′-tgcagggaacagctttaggcaa-3′ R: 5′-gattaatcccctctgaaccttcc-3′ | 500 | Sallam et al. [54] |
| seb | F: 5′-gtatggtggtgtaactgagc-3′ R: 5′-ccaaatagtgacgagttagg-3′ | 164 | Mehrotra et al. [72] |
| sec | F: 5′-agatgaagtagttgatgtgtatgg-3′ R: 5′-cacacttttagaatcaaccg-3′ | 451 | Mehrotra et al. [72] |
| sed | F: 5′-ccaataataggagaaaataaaag-3′ R: 5′-attggtattttttttcgttc-3′ | 278 | Mehrotra et al. [72] |
| tsst-1 | F: 5′-ctagactggtatagtagtggg-3′ R: 5′-cgccacttatttggaaatgg-3′ | 235 | This study |
| mecA | F: 5′-gattgggatcatagcgtca-3′ R: 5′-cagtatttcaccttgtccg-3′ | 1200 | Sallam et al. [54] |
| vanA | F: 5′-gggaaaacgacaattgc-3′ R: 5′-gtacaatgcggccgtta-3′ | 235 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsalkh, S.A.; Zakaria, A.I.; Abd-Elghany, S.M.; Imre, K.; Morar, A.; Sallam, K.I. Prevalence and Characterization of the Antimicrobial Resistance and Virulence Profiles of Staphylococcus aureus in Ready-to-Eat (Meat, Chicken, and Tuna) Pizzas in Mansoura City, Egypt. Antibiotics 2025, 14, 817. https://doi.org/10.3390/antibiotics14080817
Elsalkh SA, Zakaria AI, Abd-Elghany SM, Imre K, Morar A, Sallam KI. Prevalence and Characterization of the Antimicrobial Resistance and Virulence Profiles of Staphylococcus aureus in Ready-to-Eat (Meat, Chicken, and Tuna) Pizzas in Mansoura City, Egypt. Antibiotics. 2025; 14(8):817. https://doi.org/10.3390/antibiotics14080817
Chicago/Turabian StyleElsalkh, Sara Amgad, Amira Ibrahim Zakaria, Samir Mohammed Abd-Elghany, Kálmán Imre, Adriana Morar, and Khalid Ibrahim Sallam. 2025. "Prevalence and Characterization of the Antimicrobial Resistance and Virulence Profiles of Staphylococcus aureus in Ready-to-Eat (Meat, Chicken, and Tuna) Pizzas in Mansoura City, Egypt" Antibiotics 14, no. 8: 817. https://doi.org/10.3390/antibiotics14080817
APA StyleElsalkh, S. A., Zakaria, A. I., Abd-Elghany, S. M., Imre, K., Morar, A., & Sallam, K. I. (2025). Prevalence and Characterization of the Antimicrobial Resistance and Virulence Profiles of Staphylococcus aureus in Ready-to-Eat (Meat, Chicken, and Tuna) Pizzas in Mansoura City, Egypt. Antibiotics, 14(8), 817. https://doi.org/10.3390/antibiotics14080817









