Fully Green Particles Loaded with Essential Oils as Phytobiotics: A Review on Preparation and Application in Animal Feed
Abstract
1. Introduction
2. Essential Oils as Phytobiotics
2.1. Origin, Classification, and Structure
2.2. Mechanism of Action
2.3. Impact on Pathogenic Microflora
2.4. Impact on Probiotic Microflora
3. The Need and Benefits of EOs’ Encapsulation
4. Methods of Encapsulation
4.1. Nanoprecipitation
4.2. Emulsification–Solvent Evaporation and Emulsion–Diffusion
4.3. Electrospray Techniques
4.4. Thin Film Hydration
4.5. Ionic Gelation
4.6. Coacervation
4.7. Spray Drying
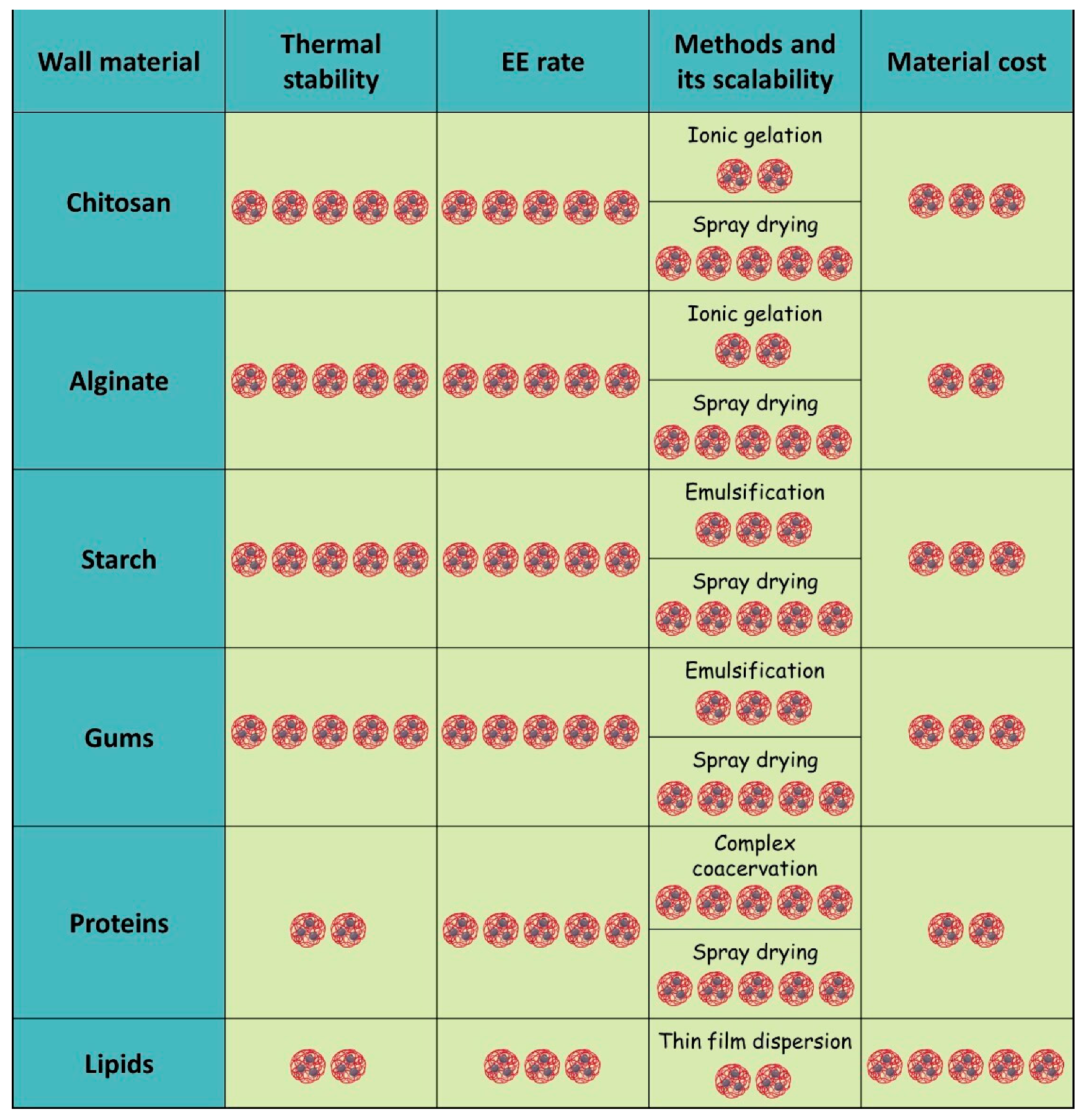
5. Wall Materials for EOs Encapsulation
5.1. Polysaccharides
5.1.1. Chitosan
| Core Material | Wall Material | Method of Preparation | Properties of Particles | References |
|---|---|---|---|---|
| Cinnamon EO | Chitosan, β-cyclodextrin | Ionic gelation | Size 300–400 nm EE 40–60% Release 25%, 50% and 28% after 120 h (pH 7.0, 4.5, 12.0) | [97] |
| Chitosan | Size 235.6 nm EE 40% | [98] | ||
| Chitosan | Size 0.1–1 µm EE 56–70% Release 25–40% after 400 min | [99] | ||
| Bitter orange oil | Chitosan | Ionic gelation | Size 40–60 nm EE 5–15% Release 20% after 20 days (pH 7.0) | [100] |
| Cumin seed oil | Chitosan | Ionic gelation | Size 150–250 nm EE 27% Release 69%, 61%, 30% and 48% after 5 h (pH 3, 5, 7, and 11) | [101] |
| Jasmine EO | Chitosan, pectin | Ionic gelation | Size 500–700 nm EE 8–30% Release 50% after 48 h (pH 7.4) | [102] |
| Achillea millefolium EO | Chitosan | Ionic gelation | Size 85–145 nm EE 85–90% | [103] |
| Cardamom EO | Chitosan | Ionic gelation | Size 50–100 nm EE 90% | [104] |
| Clove EO | Chitosan | Ionic gelation | Size 223–444 nm EE 55–70% DSC decomposition of free EO at 124 °C, decomposition of encapsulated EO at 320 °C | [105] |
| Size 100 nm EE 30–45% Release 30% after 60 days (pH 3 and 5) | [106] | |||
| Coriander EO | Chitosan | Ionic gelation | Size 57–80 nm EE 27–78% Release 90% after 175 h in PBS media | [107] |
| Spray drying | Size 400 nm–7 µm EE 5–25% Release 60% after 336 h in PBS media TGA EO decomposition at 100–200 °C, chitosan decomposition at 200–350 °C | [108] | ||
| Thyme EO | Chitosan | Nanoprecipitation | Size 10 nm EE 70% Release 100% after 360 min in water | [109] |
| Oregano (Origanum vulgare) EO | Chitosan | Ionic gelation | Size 407 nm EE 83% | [110] |
| Nettle (Urtica dioica L.) EO | Chitosan | Ionic gelation | Size 208–369 nm EE 59–68% | [111] |
| Clove (Eugenia caryophyllata) EO | Chitosan | Ionic gelation | Size 148–1287 nm EE 31–45% Release 30% after 56 days (pH 3) | [106] |
5.1.2. Gums
| Core Material | Wall Material | Method of Preparation | Properties of Particles | References |
|---|---|---|---|---|
| Ginger EO | Cashew gum | Spray drying | Size 5–30 μm EE 28% TG matrix decomposition at 250 °C; free EO evaporation below 180 °C | [115] |
| Cashew gum–Inulin | Size 4–33 μm EE 16–31% TG matrix decomposition at 250 °C; free EO evaporation below 180 °C | |||
| Lemongrass EO | Gum Arabic– Maltodextrin– OSA-starch | Spray drying | Size 5–13 μm EE 55–81% TGA free EO evaporation at 40–150 °C; matrix decomposition at 200–350 °C | [116] |
| Peppermint flavor | Gum Arabic | Spray drying | Size 45–256 nm EE 46–88% | [117] |
| Mentha longifolia L. EO | Balangu seed gum | Electrospraying | Size 96 nm EE 82–88% Release approx. 100% after 180 min in aqua media DSC complete EO decomposition at 169 °C; matrix decomposition at 291–345 °C; capsule decomposition at 223 °C | [118] |
| Fish oil Garlic EO | Persian gum–Chitosan | Electrostatic layer-by-layer deposition | Size 23–152 nm EE 63–86% DSC decomposition of matrix and capsule at 250–270 °C | [119] |
| Rosemary EO | Cashew gum galactomannan | Spray drying | EE 74–87% | [120] |
| Sweet basil EO | Lepidium sativum and Lepidium perfoliatum seed gums | Emulsification | Size 331–592 nm EE 77–89% | [121] |
| D-limonene | Alyssum homolocarpum seed gum | Electrospraying | EE 78–81% TGA free d-limonene decomposition at 150 °C; particles’ decomposition at 230 °C | [122] |
5.1.3. Alginate
| EOs | Wall Material | Method of Preparation | Properties of Particles | References |
|---|---|---|---|---|
| Perilla frutescens (L.) Britt. EO | Alginate | Ionic gelation | EE 57% Release 80% and 30% after 24 h (25 °C and 4 °C) DTG alginate matrix decomposition at 210–320 °C TG-free EO evaporation at 50–200 °C | [126] |
| Cinnamon EO | Alginate | Ionic gelation | Size 2.44 mm EE 85% | [127] |
| Thyme EO | Alginate | Ionic gelation | Size 890 µm EE 85% | [128] |
| Cumin EO | Alginate | Ionic gelation | Size 2.1 mm LC 0.22% Release 96% after 180 min in SGF and 10% after 180 min in SIF | [129] |
| Clove EO | Alginate | Emulsification | Size 1.5–3.0 mm EE 24% Release 50% after 240 min (pH 7) | [130] |
| Lavender (Lavandula angustifolia), tea tree (Melaleuca alternifolia), bergamot (Citrus bergamia), and peppermint (Mentha piperita) EOs | Alginate | Electrostatic extrusion | Size 0.9–1.2 mm | [131] |
| Tea tree EO | Chitosan and Alginate as external wall Methyl cellulose as internal wall | Spray drying | Size 7–11 µm EE 90% Release 60–90% after 72 h in PBS media | [132] |
| Carvacrol | Pectin–Alginate | Spray drying | Size 1.96 µm EE 77% Release 60% after 3 h in PBS media | [133] |
| Cinnamon EO | Alginate | Spray drying | Size 2 µm EE 88.1% Release 21% after 360 min in PBS media | [134] |
5.1.4. Starch
| EOs | Wall Material | Method | Particle’s Properties | References |
|---|---|---|---|---|
| Peppermint EO | Short linear glucan debranched from waxy maize starch | Ultrasound-assisted emulsification | Size 20 nm EE 75–88% Release 27–33% after 150 min in aqua media (80 °C) DSC melting of the particles at 84–110 °C | [142] |
| Menthone, oregano, cinnamon, lavender, and citral EO | Short linear glucans debranched from waxy maize starch | Nanoprecipitation | Size 93–113 nm EE 87% Release 85% after 48 h in PBS media | [143] |
| Lemongrass EO | OSA-starch | Spray drying | Size 13 µm TGA oil evaporation at 50–150 °C, particle decomposition at 200 °C | [116] |
| Orange EO | Short linear glucans debranched from rice starch | Spray drying | Size 30–40 µm EE 57–99% | [144] |
| Rosemary EO | OSA-starch | Electrospraying | EE 82–98% | [145] |
| Rosmarinus officinalis and Zataria multiflora EOs | OSA-starch | Spray drying | Size 8–11 µm EE 5–52% Release 90% after 30 d in the atmosphere (27 ± 3 °C and 70–75% relative humidity) | [146] |
| Vanilla EO | Jackfruit seed starch | Ultrasound-assisted emulsification | EE 79% | [147] |
| Rose EO | (OSA)-modified starch and maltodextrins (MDs) | Homogenizer-assisted emulsification | Size 2 µm EE 45% TGA decomposition of the particles at 235–309 °C, oil evaporation at 275 °C | [148] |
| Lemon EO | Chitosan and modified starch (Hicap 100) | Ultrasound-assisted emulsification | Size 339–553 nm EE 85% DSC free oil evaporation at 74–124 °C, decomposition of the particles at >200 °C | [149] |
5.2. Proteins
| EOs | Wall Material | Method | Particle’s Properties | References |
|---|---|---|---|---|
| Tuna oil and Mint (Mentha piperita) EO | Whey protein isolate-inulin | Spray drying | Size 190–280 nm EE 94% | [155] |
| Lime EO | Whey protein concentrate-maltodextrin | Spray drying | Size 3–4 µm EE 67–83% Release 60% after 150 min in mineral oil media TGA decomposition of the particles at 250 °C | [156] |
| Eugenol | Whey protein isolate- Maltodextrin | Spray drying | Size 0.1–10 µm EE 94–99% TGA free oil evaporation at 200–250 °C, decomposition of the particles at 283 °C | [157] |
| Chia EO | Whey protein concentrate–Mesquite gum or gum Arabic | Spray drying | Size 13–28 µm EE 70–81% | [158] |
| Black pepper (Piper nigrum L.) EO | Gelatin–Sodium alginate | Complex coacervation | EE 49–82% | [159] |
| Black pepper (Piper nigrum L.) EO | Lactoferrin–Sodium alginate | Complex coacervation | EE 32–85% Release 24% for 2 h in SGF media after following 85% for 2 h in SIF media | [160] |
| Shiitake (Lentinula edodes) EO | Gelatin–Carboxymethylcellulose | Complex coacervation | EE 86% | [161] |
| Citronella EO | Gelatin–Sodium alginate | Complex coacervation | Size 434 µm EE 74% TGA core material evaporation at 230–270 °C | [162] |
| Thyme EO | Soy protein–Alginate | Atomization via electrostatic extrusion | Size 0.6–1.4 mm EE 72–80% Release 42–55% after 60 min in SGF media (37 °C), 90–100% after following 60 min in SIF media | [136] |
| Carvacrol | Whey protein–Alginate | Extrusion | Size 250 and 800 µm Release 4.5% (for 250 µm) or 1.3% (for 800 µm) after 1 h in SGF media, 100% after following 4 h (250 µm) and 5 h (800 µm) in SIF media | [163] |
| Cinnamaldehyde | Gelatin–Pectin | Complex coacervation | Size 80–98 μm EE 85–89% Release 22% after 20 min in aqua media (80 °C) TGA core material evaporation at 125 °C and degradation at 225 °C; capsule decomposition at 250–400 °C | [164] |
| Rose EO | Mung bean protein Isolate–Pectin | Complex coacervation | Size 15 μm EE 90% Release 35% after 2 h in SGF media and 80% after 2 h in SIF media TGA: core material evaporation below 100 °C, capsule decomposition at 200–500 °C | [165] |
| Cardamom EO | Whey protein–Alginate | Internal gelation | Size 100 µm EE 84% Release 100% after 70 min in artificial saliva media | [166] |
5.3. Lipid-Based Systems
| Core Material | Wall Material | Method of Preparation | Properties of Particles | References |
|---|---|---|---|---|
| Chrysanthemum EO | Single-layer liposomes–Soy lecithin and cholesterol | Thin film hydration method | Size 97–232 nm EE 17–50% Release 37%, 48%, 71%, 88% after 30 min in the air media (at 4 °C, 12 °C, 25 °C, 37 °C, respectively) | |
| Double-layer liposomes–Soy lecithin and cholesterol + chitosan | Layer-by-layer electrostatic deposition method | Size 530–793 nm EE 43% Release 25%, 33%, 48%, 60% after 30 min in the air media (at 4 °C, 12 °C, 25 °C, 37 °C, respectively) | [173] | |
| Triple-layer liposomes–Soy lecithin, cholesterol, and chitosan + pectin | c | Size 642–3236 nm EE 43% Release: 12%, 17%, 22%, 25% after 30 days in the air media (at 4 °C, 12 °C, 25 °C, 37 °C, respectively) | ||
| Thyme EO | Soy lecithin and cholesterol | Thin film dispersion method and | Size 182 nm EE 35% Release 26% after 15 days in the air media | [174] |
| Eucalyptus citriodora EO | Soy lecithin and cholesterol | Thin film dispersion method | Size 150–295 nm EE 22% Release 39% and 68% after 28 days in the air media (at 4 °C and 25 °C, respectively) | [175] |
| Oliveria decumbens EO | Phosphatidylcholine | Thin film dispersion method | Size: 168 nm Release 100% after 4 days in aqua media | [176] |
| Zataria multiflora EO | Glyceryl mono stearate and Precirol® ATO5 | High-shear homogenization and ultrasound methods | Size: 255.5 nm PDI 0.369 EE 84% | [177] |
| Zataria multiflora EO | Stearic acid | High-pressure homogenizer method | Size: 134 nm PDI 0.24 EE 64.6% | [178] |
| Eugenia caryophyllata EO | Glyceryl mono stearate | High-shear homogenization and ultrasound method | Size 1231 nm PDI 0.384 EE 69.17% | [179] |
| Bergamot EO | Precirol® ATO5 | High shear homogenization | Size 194.70–437.50 nm PDI 0.17–0.70 Release 57–100% after 24 h in PBS pH 7.4 | [180] |
| Solvent diffusion | Size 133.90–365.10 nm PDI 0.18–0.39 | |||
| Cuminum cyminum L. EO | Cocoa Butter and Cacao Butter Substitute | High shear homogenization method and ultrasonic application | Size 86.11–129.53 nm PDI 0.1–0.25 EE 80.12–92.15% | [181] |
| Clove EO | Carnauba wax and beeswax | Low-energy nanoemulsification method coupled with high shear homogenization and sonication | Size 121–1489 nm PDI 0.08–0.31 EE 58–66% | [182] |
| Ziziphora clinopodioides Lam. EO | Precirol® ATO5 and Compritol® 888 ATO | High-shear homogenization and ultrasound | Size 241.1 nm PDI 0.312 EE 93% | [183] |
| Yuxingcao EO | Compritol® 888 ATO | High-shear homogenization | Size 171.2–811.9 nm PDI 0.260–0.287 EE 76.61–90.20% | [184] |
| Frankincense and myrrh EO | Compritol® 888 ATO | High-pressure homogenization | Size 113.3 nm EE 80.60% | [185] |
| Rosmarinus officinalis EO | Glyceryl tristearate | Ultrasonication | Size 103 nm EE 51.2% | [186] |
| Citral EO | Imwitor® 900 K | High-pressure homogenization | Size 97.7 nm PDI 0.249 | [187] |
| Nigella sativa L. EO | Softisan®154 and N. sativa | Hot homogenization | Size 66.27–142.70 nm PDI 0.18–0.27 | [188] |
| Cinnamon EO | Cocoa butter | High-shear homogenization | Size 100–120 nm EE 82.1% | [189] |
6. Considerations for Wall Material and Encapsulation Technique Selection
7. Application of Encapsulated EOs in Livestock Animals
7.1. Antibacterial Effect of Encapsulated EOs in Livestock Animals
7.2. Effects on Ruminant Performance
| Wall and Core Materials (Manufacturing Company) | Levels | Animals | Effects | References |
|---|---|---|---|---|
| Poultry | ||||
| Sodium alginate and whey protein isolate | 250 or 650 μg/g | Broilers | No effect on Lactobacillus spp. | [204] |
| Carvacrol | Non-significant reduction in C. perfringens | |||
| Chitosan | 60 mg/kg | Broilers | Significant increase in Lactobacillus spp. | [209] |
| Thymol | Significant reduction in E. coli | |||
| Wall material n/a * (AviPlus® P, Vetagro S.p.A., Reggio Emilia, Italy) | 0.5% | Broilers | No effect on the Listeria spp., Campylobacter spp., and Clostridium spp. counts in meat | [226] |
| Citric acid (25.0%), sorbic acid (16.7%), thymol (1.7%), and vanillin (1.0%) | ||||
| (AviPlus® P, Vetagro S.p.A., Italy) | ||||
| Sodium alginate and whey protein isolate | 500 mg/kg | Broilers | Significant increase in Lactobacillus spp. and Coprococcus spp. counts. | [208] |
| Thymol (4%), carvacrol (4%), hexanoic acid (0.5%), benzoic acid (3.5%), and butyric acid (0.5%) | Significant reduction in C. perfringens counts, Bacteroidetes, Rickenellaceae. | |||
| Wall material n/a * | 0.30 g/kg | Broilers | Significant reduction in E. coli counts. | [227] |
| Sorbic acid (200 g/kg), fumaric acid (200 g/kg), and thymol (100 g/kg) | ||||
| Soy protein isolates and soluble polysaccharides | 250 and 650 μg/g | Broilers | No effect on the Lactobacillus spp. | [228] |
| Citral EO | Significant reduction in C. perfringens | |||
| Chitosan | 100 and 200 mg/kg | Ross 308 broiler chicks | Significant increase in Lactobacillus spp. | [229] |
| Garlic EO | ||||
| Chitosan | 0.025%, 0.04% and 0.055% | Ross 308 broiler chicks | Significant increase in Lactobacillus spp. | [207] |
| Mint, thyme, and cinnamon EOs | Significant reduction in E. coli | |||
| Whey protein concentrate, maltodextrin, and modified starch. | 0.5, 1, and 2 kg/t | Ross 308 male broiler chickens | Significant increase in Lactobacillus spp. | [230] |
| Thyme, savoury, peppermint, and black pepper EOs. | Significant reduction in C. perfringens | |||
| Wall material n/a * | 0.30 g/kg | Cobb 500 chicks | Non-significant increase in Lactobacillus agilis | [231] |
| Combination of organic acids and Eos (Jefo Nutrition Inc., Saint-Hyacinthe, QC, Canada) | ||||
| Wall material n/a * | 150, 300, and 450 mg/kg | Hences | Significant increase in Bifidobacterium spp. | [232] |
| Sorbic acid (200 g/kg), fumaric acid (200 g/kg), and thymol (100 g/kg) | ||||
| Pigs | ||||
| Wall material n/a * Citric acid (25%), sorbic acid (16.7%), thymol (1.7%), and vanillin (1%) (Aviplus-S®, Vetagro S.p.A., Italy) | 0.2% | Weaned pigs | No effect on Lactobacillus spp. and E. coli counts | [233] |
| Triglycerides from hydrogenated vegetable oil Fumaric acid, citric acid, malic acid, sorbic acid, thymol, vanillin, and eugenol (Jefo Nutrition Inc., Canada) | 1 and 2 g/kg | Weaned pigs | Significant increase in Lactobacillus and Bacilli | [234] |
| Wall material n/a * Formic acid, citric acid, citrus, cinnamon, oregano, thyme, and capsicum EOs (FormaXOL™, Kemin Industries, Des Moines, IA, USA) | 4 kg/t | Finishing pigs | Significant reduction in Salmonella spp. | [235] |
| Ruminants | ||||
| Wall material n/a * Cinnamaldehyde and garlic EO (Cargill, Minnetonka, MN, USA) | 300 mg/d | Drylot beef cattle | No effect on ADG, BW No effect on fecal egg count No effect on glucose and urea nitrogen Non-significant reduction of horn fly population | [236] |
| Wall material n/a * Carvacrol, cinnamaldehyde, eugenol, and capsaicin from capsicum oleoresin (Activo® Premium, GRASP Ind. e Com. Ltd.a, Curitiba, Brazil) | 75 g/d | Steers | No effect on FBW, ADG, gain to feed ratio, DMI. No effect on blood partial pressure of carbon dioxide (pCO2) and oxygen (pO2), total concentration of CO2 (tCO2), saturation of O2 (SatO2) and CO2 (SatCO2), bicarbonate (HCO3−), total Hb, base excess (BE), pH, and packed cell volume (PCV) Non-significant increase in CH4 emission. | [218] |
| Organic carrier Thymol, eugenol, vanillin, limonene, and guaiacol (CRINA® Ruminants, DSM Nutritional Products Ltd., Kaiseraugst, Switzerland) | 1 or 2 g/d | Rumen fistulated Brahman (BBos. indicus) steers | No effect on VFA concentration No effect on CH4 emission | [219] |
| Wall material n/a * Carvacrol, cinnamaldehyde, eugenol, and capsaicin from capsicum oleoresin (Activo® Premium, GRASP Ind. e Com. Ltd.a, Brazil) | 150 mg/kg | Steers | No effect on FBW, ADG, DMI. No effect on carcass characteristics: the HCW, DP, marbling score, back fat depth, percentage of carcasses classified as low choice or greater, as well as incidence of liver abscess. Non-significant increase in YG, back fat depth. | [237] |
| Wall material n/a * Eugenol, thymol, and vanillin | 4 g/animal/day | Nellore heifers | No effect on the meat chemical composition or the muscle fatty acid profile. Increase in sarcomere length, soluble collagen content. Reduction in type III collagen. | [238] |
| Wall material n/a * Eugenol, thymol, and vanillin | 4 g/animal/day | Feedlot-finished heifers | No effect on pH of meat, fat thickness, intramuscular fat, or meat tenderness. Significant increase in antioxidant activity in meat. Significant reduction in lipid peroxidation in meat and meat color degradation. | [239] |
| Lipid matrix (80%) Anethole (10%) and carvone (10%) | 50 mg/kg | Lambs | No effect on BWG of animals infected with H. contortus compared to uninfected, H. contortus counts (at dose 20 mg/kg). Significant reduction in FEC. | [240] |
| Fat matrix Cinnamaldehyde, eugenol, carvacrol, and capsicum oleoresin | 200 and 400 mg/kg | Sheep | No effect on FI, DM. Significant reduction in CH4 emissions and protozoa counts. | [217] |
7.3. Effects on Gut Microbiota and Host Immunity
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADG | Average daily weight gain |
| AGPs | Antibiotic growth promoters |
| ATP | Adenosine triphosphate |
| BW | Body weight |
| CD | Depth of the crypts |
| DMI | Dry matter intake |
| DNA | Deoxyribonucleic acid |
| DSC | Differential scanning calorimetry |
| DTG | Differential thermogravimetric analysis |
| EE | Encapsulation efficacy |
| EOs | Essential oils |
| ESD | Emulsification–solvent diffusion |
| ESE | Emulsification–solvent evaporation |
| FCR | Feed conversion ratio |
| FDA | Food and Drug Administration |
| GI | Gastrointestinal |
| GRAS | Generally recognized as safe |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1 beta |
| MDA | Malondialdehyde |
| NF-κB | Nuclear factor kappa B |
| NLC | Nanostructured lipid carriers |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSP | Non-starch polysaccharides |
| OSA | Octenylsuccinate |
| PBS | Phosphate-buffered saline |
| PDI | Polydispersity index |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SCFA | Short-chain fatty acid |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| SLN | Solid lipid nanoparticles |
| TG | Thermogravimetry |
| TGA | Thermogravimetric analysis |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-alpha |
| Tp | Total protein |
| TPP | Triphenyl phosphate |
| VH | Height of the villi |
| VOCs | Volatile organic compounds |
References
- Mann, A.; Nehra, K.; Rana, J.S.; Dahiya, T. Antibiotic Resistance in Agriculture: Perspectives on Upcoming Strategies to Overcome Upsurge in Resistance. Curr. Res. Microb. Sci. 2021, 2, 100030. [Google Scholar] [CrossRef]
- Tóth, A.G.; Csabai, I.; Krikó, E.; Tőzsér, D.; Maróti, G.; Patai, Á.V.; Makrai, L.; Szita, G.; Solymosi, N. Antimicrobial Resistance Genes in Raw Milk for Human Consumption. Sci. Rep. 2020, 10, 7464. [Google Scholar] [CrossRef]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fèvre, E.M.; Woolhouse, M.E.J.; van Bunnik, B.A.D. Are Food Animals Responsible for Transfer of Antimicrobial-Resistant Escherichia coli or Their Resistance Determinants to Human Populations? A Systematic Review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef]
- Gonzalez Ronquillo, M.; Angeles Hernandez, J.C. Antibiotic and Synthetic Growth Promoters in Animal Diets: Review of Impact and Analytical Methods. Food Control 2017, 72, 255–267. [Google Scholar] [CrossRef]
- Anadón, A. WS14 The EU Ban of Antibiotics as Feed Additives (2006): Alternatives and Consumer Safety. J. Vet. Pharmacol. Ther. 2006, 29, 41–44. [Google Scholar] [CrossRef]
- Wen, R.; Li, C.; Zhao, M.; Wang, H.; Tang, Y. Withdrawal of Antibiotic Growth Promoters in China and Its Impact on the Foodborne Pathogen Campylobacter coli of Swine Origin. Front. Microbiol. 2022, 13, 1004725. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.-M. Potential of Essential Oils for Poultry and Pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Christaki, E.; Florou-Paneri, P. Essential Oils and Their Applications in Animal Nutrition. Med. Aromat. Plants 2013, 2, 1000140. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Bartolucci, C.; Antonacci, A.; Arduini, F.; Moscone, D.; Fraceto, L.; Campos, E.; Attaallah, R.; Amine, A.; Zanardi, C.; Cubillana-Aguilera, L.M.; et al. Green Nanomaterials Fostering Agrifood Sustainability. TrAC Trends Anal. Chem. 2020, 125, 115840. [Google Scholar] [CrossRef]
- Martin, G.B.; Kadokawa, H. “Clean, Green and Ethical” Animal Production. Case Study: Reproductive Efficiency in Small Ruminants. J. Reprod. Dev. 2006, 52, 145–152. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.D.L.; Christensen, H.; Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and Efficacy of Feed Additives Consisting of Essential Oils Derived from the Flower Buds or the Leaves of Syzygium aromaticum (L.) Merr. & L.M. Perry (Clove Bud Oil and Clove Leaf Oils) for All Animal Species (FEFANA Asbl). EFSA J. 2023, 21, e08183. [Google Scholar] [CrossRef] [PubMed]
- Feed Encapsulation Market Growth, Nutritional Delivery & Livestock Trends. Available online: https://www.futuremarketinsights.com/reports/feed-encapsulation-market (accessed on 24 July 2025).
- Mendel, M.; Chłopecka, M.; Dziekan, N.; Karlik, W. Phytogenic Feed Additives as Potential Gut Contractility Modifiers—A Review. Anim. Feed. Sci. Technol. 2017, 230, 30–46. [Google Scholar] [CrossRef]
- Gauthier, R. Organic Acids and Essential Oils, a Realistic Alternative to Antibiotic Growth Promoters in Pigs and Poultry. 2005. Available online: https://www.researchgate.net/publication/237310259_ORGANIC_ACIDS_AND_ESSENTIAL_OILS_A_REALISTIC_ALTERNATIVE_TO_ANTIBIOTIC_GROWTH_PROMOTERS_IN_PIGS_AND_POULTRY (accessed on 28 June 2025).
- Lingan, K. A Review on Major Constituents of Various Essential Oils and Its Application. Transl. Med. 2018, 8, 1000201. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Kunnath, K.; Alfarhan, A.; Rajagopal, R.; Ramesh, V. Chemical Composition of Cinnamomum verum Leaf and Flower Essential Oils and Analysis of Their Antibacterial, Insecticidal, and Larvicidal Properties. Molecules 2021, 26, 6303. [Google Scholar] [CrossRef]
- Morsy, N.F.S. Chemical Structure, Quality Indices and Bioactivity of Essential Oil Constituents. In Active Ingredients from Aromatic and Medicinal Plants; IntechOpen: London, UK, 2017; ISBN 978-953-51-2976-9. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.R.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A Review of Plant-Derived Essential Oils in Ruminant Nutrition and Production. Anim. Feed. Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- de Lange, C.F.M.; Pluske, J.; Gong, J.; Nyachoti, C.M. Strategic Use of Feed Ingredients and Feed Additives to Stimulate Gut Health and Development in Young Pigs. Livest. Sci. 2010, 134, 124–134. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-K.; Tan, N.-P.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. The Missing Piece: Recent Approaches Investigating the Antimicrobial Mode of Action of Essential Oils. Evol. Bioinform. Online 2021, 17, 1176934320938391. [Google Scholar] [CrossRef]
- Yap, P.; Yusoff, K.; Lim, E.; Chong, C.M.; Koksong, L. Membrane Disruption Properties of Essential Oils—A Double-Edged Sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Tiihonen, K.; Kettunen, H.; Peuranen, S.; Schulze, H.; Rautonen, N. In Vitro Effects of Essential Oils on Potential Pathogens and Beneficial Members of the Normal Microbiota. Vet. Med. 2010, 55, 71–78. [Google Scholar] [CrossRef]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial Activity of Essential Oils and Structurally Related Synthetic Food Additives towards Selected Pathogenic and Beneficial Gut Bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef]
- Kivanç, M.; Akgül, A.; Doğan, A. Inhibitory and Stimulatory Effects of Cumin, Oregano and Their Essential Oils on Growth and Acid Production of Lactobacillus plantarum and Leuconostoc mesenteroides. Int. J. Food Microbiol. 1991, 13, 81–85. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Tarapoulouzi, M.; Varzakas, T.; Jafari, S.M. Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms 2023, 11, 2896. [Google Scholar] [CrossRef]
- Reis, D.R.; Ambrosi, A.; Luccio, M.D. Encapsulated Essential Oils: A Perspective in Food Preservation. Future Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial Activity and Mechanism of Limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- İşcan, G.; Kirimer, N.; Demirci, F.; Demirci, B.; Noma, Y.; Başer, K.H.C. Biotransformation of (-)-(R)-α-Phellandrene: Antimicrobial Activity of Its Major Metabolite. Chem. Biodivers. 2012, 9, 1525–1532. [Google Scholar] [CrossRef]
- De Freitas, B.C.; Queiroz, P.A.; Baldin, V.P.; Do Amaral, P.H.; Rodrigues, L.L.; Vandresen, F.; Caleffi-Ferracioli, K.R.; De L Scodro, R.B.; Cardoso, R.F.; Siqueira, V.L. (-)-Camphene-Based Derivatives as Potential Antibacterial Agents against Staphylococcus aureus and Enterococcus spp. Future Microbiol. 2020, 15, 1527–1534. [Google Scholar] [CrossRef]
- Nematollahi, N.; Kolev, S.D.; Steinemann, A. Volatile Chemical Emissions from Essential Oils. Air Qual. Atmos. Health 2018, 11, 949–954. [Google Scholar] [CrossRef]
- Borges, M.; Lacerda, R.; Correia, J.; Melo, T.; Ferreira, S.B. Potential Antibacterial Action of Alpha-Pinene. Med. Sci. Forum 2022, 12, 11. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Comp. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Stevanović, Z.D.; Bošnjak-Neumüller, J.; Pajić-Lijaković, I.; Raj, J.; Vasiljević, M. Essential Oils as Feed Additives-Future Perspectives. Molecules 2018, 23, 1717. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Singh, B.K.; Dubey, N.K. Encapsulation of Essential Oils—A Booster to Enhance Their Bio-Efficacy as Botanical Preservatives. J. Sci. Res. 2020, 64, 175–178. [Google Scholar] [CrossRef]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef]
- de Souza, H.F.; dos Santos, F.R.; Cunha, J.S.; Pacheco, F.C.; Pacheco, A.F.C.; Soutelino, M.E.M.; Martins, C.C.N.; Andressa, I.; Rocha, R.d.S.; da Cruz, A.G.; et al. Microencapsulation to Harness the Antimicrobial Potential of Essential Oils and Their Applicability in Dairy Products: A Comprehensive Review of the Literature. Foods 2024, 13, 2197. [Google Scholar] [CrossRef]
- Gulyaev, I.A.; Sokol, M.B.; Mollaeva, M.R.; Klimenko, M.A.; Yabbarov, N.G.; Chirkina, M.V.; Nikolskaya, E.D. Polymeric Drug Delivery Systems in Biomedicine. Biochemistry 2025, 90, S233–S262. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Microparticles, Microcapsules and Microspheres: A Review of Recent Developments and Prospects for Oral Delivery of Insulin. Int. J. Pharm. 2018, 537, 223–244. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced Gelation of Alginate: Mechanisms and Applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Sokol, M.B.; Beganovskaya, V.A.; Mollaeva, M.R.; Yabbarov, N.G.; Chirkina, M.V.; Belykh, D.V.; Startseva, O.M.; Egorov, A.E.; Kostyukov, A.A.; Kuzmin, V.A.; et al. Pharmaceutical Approach to Develop Novel Photosensitizer Nanoformulation: An Example of Design and Characterization Rationale of Chlorophyll α Derivative. Pharmaceutics 2024, 16, 126. [Google Scholar] [CrossRef] [PubMed]
- Sokol, M.B.; Sokhraneva, V.A.; Groza, N.V.; Mollaeva, M.R.; Yabbarov, N.G.; Chirkina, M.V.; Trufanova, A.A.; Popenko, V.I.; Nikolskaya, E.D. Thymol-Modified Oleic and Linoleic Acids Encapsulated in Polymeric Nanoparticles: Enhanced Bioactivity, Stability, and Biomedical Potential. Polymers 2024, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Gulyaev, I.A.; Sokol, M.B.; Klimenko, M.A.; Mollaeva, M.R.; Yabbarov, N.G.; Chirkina, M.V.; Nikolskaya, E.D. Design of SAHA-Loaded PLGA Nanoparticles Aimed for Use in Breast Cancer Combination Therapy. Phys. At. Nucl. 2023, 86, 2490–2495. [Google Scholar] [CrossRef]
- Paolino, D.; Mancuso, A.; Cristiano, M.C.; Froiio, F.; Lammari, N.; Celia, C.; Fresta, M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials 2021, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Encapsulation to Drug Delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.Â.; Pinheiro, A.C.; Ramos, O.L.; Silva, H.; Bourbon, A.I.; Vicente, A.A. Advances in Food Nanotechnology. In Emerging Nanotechnologies in Food Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 11–38. ISBN 978-0-323-42980-1. [Google Scholar]
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle Formation and Its Mechanism in Single and Double Emulsion Solvent Evaporation. J. Control. Release 2004, 99, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Antipina, M.N. Emulsion-Based Techniques for Encapsulation in Biomedicine, Food and Personal Care. Curr. Opin. Pharmacol. 2014, 18, 47–55. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; Hernández Delgado, M.; Zambrano-Zaragoza, M.D.L.L.; Leyva-Gómez, G.; Mendoza-Muñoz, N.; Quintanar-Guerrero, D. Implementation of the Emulsification-Diffusion Method by Solvent Displacement for Polystyrene Nanoparticles Prepared from Recycled Material. RSC Adv. 2021, 11, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Muñoz, N.; Alcalá-Alcalá, S.; Quintanar-Guerrero, D. Preparation of Polymer Nanoparticles by the Emulsification-Solvent Evaporation Method: From Vanderhoff’s Pioneer Approach to Recent Adaptations. In Polymer Nanoparticles for Nanomedicines; Vauthier, C., Ponchel, G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 87–121. ISBN 978-3-319-41419-5. [Google Scholar]
- Mitri, K.; Vauthier, C.; Huang, N.; Menas, A.; Ringard-Lefebvre, C.; Anselmi, C.; Stambouli, M.; Rosilio, V.; Vachon, J.-J.; Bouchemal, K. Scale-up of Nanoemulsion Produced by Emulsification and Solvent Diffusion. J. Pharm. Sci. 2012, 101, 4240–4247. [Google Scholar] [CrossRef]
- Sokol, M.B.; Chirkina, M.V.; Yabbarov, N.G.; Mollaeva, M.R.; Podrugina, T.A.; Pavlova, A.S.; Temnov, V.V.; Hathout, R.M.; Metwally, A.A.; Nikolskaya, E.D. Structural Optimization of Platinum Drugs to Improve the Drug-Loading and Antitumor Efficacy of PLGA Nanoparticles. Pharmaceutics 2022, 14, 2333. [Google Scholar] [CrossRef]
- Sokol, M.B.; Yabbarov, N.G.; Mollaeva, M.R.; Chirkina, M.V.; Mollaev, M.D.; Zabolotsky, A.I.; Kuznetsov, S.L.; Nikolskaya, E.D. Alpha-Fetoprotein Mediated Targeting of Polymeric Nanoparticles to Treat Solid Tumors. Nanomedicine 2022, 17, 1217–1235. [Google Scholar] [CrossRef]
- Mollaeva, M.R.; Yabbarov, N.; Sokol, M.; Chirkina, M.; Mollaev, M.D.; Zabolotskii, A.; Seregina, I.; Bolshov, M.; Kaplun, A.; Nikolskaya, E. Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12261. [Google Scholar] [CrossRef]
- Alfatama, M.; Shahzad, Y.; Choukaife, H. Recent Advances of Electrospray Technique for Multiparticulate Preparation: Drug Delivery Applications. Adv. Colloid Interface Sci. 2024, 325, 103098. [Google Scholar] [CrossRef]
- Morais, A.Í.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of Polymeric Microparticles by Electrospray: The Impact of Experimental Parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jansen, J.A.; Yang, F. Electrospraying: Possibilities and Challenges of Engineering Carriers for Biomedical Applications—A Mini Review. Front. Chem. 2019, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Hoerr, R.A. Electrospray: Scaling-Up. In Dekker Encyclopedia of Nanoscience and Nanotechnology, 3rd ed.; Taylor & Francis: Abingdon, UK, 2015; pp. 1–11. ISBN 978-1-4398-9134-6. [Google Scholar]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Zhang, T.; Song, Y.; She, Z.; Li, J.; Deng, Y. Progress Involving New Techniques for Liposome Preparation. Asian J. Pharm. Sci. 2014, 9, 176–182. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil Encapsulation Techniques Using Alginate as Encapsulating Agent: Applications and Drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef]
- Leong, J.-Y.; Lam, W.-H.; Ho, K.-W.; Voo, W.-P.; Lee, M.F.-X.; Lim, H.-P.; Lim, S.-L.; Tey, B.-T.; Poncelet, D.; Chan, E.-S. Advances in Fabricating Spherical Alginate Hydrogels with Controlled Particle Designs by Ionotropic Gelation as Encapsulation Systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Chadha, S. Recent Advances in Nano-Encapsulation Technologies for Controlled Release of Biostimulants and Antimicrobial Agents. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., de Lima, R., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2021; pp. 29–55. ISBN 978-0-12-820092-6. [Google Scholar]
- Bayraktar, O.; Erdoğan, İ.; Köse, M.D.; Kalmaz, G. Nanocarriers for Plant-Derived Natural Compounds. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 395–412. ISBN 978-0-323-46152-8. [Google Scholar]
- Chen, K.-Y.; Zeng, S.-Y. Fabrication of Quaternized Chitosan Nanoparticles Using Tripolyphosphate/Genipin Dual Cross-Linkers as a Protein Delivery System. Polymers 2018, 10, 1226. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Zhang, X. Gelatin and High Methyl Pectin Coacervates Crosslinked with Tannic Acid: The Characterization, Rheological Properties, and Application for Peppermint Oil Microencapsulation. Food Hydrocoll. 2019, 97, 105174. [Google Scholar] [CrossRef]
- Da Silva, T.M.; De Deus, C.; De Souza Fonseca, B.; Lopes, E.J.; Cichoski, A.J.; Esmerino, E.A.; De Bona Da Silva, C.; Muller, E.I.; Moraes Flores, E.M.; De Menezes, C.R. The Effect of Enzymatic Crosslinking on the Viability of Probiotic Bacteria (Lactobacillus acidophilus) Encapsulated by Complex Coacervation. Food Res. Int. 2019, 125, 108577. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Kim, A. Coacervates: Recent Developments as Nanostructure Delivery Platforms for Therapeutic Biomolecules. Int. J. Pharm. 2022, 624, 122058. [Google Scholar] [CrossRef]
- Tang, Y.; Scher, H.B.; Jeoh, T. Industrially Scalable Complex Coacervation Process to Microencapsulate Food Ingredients. Innov. Food Sci. Emerg. Technol. 2020, 59, 102257. [Google Scholar] [CrossRef]
- Lemetter, C.Y.G.; Meeuse, F.M.; Zuidam, N.J. Control of the Morphology and the Size of Complex Coacervate Microcapsules during Scale-up. AIChE J. 2009, 55, 1487–1496. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils-A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef]
- Chopde, S.; Datir, R.; Deshmukh, G.; Dhotre, A.; Patil, M. Nanoparticle Formation by Nanospray Drying & Its Application in Nanoencapsulation of Food Bioactive Ingredients. J. Agric. Food Res. 2020, 2, 100085. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- van Boven, A.P.; Calderon Novoa, S.M.; Kohlus, R.; Schutyser, M.A.I. Investigation on Nozzle Zone Agglomeration during Spray Drying Using Response Surface Methodology. Powder Technol. 2023, 429, 118910. [Google Scholar] [CrossRef]
- Visan, A.I.; Cristescu, R. Polysaccharide-Based Coatings as Drug Delivery Systems. Pharmaceutics 2023, 15, 2227. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer Based Nanomaterials in Drug Delivery Systems: A Review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, W.; Zhu, G.; Zhou, R.; Niu, Y. A Review of the Preparation and Application of Flavour and Essential Oils Microcapsules Based on Complex Coacervation Technology. J. Sci. Food Agric. 2014, 94, 1482–1494. [Google Scholar] [CrossRef]
- Singh, V.; Indoria, S.; Jisha, K.J.; Gardas, R.L. Structure and Solubility of Polysaccharides. In Polysaccharides; Inamuddin, Ahamed, M.I., Boddula, R., Altalhi, T., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 325–336. ISBN 978-1-119-71138-4. [Google Scholar]
- Putro, J.N.; Soetaredjo, F.E.; Lunardi, V.B.; Irawaty, W.; Yuliana, M.; Santoso, S.P.; Puspitasari, N.; Wenten, I.G.; Ismadji, S. Polysaccharides Gums in Drug Delivery Systems: A Review. Int. J. Biol. Macromol. 2023, 253, 127020. [Google Scholar] [CrossRef]
- Shah, A.; Qazi, I.; Wanapat, M. Role of Chitin and Chitosan in Ruminant Diets and Their Impact on Digestibility, Microbiota and Performance of Ruminants. Fermentation 2022, 8, 549. [Google Scholar] [CrossRef]
- Hirano, S. Chitin Biotechnology Applications. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 1996; Volume 2, pp. 237–258. ISBN 978-0-444-82444-8. [Google Scholar]
- Qin, C.; Gao, J.; Wang, L.; Zeng, L.; Liu, Y. Safety Evaluation of Short-Term Exposure to Chitooligomers from Enzymic Preparation. Food Chem. Toxicol. 2006, 44, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Human Enzymatic Activities Related to the Therapeutic Administration of Chitin Derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Maroto, M. Advanced Nutrition and Human Metabolism. J. Nutr. Educ. Behav. 2022, 54, 804. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A Review on the Preparation of Chitosan Oligosaccharides and Application to Human Health, Animal Husbandry and Agricultural Production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Mirbagheri, V.S.; Alishahi, A.; Ahmadian, G.; Hashemi Petroudi, S.H.; Ojagh, S.M.; Romanazzi, G. Toward Understanding the Antibacterial Mechanism of Chitosan: Experimental Approach and in Silico Analysis. Food Hydrocoll. 2024, 147, 109382. [Google Scholar] [CrossRef]
- Mei, L.; Xu, Z.; Shi, Y.; Lin, C.; Jiao, S.; Zhang, L.; Li, P. Multivalent and Synergistic Chitosan Oligosaccharide-Ag Nanocomposites for Therapy of Bacterial Infection. Sci. Rep. 2020, 10, 10011. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters Influencing the Size of Chitosan-TPP Nano- and Microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef]
- Matshetshe, K.I.; Parani, S.; Manki, S.M.; Oluwafemi, O.S. Preparation, Characterization and in Vitro Release Study of β-Cyclodextrin/Chitosan Nanoparticles Loaded Cinnamomum zeylanicum Essential Oil. Int. J. Biol. Macromol. 2018, 118, 676–682. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-Cinnamon Essential Oil Nano-Formulation: Application as a Novel Additive for Controlled Release and Shelf Life Extension of Beef Patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef]
- Subasinghe, U.G.P.P.; Wickramarachchi, S. Encapsulation of Cinnamon Leaf Oil within Chitosan: Formulation and Characterization. Ceylon J. Sci. 2019, 48, 279. [Google Scholar] [CrossRef]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Bitter Orange Oil Incorporated into Chitosan Nanoparticles: Preparation, Characterization and Their Potential Application on Antioxidant and Antimicrobial Characteristics of White Button Mushroom. Food Hydrocoll. 2020, 100, 105387. [Google Scholar] [CrossRef]
- Amiri, A.; Mousakhani-Ganjeh, A.; Amiri, Z.; Guo, Y.; Pratap Singh, A.; Esmaeilzadeh Kenari, R. Fabrication of Cumin Loaded-Chitosan Particles: Characterized by Molecular, Morphological, Thermal, Antioxidant and Anticancer Properties as Well as Its Utilization in Food System. Food Chem. 2020, 310, 125821. [Google Scholar] [CrossRef]
- Attallah, O.A.; Shetta, A.; Elshishiny, F.; Mamdouh, W. Essential Oil Loaded Pectin/Chitosan Nanoparticles Preparation and Optimization via Box–Behnken Design against MCF-7 Breast Cancer Cell Lines. RSC Adv. 2020, 10, 8703–8708. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Saber, M.; Bagheri, M.; Mahdavinia, G.R. Achillea millefolium Essential Oil and Chitosan Nanocapsules with Enhanced Activity against Tetranychus urticae. J. Pest Sci. 2018, 91, 837–848. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H.; Imran, M. Encapsulation of Cardamom Essential Oil in Chitosan Nano-Composites: In-Vitro Efficacy on Antibiotic-Resistant Bacterial Pathogens and Cytotoxicity Studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan Nanoparticles Loaded with Clove Essential Oil: Characterization, Antioxidant and Antibacterial Activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the Antifungal Activity of Clove Essential Oil Encapsulated by Chitosan Nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Upadhyay, N.; Singh, P.; Sharma, S.; Dubey, N.K. Encapsulation in Chitosan-Based Nanomatrix as an Efficient Green Technology to Boost the Antimicrobial, Antioxidant and in Situ Efficacy of Coriandrum Sativum Essential Oil. Int. J. Biol. Macromol. 2019, 133, 294–305. [Google Scholar] [CrossRef]
- Duman, F.; Kaya, M. Crayfish Chitosan for Microencapsulation of Coriander (Coriandrum sativum L.) Essential Oil. Int. J. Biol. Macromol. 2016, 92, 125–133. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Gómez Y Gómez, Y. Release Study and Inhibitory Activity of Thyme Essential Oil-Loaded Chitosan Nanoparticles and Nanocapsules against Foodborne Bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Ariaii, P.; Motamedzadegan, A. Characterization, Antioxidant and Antibacterial Activities of Chitosan Nanoparticles Loaded with Nettle Essential Oil. Food Meas. 2021, 15, 1395–1402. [Google Scholar] [CrossRef]
- Taheri, A.; Jafari, S.M. Nanostructures of Gums for Encapsulation of Food Ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 521–578. ISBN 978-0-12-815663-6. [Google Scholar]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Al-Baadani, H.H.; Al-Mufarrej, S.I.; Al-Garadi, M.A.; Alhidary, I.A.; Al-Sagan, A.A.; Azzam, M.M. The Use of Gum Arabic as a Natural Prebiotic in Animals: A Review. Anim. Feed. Sci. Technol. 2021, 274, 114894. [Google Scholar] [CrossRef]
- Fernandes, R.V.D.B.; Botrel, D.A.; Silva, E.K.; Borges, S.V.; Oliveira, C.R.D.; Yoshida, M.I.; Feitosa, J.P.D.A.; De Paula, R.C.M. Cashew Gum and Inulin: New Alternative for Ginger Essential Oil Microencapsulation. Carbohydr. Polym. 2016, 153, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.R.; Fernandes, R.V.D.B.; De Castro E Silva, P.; Dessimoni, A.L.D.A.; Oliveira, C.R.; Borges, S.V.; Botrel, D.A. Influence of Modified Starches as Wall Materials on the Properties of Spray-Dried Lemongrass Oil. J. Food Sci. Technol. 2019, 56, 4972–4981. [Google Scholar] [CrossRef] [PubMed]
- Sangolkar, R.D.; Kawadkar, D.K.; Bhanvase, B.A.; Sonawane, S.H.; Potoroko, I. Ultrasound Assisted Encapsulation of Peppermint Flavor in Gum Arabic: Study of Process Parameters. J. Food Process. Eng. 2019, 42, e13269. [Google Scholar] [CrossRef]
- Rezaeinia, H.; Ghorani, B.; Emadzadeh, B.; Tucker, N. Electrohydrodynamic Atomization of Balangu (Lallemantia royleana) Seed Gum for the Fast-Release of Mentha longifolia L. Essential Oil: Characterization of Nano-Capsules and Modeling the Kinetics of Release. Food Hydrocoll. 2019, 93, 374–385. [Google Scholar] [CrossRef]
- Raeisi, S.; Ojagh, S.M.; Quek, S.Y.; Pourashouri, P.; Salaün, F. Nano-Encapsulation of Fish Oil and Garlic Essential Oil by a Novel Composition of Wall Material: Persian Gum-Chitosan. LWT 2019, 116, 108494. [Google Scholar] [CrossRef]
- Mendes, L.G.; Mendes, F.R.d.S.; Furtado, R.F.; Freire, G.A.; Sales, G.W.P.; da Costa, J.M.C.; Nogueira, N.A.P.; Monteiro-Moreira, A.C.d.O.; Moreira, R.d.A. Use of Cashew Gum Combined with Galactomannan for Encapsulation of Rosmarinus officinalis Essential Oil. J. Environ. Anal. Prog. 2020, 5, 369–380. [Google Scholar] [CrossRef]
- Aboutalebzadeh, S.; Esmaeilzadeh-Kenari, R.; Jafarpour, A. Nano-Encapsulation of Sweet Basil Essential Oil Based on Native Gums and Its Application in Controlling the Oxidative Stability of Kilka Fish Oil. Food Meas. 2022, 16, 2386–2399. [Google Scholar] [CrossRef]
- Khoshakhlagh, K.; Koocheki, A.; Mohebbi, M.; Allafchian, A. Development and Characterization of Electrosprayed Alyssum homolocarpum Seed Gum Nanoparticles for Encapsulation of D-Limonene. J. Colloid Interface Sci. 2017, 490, 562–575. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential Food and Nutraceutical Applications of Alginate: A Review. Mar Drugs 2022, 20, 564. [Google Scholar] [CrossRef]
- Guarino, V.; Caputo, T.; Altobelli, R.; Ambrosio, L. Degradation Properties and Metabolic Activity of Alginate and Chitosan Polyelectrolytes for Drug Delivery and Tissue Engineering Applications. AIMS Mater. Sci. 2015, 2, 497–502. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of Alginic Acid and Its Sodium, Potassium, Ammonium and Calcium Salts (E 400–E 404) as Food Additives. EFSA J. 2017, 15, e05049. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Z.; Li, X.; Li, H.; Cui, L.; He, D. Microcapsules Biologically Prepared Using Perilla frutescens (L.) Britt. Essential Oil and Their Use for Extension of Fruit Shelf Life. J. Sci. Food Agric. 2018, 98, 1033–1041. [Google Scholar] [CrossRef]
- Paris, M.J.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Modelling Release Mechanisms of Cinnamon (Cinnamomum zeylanicum) Essential Oil Encapsulated in Alginate Beads during Vapor-Phase Application. J. Food Eng. 2020, 282, 110024. [Google Scholar] [CrossRef]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of Alginate Microspheres Containing Thyme Essential Oil Using Ionic Gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Gholamian, S.; Nourani, M.; Bakhshi, N. Formation and Characterization of Calcium Alginate Hydrogel Beads Filled with Cumin Seeds Essential Oil. Food Chem. 2021, 338, 128143. [Google Scholar] [CrossRef] [PubMed]
- Faidi, A.; Lassoued, M.A.; Becheikh, M.E.H.; Touati, M.; Stumbé, J.-F.; Farhat, F. Application of Sodium Alginate Extracted from a Tunisian Brown Algae Padina pavonica for Essential Oil Encapsulation: Microspheres Preparation, Characterization and in Vitro Release Study. Int. J. Biol. Macromol. 2019, 136, 386–394. [Google Scholar] [CrossRef]
- Kokina, M.; Salević, A.; Kalušević, A.; Lević, S.; Pantić, M.; Pljevljakušić, D.; Šavikin, K.; Shamtsyan, M.; Nikšić, M.; Nedović, V. Characterization, Antioxidant and Antibacterial Activity of Essential Oils and Their Encapsulation into Biodegradable Material Followed by Freeze Drying. Food Technol. Biotechnol. 2019, 57, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Yin, X.; Wang, X.; Qiu, B.; Zhu, L.; Lin, Q. Microencapsulation of Tea Tree Oil by Spray-drying with Methyl Cellulose as the Emulsifier and Wall Material Together with Chitosan/Alginate. J. Appl. Polym. Sci. 2017, 134, app.44662. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Bai, J. Microencapsulation and Antimicrobial Activity of Carvacrol in a Pectin-Alginate Matrix. Food Hydrocoll. 2019, 92, 69–73. [Google Scholar] [CrossRef]
- Makimori, R.Y.; Endo, E.H.; Makimori, J.W.; Zanqueta, E.B.; Ueda-Nakamura, T.; Leimann, F.V.; Gonçalves, O.H.; Dias Filho, B.P. Preparation, Characterization and Antidermatophytic Activity of Free- and Microencapsulated Cinnamon Essential Oil. J. Mycol. Médicale 2020, 30, 100933. [Google Scholar] [CrossRef]
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of Black Seed Oil in Alginate Beads as a pH-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, In Vivo and Ex Vivo Study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef]
- Volić, M.; Pajić-Lijaković, I.; Djordjević, V.; Knežević-Jugović, Z.; Pećinar, I.; Stevanović-Dajić, Z.; Veljović, Đ.; Hadnadjev, M.; Bugarski, B. Alginate/Soy Protein System for Essential Oil Encapsulation with Intestinal Delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Alvarez-Ramirez, J.; Garcia, H.S. Microencapsulation Using Starch as Wall Material: A Review. Food Rev. Int. 2018, 34, 148–161. [Google Scholar] [CrossRef]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of Starch Digestion by α-Amylase—Structural Basis for Kinetic Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of Oxidised Starch (E 1404), Monostarch Phosphate (E 1410), Distarch Phosphate (E 1412), Phosphated Distarch Phosphate (E 1413), Acetylated Distarch Phosphate (E 1414), Acetylated Starch (E 1420), Acetylated Distarch Adipate (E 1422), Hydroxypropyl Starch (E 1440), Hydroxypropyl Distarch Phosphate (E 1442), Starch Sodium Octenyl Succinate (E 1450), Acetylated Oxidised Starch (E 1451) and Starch Aluminium Octenyl Succinate (E 1452) as Food Additives. EFSA J. 2017, 15, e04911. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; et al. Opinion on the Re-evaluation of Starch Sodium Octenyl Succinate (E 1450) as a Food Additive in Foods for Infants below 16 Weeks of Age and the Follow-up of Its Re-evaluation as a Food Additive for Uses in Foods for All Population Groups. EFSA J. 2020, 18, e05874. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Yamarik, T.A.; Cosmetic Ingredient Review Expert Panel. Final Report on the Safety Assessment of Aluminum Starch Octenylsuccinate. Int. J. Toxicol. 2002, 21, 1–7. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Ji, N.; Liu, J.; Xiong, L.; Sun, Q. Morphology and Characteristics of Starch Nanoparticles Self-Assembled via a Rapid Ultrasonication Method for Peppermint Oil Encapsulation. J. Agric. Food Chem. 2017, 65, 8363–8373. [Google Scholar] [CrossRef]
- Qiu, C.; Chang, R.; Yang, J.; Ge, S.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q. Preparation and Characterization of Essential Oil-Loaded Starch Nanoparticles Formed by Short Glucan Chains. Food Chem. 2017, 221, 1426–1433. [Google Scholar] [CrossRef]
- Márquez-Gómez, M.; Galicia-García, T.; Márquez-Meléndez, R.; Ruiz-Gutiérrez, M.; Quintero-Ramos, A. Spray-dried Microencapsulation of Orange Essential Oil Using Modified Rice Starch as Wall Material. J. Food Process. Preserv. 2018, 42, e13428. [Google Scholar] [CrossRef]
- Biduski, B.; Kringel, D.H.; Colussi, R.; Hackbart, H.C.D.S.; Lim, L.-T.; Dias, A.R.G.; Zavareze, E.D.R. Electrosprayed Octenyl Succinic Anhydride Starch Capsules for Rosemary Essential Oil Encapsulation. Int. J. Biol. Macromol. 2019, 132, 300–307. [Google Scholar] [CrossRef]
- Ahsaei, S.M.; Rodríguez-Rojo, S.; Salgado, M.; Cocero, M.J.; Talebi-Jahromi, K.; Amoabediny, G. Insecticidal Activity of Spray Dried Microencapsulated Essential Oils of Rosmarinus officinalis and Zataria multiflora against Tribolium confusum. Crop Prot. 2020, 128, 104996. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Tian, J.; Chu, Z. Effect of a New Shell Material—Jackfruit Seed Starch on Novel Flavor Microcapsules Containing Vanilla Oil. Ind. Crops Prod. 2018, 112, 47–52. [Google Scholar] [CrossRef]
- Xiao, Z.; Kang, Y.; Hou, W.; Niu, Y.; Kou, X. Microcapsules Based on Octenyl Succinic Anhydride (OSA)-Modified Starch and Maltodextrins Changing the Composition and Release Property of Rose Essential Oil. Int. J. Biol. Macromol. 2019, 137, 132–138. [Google Scholar] [CrossRef]
- Hasani, S.; Ojagh, S.M.; Ghorbani, M. Nanoencapsulation of Lemon Essential Oil in Chitosan-Hicap System. Part 1: Study on Its Physical and Structural Characteristics. Int. J. Biol. Macromol. 2018, 115, 143–151. [Google Scholar] [CrossRef]
- Lei, M.; Jiang, F.-C.; Cai, J.; Hu, S.; Zhou, R.; Liu, G.; Wang, Y.-H.; Wang, H.-B.; He, J.-R.; Xiong, X.-G. Facile Microencapsulation of Olive Oil in Porous Starch Granules: Fabrication, Characterization, and Oxidative Stability. Int. J. Biol. Macromol. 2018, 111, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of Spray Drying Microencapsulation of Almond Oil into Taro Starch Spherical Aggregates. LWT 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Han, M.; Liu, K.; Liu, X.; Rashid, M.T.; Zhang, H.; Wang, M. Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods 2023, 12, 2999. [Google Scholar] [CrossRef]
- Martins, J.T.; Bourbon, A.I.; Pinheiro, A.C.; Fasolin, L.H.; Vicente, A.A. Protein-Based Structures for Food Applications: From Macro to Nanoscale. Front. Sustain. Food Syst. 2018, 2, 77. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Al-Sagheer, A.; Noreldin, A.E.; Abd El-Hack, M.E.; Khafaga, A.F.; Abdel-Latif, M.A.; Swelum, A.A.; Arif, M.; Salem, A.Z.M. Beneficial Effects of Rumen-Protected Methionine on Nitrogen-Use Efficiency, Histological Parameters, Productivity and Reproductive Performance of Ruminants. Anim. Biotechnol. 2021, 32, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Fang, Z.; Khan, M.A.; Chen, Y.; Chen, Y.Q.; Liang, L. Tuna Oil and Mentha piperita Oil Emulsions and Microcapsules Stabilised by Whey Protein Isolate and Inulin: Characterisation and Stability. Int. J. Food Sci. Tech. 2017, 52, 494–503. [Google Scholar] [CrossRef]
- Campelo, P.H.; Sanches, E.A.; Fernandes, R.V.D.B.; Botrel, D.A.; Borges, S.V. Stability of Lime Essential Oil Microparticles Produced with Protein-Carbohydrate Blends. Food Res. Int. 2018, 105, 936–944. [Google Scholar] [CrossRef]
- Talón, E.; Lampi, A.-M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of Eugenol by Spray-Drying Using Whey Protein Isolate or Lecithin: Release Kinetics, Antioxidant and Antimicrobial Properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef]
- Rodea-González, D.A.; Cruz-Olivares, J.; Román-Guerrero, A.; Rodríguez-Huezo, M.E.; Vernon-Carter, E.J.; Pérez-Alonso, C. Spray-Dried Encapsulation of Chia Essential Oil (Salvia hispanica L.) in Whey Protein Concentrate-Polysaccharide Matrices. J. Food Eng. 2012, 111, 102–109. [Google Scholar] [CrossRef]
- Heckert Bastos, L.P.; Vicente, J.; Corrêa dos Santos, C.H.; Geraldo de Carvalho, M.; Garcia-Rojas, E.E. Encapsulation of Black Pepper (Piper nigrum L.) Essential Oil with Gelatin and Sodium Alginate by Complex Coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; Dos Santos, C.H.C.; de Carvalho, M.G.; Garcia-Rojas, E.E. Encapsulation of the Black Pepper (Piper nigrum L.) Essential Oil by Lactoferrin-Sodium Alginate Complex Coacervates: Structural Characterization and Simulated Gastrointestinal Conditions. Food Chem. 2020, 316, 126345. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, M.-F.; Chen, W.-S.; Zeng, Q.-Z.; Su, D.-X.; Tian, B.; He, S. Microencapsulation of Shiitake (Lentinula edodes) Essential Oil by Complex Coacervation: Formation, Rheological Property, Oxidative Stability and Odour Attenuation Effect. Int. J. Food Sci. Technol. 2018, 53, 1681–1688. [Google Scholar] [CrossRef]
- De Matos, E.F.; Scopel, B.S.; Dettmer, A. Citronella Essential Oil Microencapsulation by Complex Coacervation with Leather Waste Gelatin and Sodium Alginate. J. Environ. Chem. Eng. 2018, 6, 1989–1994. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.C.; Yu, H.; Zhu, J.; de Lange, K.; Yin, Y.; Wang, Q.; Gong, J. Evaluation of Alginate-Whey Protein Microcapsules for Intestinal Delivery of Lipophilic Compounds in Pigs. J. Sci. Food Agric. 2016, 96, 2674–2681. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Cai, J.; Zhang, X.; Duhoranimana, E.; Su, J. Gelatin and Pectin Complex Coacervates as Carriers for Cinnamaldehyde: Effect of Pectin Esterification Degree on Coacervate Formation, and Enhanced Thermal Stability. Food Hydrocoll. 2019, 87, 712–722. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Adhikari, B.; Chang, L. Microencapsulation of Rose Essential Oil in Mung Bean Protein Isolate-Apricot Peel Pectin Complex Coacervates and Characterization of Microcapsules. Food Hydrocoll. 2022, 124, 107366. [Google Scholar] [CrossRef]
- Zandi, M.; Dardmeh, N.; Pirsa, S.; Almasi, H. Identification of Cardamom Encapsulated Alginate–Whey Protein Concentrates Microcapsule Release Kinetics and Mechanism during Storage, Stew Process and Oral Consumption. J. Food Process. Eng. 2017, 40, e12314. [Google Scholar] [CrossRef]
- Da Silva, M.T.S.; Pinto, J.C. Influence of Encapsulated Aroma Compounds on the Formation and Morphology of Gelatin Microparticles. Macromol. Symp. 2019, 383, 1800061. [Google Scholar] [CrossRef]
- Zelikina, D.; Chebotarev, S.; Antipova, A.; Martirosova, E.; Anokhina, M.; Palmina, N.; Bogdanova, N.; Khvatov, A.; Tsaplev, Y.; Trofimov, A.; et al. Efficacy of a Maillard-Type Conjugate of Whey Protein Isolate with Chitosan as a Carrier for a Liposomal Form of a Combination of Curcumin and Balanced Amounts of n-3 and n-6 PUFAs. Part I. Structure–Functionality Relationships. Int. Dairy J. 2024, 154, 105923. [Google Scholar] [CrossRef]
- Ahlström, C.; Thuvander, J.; Rayner, M.; Matos, M.; Gutiérrez, G.; Östbring, K. The Effect of Precipitation pH on Protein Recovery Yield and Emulsifying Properties in the Extraction of Protein from Cold-Pressed Rapeseed Press Cake. Molecules 2022, 27, 2957. [Google Scholar] [CrossRef]
- Ashkar, A.; Sosnik, A.; Davidovich-Pinhas, M. Structured Edible Lipid-Based Particle Systems for Oral Drug-Delivery. Biotechnol. Adv. 2022, 54, 107789. [Google Scholar] [CrossRef] [PubMed]
- Zöller, K.; To, D.; Knoll, P.; Bernkop-Schnürch, A. Digestion of Lipid Excipients and Lipid-Based Nanocarriers by Pancreatic Lipase and Pancreatin. Eur. J. Pharm. Biopharm. 2022, 176, 32–42. [Google Scholar] [CrossRef]
- Final Report on the Safety Assessment of Lecithin and Hydrogenated Lecithin. Int. J. Toxicol. 2001, 20, 21–45. [CrossRef]
- Lin, L.; Gu, Y.; Sun, Y.; Cui, H. Characterization of Chrysanthemum Essential Oil Triple-Layer Liposomes and Its Application against Campylobacter Jejuni on Chicken. LWT 2019, 107, 16–24. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Thangaraj, B.; Abdel-Samie, M.A.S.; Cui, H. Improving the Stability of Thyme Essential Oil Solid Liposome by Using β-Cyclodextrin as a Cryoprotectant. Carbohydr. Polym. 2018, 188, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, W.; Li, C.; Cui, H. Enhancing Stability of Eucalyptus Citriodora Essential Oil by Solid Nanoliposomes Encapsulation. Ind. Crops Prod. 2019, 140, 111615. [Google Scholar] [CrossRef]
- Chavoshi, F.; Didar, Z.; Vazifedoost, M.; Shahidi Noghabi, M.; Zendehdel, A. Psyllium Seed Gum Films Loading Oliveria Decumbens Essential Oil Encapsulated in Nanoliposomes: Preparation and Characterization. Food Meas. 2022, 16, 4318–4330. [Google Scholar] [CrossRef]
- Nasseri, M.; Golmohammadzadeh, S.; Arouiee, H.; Jaafari, M.R.; Neamati, H. Antifungal Activity of Zataria multiflora Essential Oil-Loaded Solid Lipid Nanoparticles in-Vitro Condition. Iran. J. Basic Med. Sci. 2016, 19, 1231–1237. [Google Scholar] [PubMed]
- Kelidari, H.R.; Moemenbellah-Fard, M.D.; Morteza-Semnani, K.; Amoozegar, F.; Shahriari-Namadi, M.; Saeedi, M.; Osanloo, M. Solid-Lipid Nanoparticles (SLN)s Containing Zataria multiflora Essential Oil with No-Cytotoxicity and Potent Repellent Activity against Anopheles stephensi. J. Parasit. Dis. 2021, 45, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fazly Bazzaz, B.S.; Khameneh, B.; Namazi, N.; Iranshahi, M.; Davoodi, D.; Golmohammadzadeh, S. Solid Lipid Nanoparticles Carrying Eugenia caryophyllata Essential Oil: The Novel Nanoparticulate Systems with Broad-Spectrum Antimicrobial Activity. Lett. Appl. Microbiol. 2018, 66, 506–513. [Google Scholar] [CrossRef]
- Shaaban, M.; Nasr, M.; Tawfik, A.A.; Fadel, M.; Sammour, O. Bergamot Oil as an Integral Component of Nanostructured Lipid Carriers and a Photosensitizer for Photodynamic Treatment of Vitiligo: Characterization and Clinical Experimentation. Expert Opin. Drug Deliv. 2021, 18, 139–150. [Google Scholar] [CrossRef]
- Khosh manzar, M.; Pirouzifard, M.K.; Hamishehkar, H.; Pirsa, S. Cocoa Butter and Cocoa Butter Substitute as a Lipid Carrier of Cuminum cyminum L. Essential Oil; Physicochemical Properties, Physical Stability and Controlled Release Study. J. Mol. Liq. 2020, 314, 113638. [Google Scholar] [CrossRef]
- De Meneses, A.C.; Marques, E.B.P.; Leimann, F.V.; Gonçalves, O.H.; Ineu, R.P.; De Araújo, P.H.H.; De Oliveira, D.; Sayer, C. Encapsulation of Clove Oil in Nanostructured Lipid Carriers from Natural Waxes: Preparation, Characterization and in Vitro Evaluation of the Cholinesterase Enzymes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123879. [Google Scholar] [CrossRef]
- Hosseinpour Jajarm, F.; Moravvej, G.; Modarres Awal, M.; Golmohammadzadeh, S. Insecticidal Activity of Solid Lipid Nanoparticle Loaded by Ziziphora clinopodioides Lam. against Tribolium castaneum (Herbst, 1797) (Coleoptera: Tenebrionidae). Int. J. Pest Manag. 2021, 67, 147–154. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, Y.-X.; Hu, X.; Liu, C.-Y.; Quan, L.-H.; Liao, Y.-H. Solid Lipid Nanoparticles for Sustained Pulmonary Delivery of Yuxingcao Essential Oil: Preparation, Characterization and in Vivo Evaluation. Int. J. Pharm. 2017, 516, 364–371. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, J.-H.; Liu, Y.; Wang, Z.; Zhang, Y.-T.; Feng, N.-P. Preparation and Characterization of Solid Lipid Nanoparticles Loaded with Frankincense and Myrrh Oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar] [CrossRef]
- Ben-Khalifa, R.; Gaspar, F.B.; Pereira, C.; Chekir-Ghedira, L.; Rodríguez-Rojo, S. Essential Oil and Hydrophilic Antibiotic Co-Encapsulation in Multiple Lipid Nanoparticles: Proof of Concept and In Vitro Activity against Pseudomonas aeruginosa. Antibiotics 2021, 10, 1300. [Google Scholar] [CrossRef]
- Zielińska, A.; Martins-Gomes, C.; Ferreira, N.R.; Silva, A.M.; Nowak, I.; Souto, E.B. Anti-Inflammatory and Anti-Cancer Activity of Citral: Optimization of Citral-Loaded Solid Lipid Nanoparticles (SLN) Using Experimental Factorial Design and LUMiSizer®. Int. J. Pharm. 2018, 553, 428–440. [Google Scholar] [CrossRef]
- ALHaj, N.A.; Shamsudin, M.N.; Alipiah, N.M.; Zamri, H.F.; Bustamam, A.; Ibrahim, S.; Abdullah, R. Characterization of Nigella sativa L. Essential Oil-Loaded Solid Lipid Nanoparticles. Am. J. Pharmacol. Toxicol. 2010, 5, 52–57. [Google Scholar] [CrossRef]
- Bashiri, S.; Ghanbarzadeh, B.; Ayaseh, A.; Dehghannya, J.; Ehsani, A.; Ozyurt, H. Essential Oil-Loaded Nanostructured Lipid Carriers: The Effects of Liquid Lipid Type on the Physicochemical Properties in Beverage Models. Food Biosci. 2020, 35, 100526. [Google Scholar] [CrossRef]
- Ashraf, M.Z.; Srivastava, S. Oxidized Phospholipids: Introduction and Biological Significance. In Lipoproteins—Role in Health and Diseases; Kostner, G., Ed.; InTechOpen: London, UK, 2012; ISBN 978-953-51-0773-6. [Google Scholar]
- Cengiz, A.; Schroën, K.; Berton-Carabin, C. Towards Oxidatively Stable Emulsions Containing Iron-Loaded Liposomes: The Key Role of Phospholipid-to-Iron Ratio. Foods 2021, 10, 1293. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation Techniques, Factors Influencing Encapsulation Efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, M.C.S.; das Graças Fernandes da Silva, M.F.; Fernandes, J.B.; Forim, M.R. Evaluation of the Microencapsulation of Orange Essential Oil in Biopolymers by Using a Spray-Drying Process. Sci. Rep. 2020, 10, 11799. [Google Scholar] [CrossRef]
- Bastiaansen, T.M.M.; Benders, R.T.; Dijksman, J.A.; Thomas, M.; Hendriks, W.H.; de Vries, S.; Bosch, G. Changes in Thermomechanical Properties of Feed in Relation to Composition and Their Effect on Pellet Manufacturing. Anim. Feed. Sci. Technol. 2023, 295, 115540. [Google Scholar] [CrossRef]
- Somagond, A.; Aderao, G.N.; Girimal, D.; Singh, M. Animal Feeding and Watering Technologies⍟. In Engineering Applications in Livestock Production; Tarafdar, A., Pandey, A., Gaur, G.K., Singh, M., Pandey, H.O., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 37–62. ISBN 978-0-323-98385-3. [Google Scholar]
- Silva, E.K.; Azevedo, V.M.; Cunha, R.L.; Hubinger, M.D.; Meireles, M.A.A. Ultrasound-Assisted Encapsulation of Annatto Seed Oil: Whey Protein Isolate versus Modified Starch. Food Hydrocoll. 2016, 56, 71–83. [Google Scholar] [CrossRef]
- Merck|Russian Federation|Life Science Products & Service Solutions. Available online: https://www.sigmaaldrich.com/RU/en (accessed on 29 January 2025).
- Patra, P.; Upadhyay, T.K.; Alshammari, N.; Saeed, M.; Kesari, K.K. Alginate-Chitosan Biodegradable and Biocompatible Based Hydrogel for Breast Cancer Immunotherapy and Diagnosis: A Comprehensive Review. ACS Appl. Bio Mater. 2024, 7, 3515–3534. [Google Scholar] [CrossRef]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Dai Tran, L.; Long Bach, G.; et al. Characterization of Chitosan/Alginate/Lovastatin Nanoparticles and Investigation of Their Toxic Effects in Vitro and in Vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Wathoni, N.; Herdiana, Y.; Suhandi, C.; Mohammed, A.F.A.; El-Rayyes, A.; Narsa, A.C. Chitosan/Alginate-Based Nanoparticles for Antibacterial Agents Delivery. Int. J. Nanomed. 2024, 19, 5021–5044. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Männer, K.; Schieder, C.; Zentek, J. Effect of Supplementation of Phytogenic Feed Additives (Powdered vs. Encapsulated) on Performance and Nutrient Digestibility in Broiler Chickens. Poultry Science 2016, 95, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.; Al-Harthi, M.; El-Kelawy, M. Utilisation of Essential Oils as a Natural Growth Promoter for Broiler Chickens. Ital. J. Anim. Sci. 2019, 18, 1005–1012. [Google Scholar] [CrossRef]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef]
- Liu, X.; Diarra, M.S.; Zhang, Y.; Wang, Q.; Yu, H.; Nie, S.-P.; Xie, M.-Y.; Gong, J. Effect of Encapsulated Carvacrol on the Incidence of Necrotic Enteritis in Broiler Chickens. Avian Pathol. 2016, 45, 357–364. [Google Scholar] [CrossRef]
- Mak, P.H.W.; Rehman, M.A.; Kiarie, E.G.; Topp, E.; Diarra, M.S. Production Systems and Important Antimicrobial Resistant-Pathogenic Bacteria in Poultry: A Review. J. Anim. Sci. Biotechnol. 2022, 13, 148. [Google Scholar] [CrossRef]
- Moharreri, M.; Vakili, R.; Oskoueian, E.; Rajabzadeh, G. Phytobiotic Role of Essential Oil-Loaded Microcapsules in Improving the Health Parameters in Clostridium perfringens-Infected Broiler Chickens. Ital. J. Anim. Sci. 2021, 20, 2075–2085. [Google Scholar] [CrossRef]
- Nouri, A. Chitosan Nano-Encapsulation Improves the Effects of Mint, Thyme, and Cinnamon Essential Oils in Broiler Chickens. Br. Poult. Sci. 2019, 60, 530–538. [Google Scholar] [CrossRef]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary Encapsulated Essential Oils and Organic Acids Mixture Improves Gut Health in Broiler Chickens Challenged with Necrotic Enteritis. J. Anim. Sci. Biotechnol. 2020, 11, 18. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Meimandipour, A. Feeding Broilers with Thyme Essential Oil Loaded in Chitosan Nanoparticles: An Efficient Strategy for Successful Delivery. Br. Poult. Sci. 2018, 59, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Shohe, A.; Vidyarthi, V.K.; Zuyie, R. Performance of Broiler Chicken on Diet Supplemented with Turmeric Powder (Curcuma longa). Livest. Res. Int. 2019, 7, 77–82. [Google Scholar]
- Willems, O.W.; Miller, S.P.; Wood, B.J. Assessment of Residual Body Weight Gain and Residual Intake and Body Weight Gain as Feed Efficiency Traits in the Turkey (Meleagris gallopavo). Genet. Sel. Evol. 2013, 45, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, W.; Yuan, S.; Chen, H. The Effect of Different Dietary Levels of Thyme Essential Oil on Serum Biochemical Indices in Mahua Broiler Chickens. Ital. J. Anim. Sci. 2014, 13, 3238. [Google Scholar] [CrossRef]
- Nguyen, T.N.D.; Le, H.N.; Pham, V.V.; Eva, P.; Alberto, F.; Le, T.H. Relationship between the Ratio of Villous Height:Crypt Depth and Gut Bacteria Counts as Well Production Parameters in Broiler Chickens. J. Agric. Dev. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Dorantes-Iturbide, G.; Orzuna-Orzuna, J.F.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Lee-Rangel, H.A. Essential Oils as a Dietary Additive for Small Ruminants: A Meta-Analysis on Performance, Rumen Parameters, Serum Metabolites, and Product Quality. Vet. Sci. 2022, 9, 475. [Google Scholar] [CrossRef]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The Ruminal Microbiome Associated with Methane Emissions from Ruminant Livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Khairunisa, B.H.; Heryakusuma, C.; Ike, K.; Mukhopadhyay, B.; Susanti, D. Evolving Understanding of Rumen Methanogen Ecophysiology. Front. Microbiol. 2023, 14, 1296008. [Google Scholar] [CrossRef] [PubMed]
- Soltan, Y.A.; Natel, A.S.; Araujo, R.C.; Morsy, A.S.; Abdalla, A.L. Progressive Adaptation of Sheep to a Microencapsulated Blend of Essential Oils: Ruminal Fermentation, Methane Emission, Nutrient Digestibility, and Microbial Protein Synthesis. Anim. Feed. Sci. Technol. 2018, 237, 8–18. [Google Scholar] [CrossRef]
- Alemu, A.W.; Romero-Pérez, A.; Araujo, R.C.; Beauchemin, K.A. Effect of Encapsulated Nitrate and Microencapsulated Blend of Essential Oils on Growth Performance and Methane Emissions from Beef Steers Fed Backgrounding Diets. Animals 2019, 9, 21. [Google Scholar] [CrossRef]
- Tomkins, N.W.; Denman, S.E.; Pilajun, R.; Wanapat, M.; McSweeney, C.S.; Elliott, R. Manipulating Rumen Fermentation and Methanogenesis Using an Essential Oil and Monensin in Beef Cattle Fed a Tropical Grass Hay. Anim. Feed. Sci. Technol. 2015, 200, 25–34. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Chaudhary, L.C.; Agarwal, N.; Chaturvedi, V.B.; Kamra, D.N. Effect of Feeding of Blend of Essential Oils on Methane Production, Growth, and Nutrient Utilization in Growing Buffaloes. Asian-Australas J. Anim. Sci. 2018, 31, 672–676. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M. Methane Emissions from Beef Cattle: Effects of Fumaric Acid, Essential Oil, and Canola Oil1. J. Anim. Sci. 2006, 84, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Yu, Z. Effects of Essential Oils on Methane Production and Fermentation by, and Abundance and Diversity of, Rumen Microbial Populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Essential Oils Affect Populations of Some Rumen Bacteria in Vitro as Revealed by Microarray (RumenBactArray) Analysis. Front. Microbiol. 2015, 6, 297. [Google Scholar] [CrossRef]
- Nasir, M.; Rodríguez-Prado, M.; Simoni, M.; Martín-Orúe, S.M.; Pérez, J.F.; Calsamiglia, S. Optimizing Essential Oil Mixtures: Synergistic Effects on Cattle Rumen Fermentation and Methane Emission. Animals 2025, 15, 2105. [Google Scholar] [CrossRef]
- Benchaar, C.; Greathead, H. Essential Oils and Opportunities to Mitigate Enteric Methane Emissions from Ruminants. Anim. Feed. Sci. Technol. 2011, 166–167, 338–355. [Google Scholar] [CrossRef]
- Stamilla, A.; Russo, N.; Messina, A.; Spadaro, C.; Natalello, A.; Caggia, C.; Randazzo, C.L.; Lanza, M. Effects of Microencapsulated Blend of Organic Acids and Essential Oils as a Feed Additive on Quality of Chicken Breast Meat. Animals 2020, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Yan, F.; Yang, C.; Yang, X. Effects of Encapsulated Organic Acids and Essential Oils on Intestinal Barrier, Microbial Count, and Bacterial Metabolites in Broiler Chickens. Poult. Sci. 2019, 98, 2858–2865. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Diarra, M.S.; Yu, H.; Hua, Y.; Gong, J. Functional Assessment of Encapsulated Citral for Controlling Necrotic Enteritis in Broiler Chickens. Poult. Sci. 2016, 95, 780–789. [Google Scholar] [CrossRef]
- Amiri, N.; Afsharmanesh, M.; Salarmoini, M.; Meimandipour, A.; Hosseini, S.A.; Ebrahimnejad, H. Nanoencapsulation (in Vitro and in Vivo) as an Efficient Technology to Boost the Potential of Garlic Essential Oil as Alternatives for Antibiotics in Broiler Nutrition. Animal 2021, 15, 100022. [Google Scholar] [CrossRef]
- Moharreri, M.; Vakili, R.; Oskoueian, E.; Rajabzadeh, G. Effects of Microencapsulated Essential Oils on Growth Performance and Biomarkers of Inflammation in Broiler Chickens Challenged with Salmonella Enteritidis. J. Saudi Soc. Agric. Sci. 2022, 21, 349–357. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Xin, H.; Chen, S.; Yang, C.; Duan, Y.; Yang, X. Effects of a Protected Inclusion of Organic Acids and Essential Oils as Antibiotic Growth Promoter Alternative on Growth Performance, Intestinal Morphology and Gut Microflora in Broilers. Anim. Sci. J. 2017, 88, 1414–1424. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Li, X.; Yang, X.; Long, F.; Yang, X. Effects of Encapsulated Essential Oils and Organic Acids on Laying Performance, Egg Quality, Intestinal Morphology, Barrier Function, and Microflora Count of Hens during the Early Laying Period. Poult. Sci. 2019, 98, 6751–6760. [Google Scholar] [CrossRef] [PubMed]
- Kwak, W.G.; Song, M.H.; Lee, D.H.; Yun, W.; Lee, J.H.; Lee, C.H.; Oh, H.J.; Liu, S.; An, J.S.; Kim, H.B.; et al. The Effects of Microencapsulated Compounds Supplementation on Growth Performance, Immune Cells, and Rectal Temperature in Weaned Pigs by Lipopolysaccharides. Can. J. Anim. Sci. 2019, 99, 505–513. [Google Scholar] [CrossRef]
- Xu, Y.; Lahaye, L.; He, Z.; Zhang, J.; Yang, C.; Piao, X. Micro-Encapsulated Essential Oils and Organic Acids Combination Improves Intestinal Barrier Function, Inflammatory Responses and Microbiota of Weaned Piglets Challenged with Enterotoxigenic Escherichia coli F4 (K88+). Anim. Nutr. 2020, 6, 269–277. [Google Scholar] [CrossRef]
- Walia, K.; Argüello, H.; Lynch, H.; Leonard, F.C.; Grant, J.; Yearsley, D.; Kelly, S.; Duffy, G.; Gardiner, G.E.; Lawlor, P.G. Effect of Strategic Administration of an Encapsulated Blend of Formic Acid, Citric Acid, and Essential Oils on Salmonella Carriage, Seroprevalence, and Growth of Finishing Pigs. Prev. Vet. Med. 2017, 137, 28–35. [Google Scholar] [CrossRef]
- Moriel, P.; Silva, G.M.; Piccolo, M.B.; Ranches, J.; Vendramini, J.M.B.; Arthington, J.D. Supplementation of Encapsulated Cinnamaldehyde and Garlic Oil on Pre- and Postweaning Growth Performance of Beef Cattle Fed Warm-Season Forages. Prof. Anim. Sci. 2018, 34, 275–283. [Google Scholar] [CrossRef]
- Araujo, R.C.; Daley, D.R.; Goodall, S.R.; Jalali, S.; Guimarães Bisneto, O.A.; Budde, A.M.; Wagner, J.J.; Engle, T.E. Effects of a Microencapsulated Blend of Essential Oils Supplemented Alone or in Combination with Monensin on Performance and Carcass Characteristics of Growing and Finishing Beef Steers. Appl. Anim. Sci. 2019, 35, 177–184. [Google Scholar] [CrossRef]
- Monteschio, J.O.; Vargas-Junior, F.M.; Almeida, F.L.A.; Pinto, L.A.D.M.; Kaneko, I.N.; Almeida, A.A.; Freitas, L.W.; Alves, S.P.A.; Bessa, R.J.B.; Prado, I.N. The Effect of Encapsulated Active Principles (Eugenol, Thymol and Vanillin) and Clove and Rosemary Essential Oils on the Structure, Collagen Content, Chemical Composition and Fatty Acid Profile of Nellore Heifers Muscle. Meat Sci. 2019, 155, 27–35. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Monteschio, J.; De Souza, K.A.; Vital, A.C.P.; Guerrero, A.; Valero, M.V.; Kempinski, E.M.B.C.; Barcelos, V.C.; Nascimento, K.F.; Do Prado, I.N. Clove and Rosemary Essential Oils and Encapsuled Active Principles (Eugenol, Thymol and Vanillin Blend) on Meat Quality of Feedlot-Finished Heifers. Meat Sci. 2017, 130, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Katiki, L.M.; Araujo, R.C.; Ziegelmeyer, L.; Gomes, A.C.P.; Gutmanis, G.; Rodrigues, L.; Bueno, M.S.; Veríssimo, C.J.; Louvandini, H.; Ferreira, J.F.S.; et al. Evaluation of Encapsulated Anethole and Carvone in Lambs Artificially- and Naturally-Infected with Haemonchus Contortus. Exp. Parasitol. 2019, 197, 36–42. [Google Scholar] [CrossRef]
- Everitt, J.I. The Future of Preclinical Animal Models in Pharmaceutical Discovery and Development: A Need to Bring in Cerebro to the in Vivo Discussions. Toxicol. Pathol. 2015, 43, 70–77. [Google Scholar] [CrossRef]
- Liu, A.; Li, Z.; Jin, X.; Wu, Q.; Hu, H.; Zhang, C. An Encapsulated Organic Acid and Essential Oil Mixture Improves the Intestinal Health of Weaned Piglets by Altering Intestinal Inflammation and Antioxidative Capacity. Animals 2022, 12, 2426. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Zhang, X.-L.; Xu, L.-H.; Peng, H.; Wang, C.-K.; Bi, Y.-Z. Encapsulated Blends of Essential Oils and Organic Acids Improved Performance, Intestinal Morphology, Cecal Microflora, and Jejunal Enzyme Activity of Broilers. Czech J. Anim. Sci. 2019, 64, 189–198. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Huang, S.; Huang, Y.; Shi, H.; Bai, X. Effects of Dietary Essential Oil Supplementation on Growth Performance, Carcass Yield, Meat Quality, and Intestinal Tight Junctions of Broilers with or without Eimeria Challenge. Poult. Sci. 2023, 102, 102874. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Alvim, I.D.; Wen, C.; Gómez Expósito, R.; Aalvink, S.; Contreras Castillo, C.J.; Da Gloria, E.M.; Smidt, H. Exploring the Effect of a Microencapsulated Citrus Essential Oil on in Vitro Fermentation Kinetics of Pig Gut Microbiota. Front. Microbiol. 2022, 13, 952706. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Holban, A.-M.; Curutiu, C.; Ditu, L.M. Modulation of Gut Microbiota by Essential Oils and Inorganic Nanoparticles: Impact in Nutrition and Health. Front. Nutr. 2022, 9, 920413. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, W.; Xiong, P.; Cheng, D.; Wei, W.; Zhou, Q.; Xu, C.; Song, Q.; Ji, H.; Hu, Y.; et al. Effects of Microencapsulated Plant Essential Oils on Growth Performance, Immunity, and Intestinal Health of Weaned Tibetan Piglets. Front. Vet. Sci. 2024, 11, 1456181. [Google Scholar] [CrossRef] [PubMed]
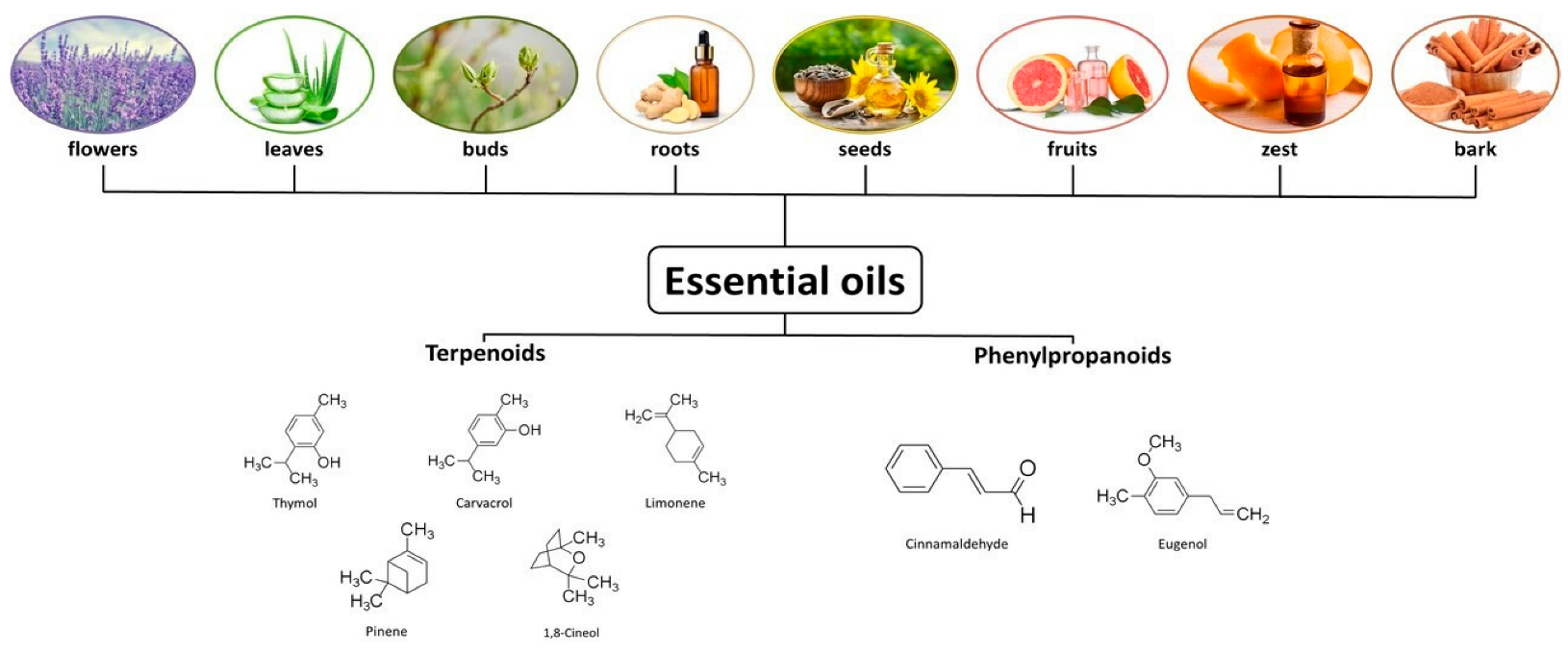
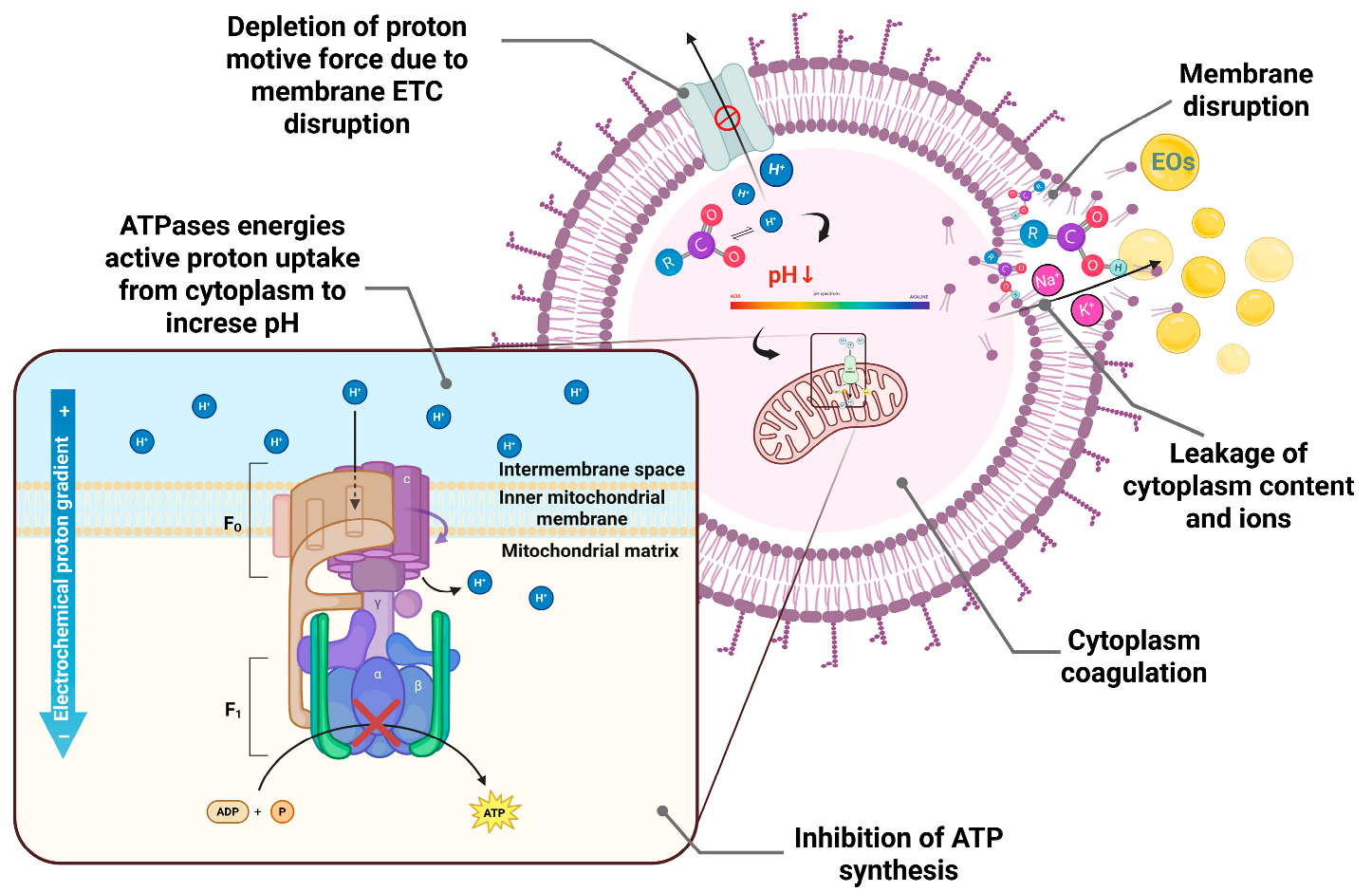
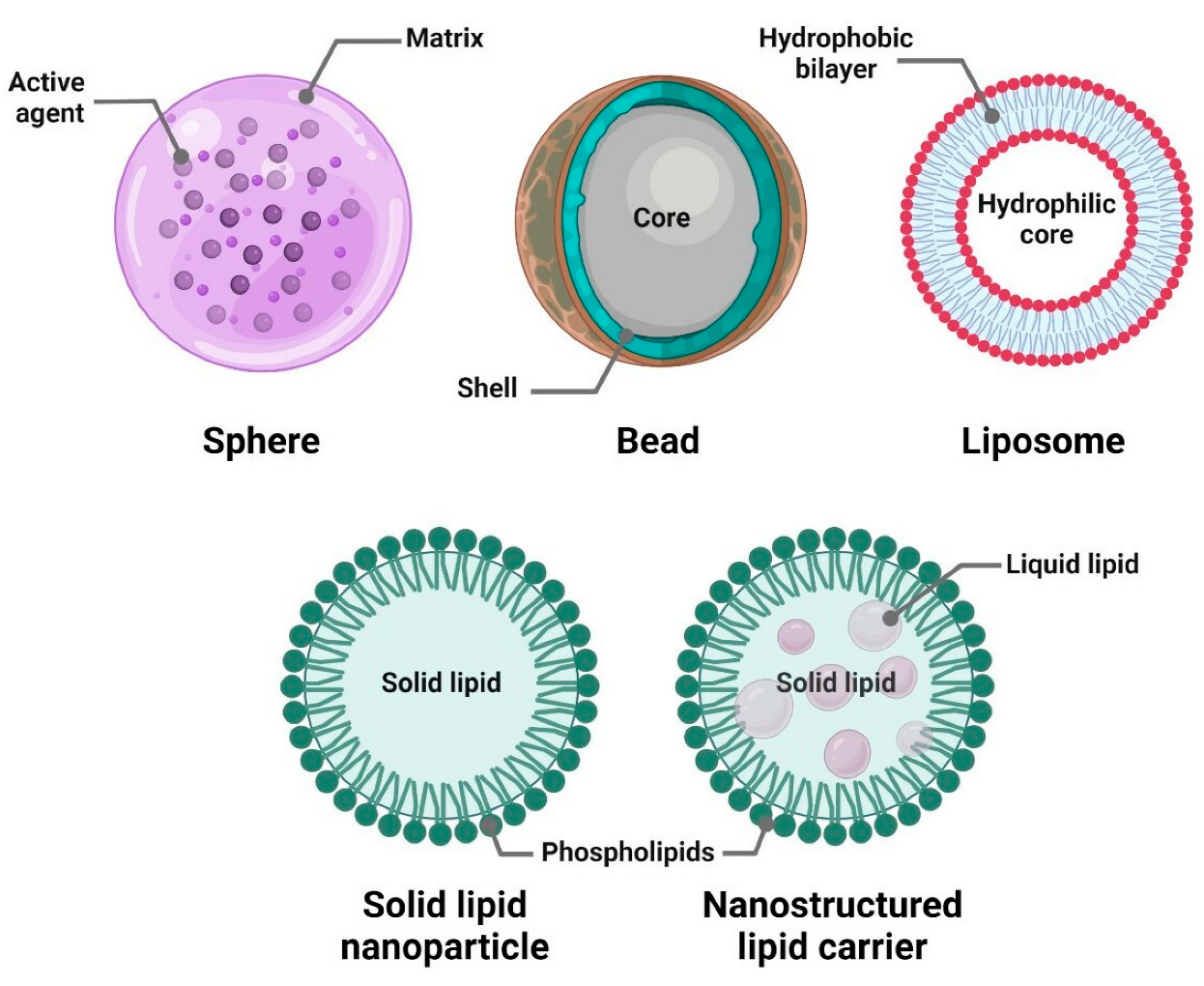
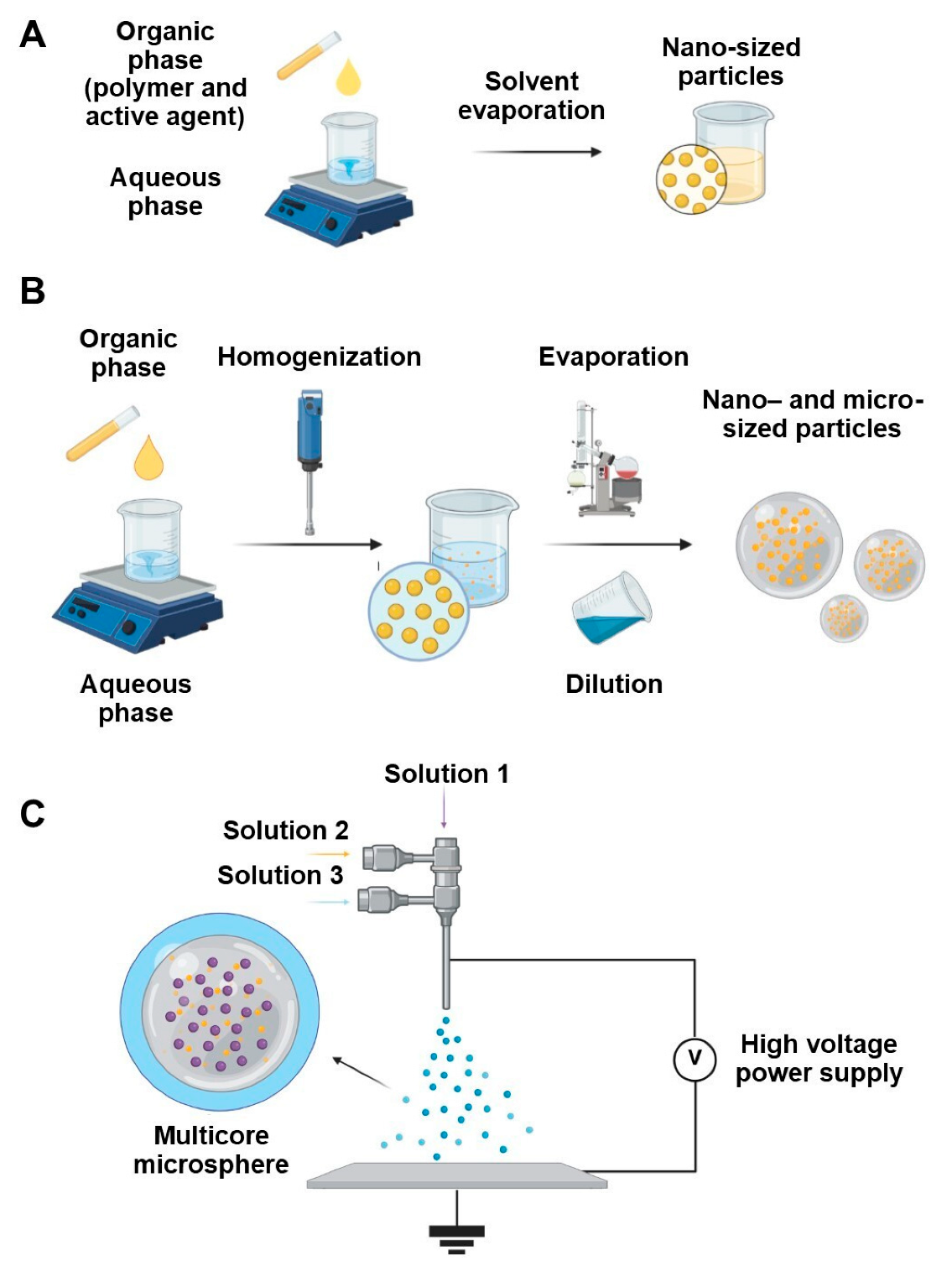
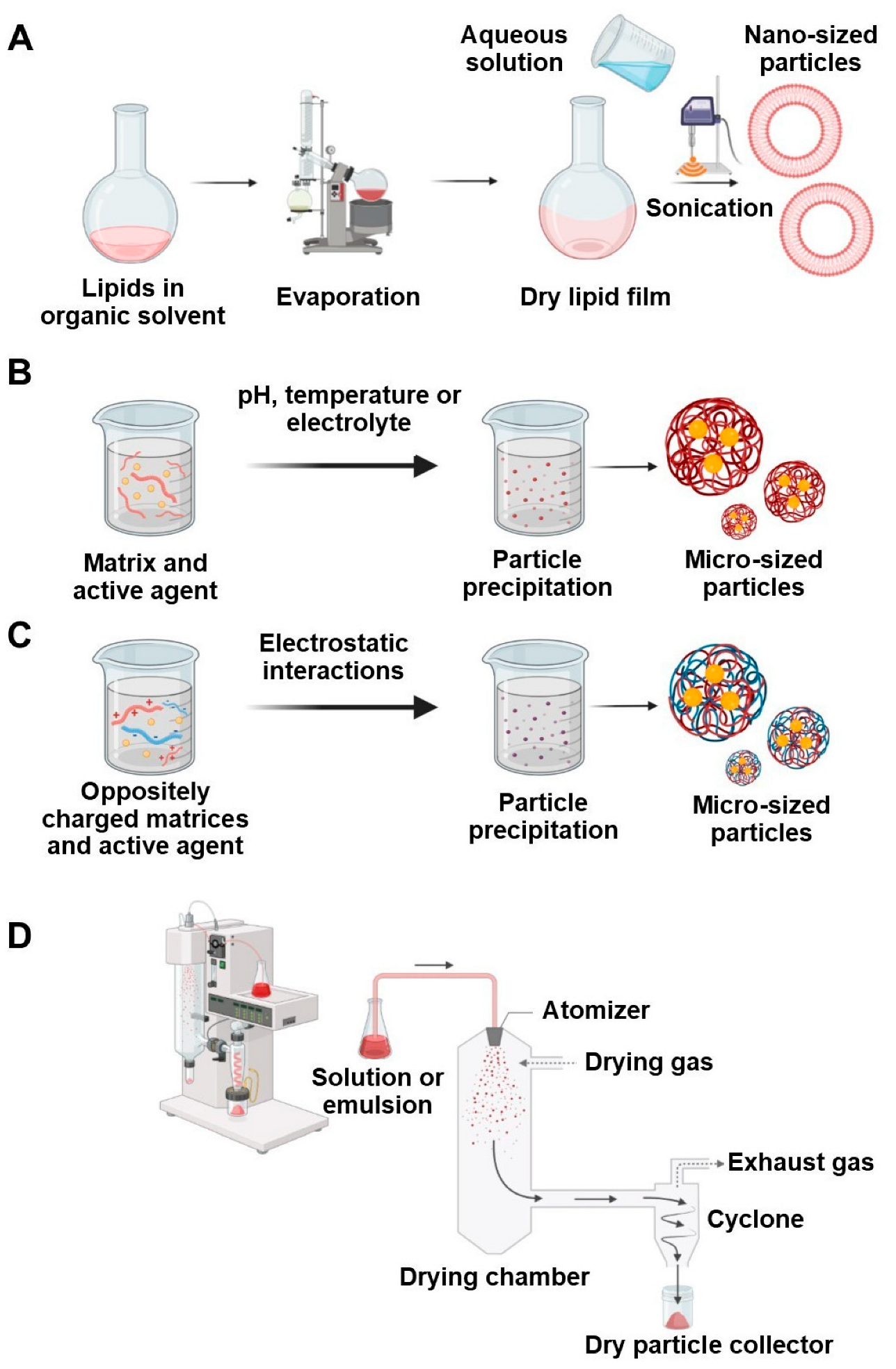
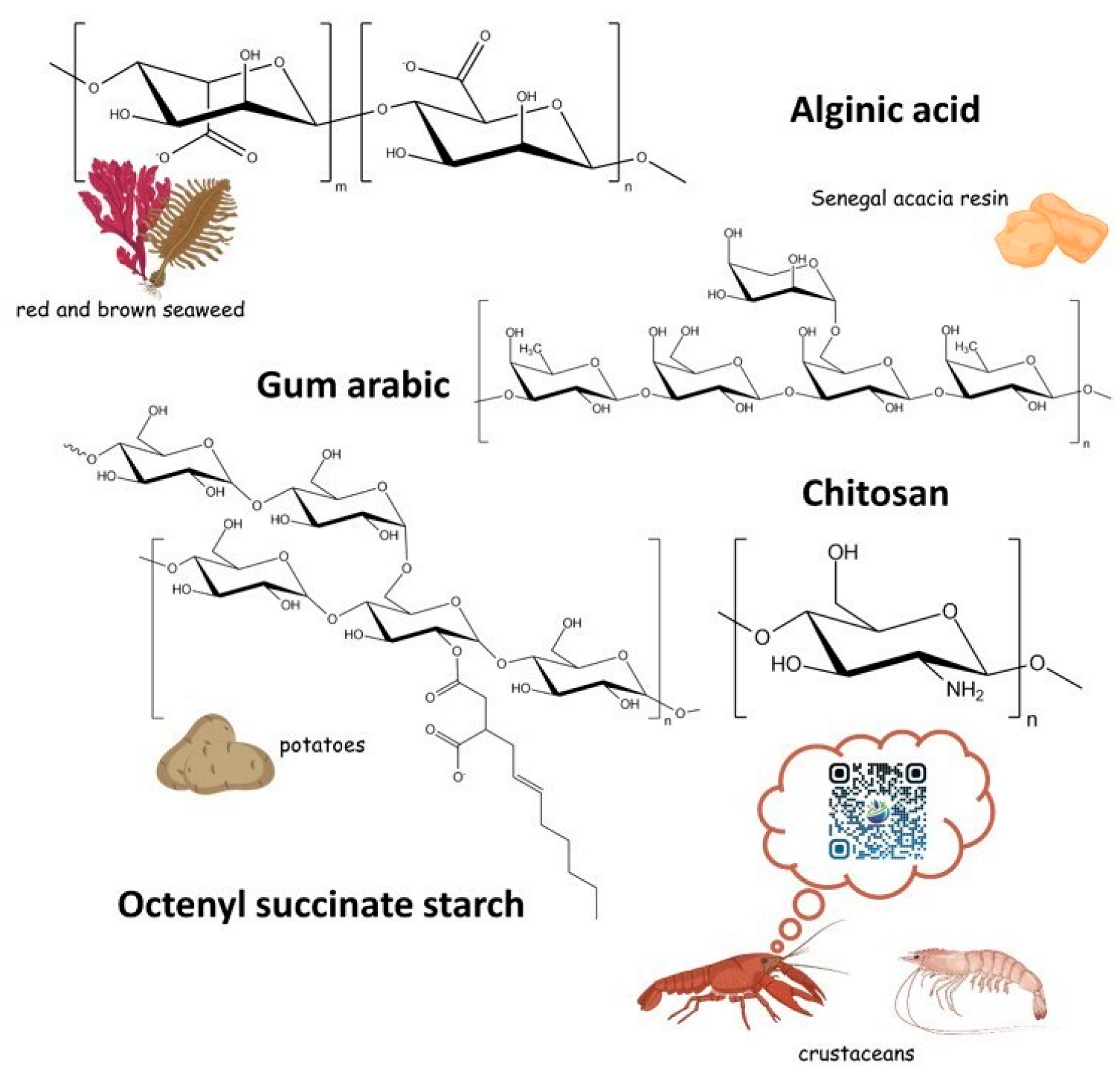
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokol, M.; Gulayev, I.; Chirkina, M.; Klimenko, M.; Kamaeva, O.; Yabbarov, N.; Mollaeva, M.; Nikolskaya, E. Fully Green Particles Loaded with Essential Oils as Phytobiotics: A Review on Preparation and Application in Animal Feed. Antibiotics 2025, 14, 803. https://doi.org/10.3390/antibiotics14080803
Sokol M, Gulayev I, Chirkina M, Klimenko M, Kamaeva O, Yabbarov N, Mollaeva M, Nikolskaya E. Fully Green Particles Loaded with Essential Oils as Phytobiotics: A Review on Preparation and Application in Animal Feed. Antibiotics. 2025; 14(8):803. https://doi.org/10.3390/antibiotics14080803
Chicago/Turabian StyleSokol, Maria, Ivan Gulayev, Margarita Chirkina, Maksim Klimenko, Olga Kamaeva, Nikita Yabbarov, Mariia Mollaeva, and Elena Nikolskaya. 2025. "Fully Green Particles Loaded with Essential Oils as Phytobiotics: A Review on Preparation and Application in Animal Feed" Antibiotics 14, no. 8: 803. https://doi.org/10.3390/antibiotics14080803
APA StyleSokol, M., Gulayev, I., Chirkina, M., Klimenko, M., Kamaeva, O., Yabbarov, N., Mollaeva, M., & Nikolskaya, E. (2025). Fully Green Particles Loaded with Essential Oils as Phytobiotics: A Review on Preparation and Application in Animal Feed. Antibiotics, 14(8), 803. https://doi.org/10.3390/antibiotics14080803









