Investigation of WQ-3810, a Fluoroquinolone with a High Potential Against Fluoroquinolone-Resistant Mycobacterium avium
Abstract
1. Introduction
2. Results
2.1. Inhibitory Effects of WQ-3810 Against M. avium WT and Mutant DNA Gyrases
2.2. Minimum Inhibitory Concentration (MIC) of WQ-3810
2.3. Effects of Cell Wall Synthesis Inhibitors on the MIC of WQ-3810
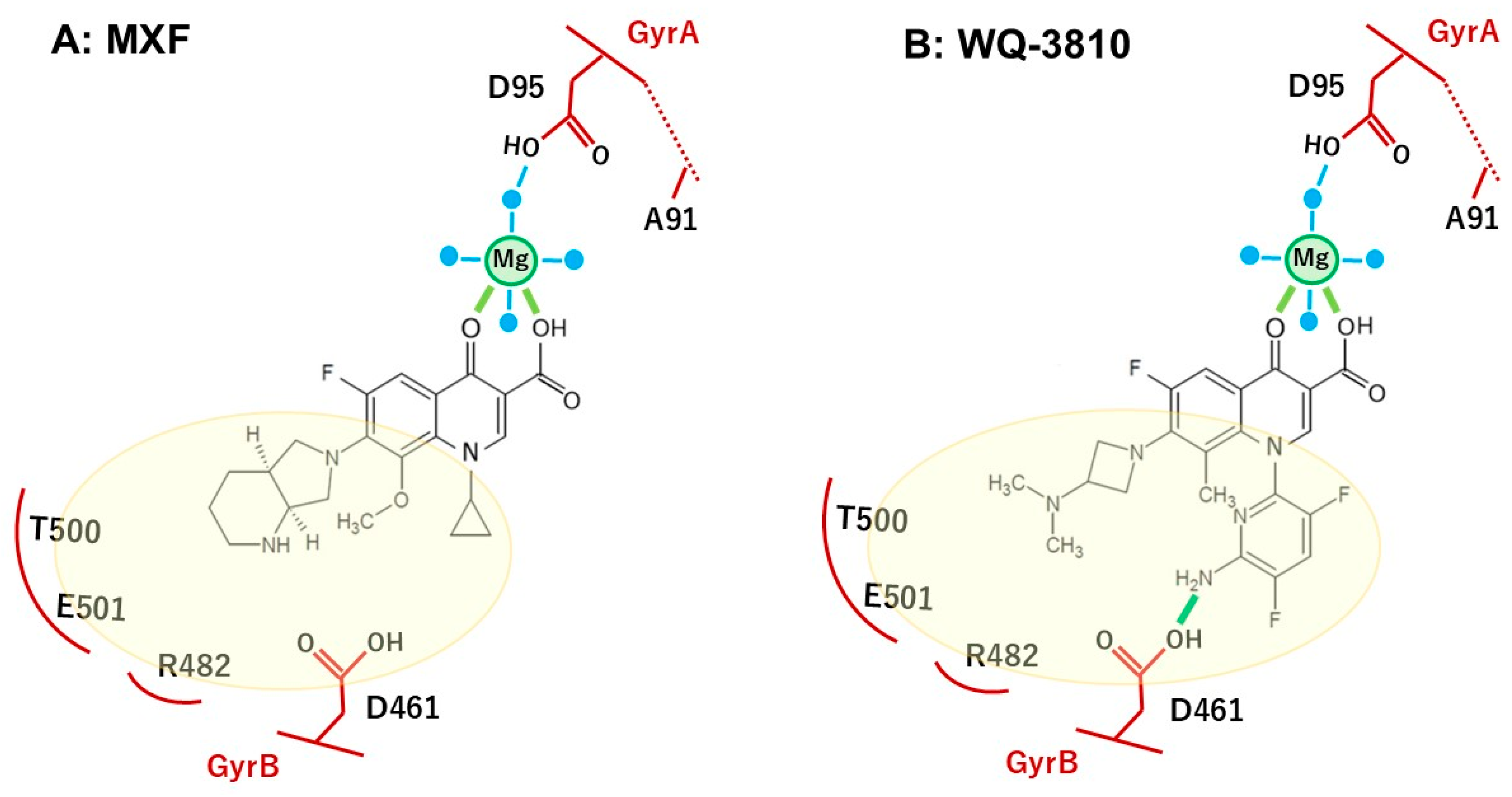
3. Discussion
4. Materials and Methods
4.1. Recombinant M. avium Subsp. Hominissuis DNA Gyrases
4.2. Clinical Isolates of M. avium Subsp. Hominissuis
4.3. Antimicrobial Compounds and Reagents
4.4. FQs Inhibited DNA Gyrase Supercoiling Assay
4.5. Minimum Inhibitory Concentration (MIC) Assay
4.6. Checkerboard Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizzi, R.; Plain, K.M.; Whittington, R.; Timms, V.J. Global Phylogeny of Mycobacterium avium and Identification of Mutation Hotspots During Niche Adaptation. Front. Microbiol. 2022, 13, 892333. [Google Scholar] [CrossRef] [PubMed]
- Boonjetsadaruhk, W.; Kaewprasert, O.; Nithichanon, A.; Ananta, P.; Chaimanee, P.; Salao, K.; Phoksawat, W.; Laohaviroj, M.; Sirichoat, A.; Fong, Y.; et al. High rate of reinfection and possible transmission of Mycobacterium avium complex in Northeast Thailand. One Health 2022, 14, 100374. [Google Scholar] [CrossRef] [PubMed]
- Busatto, C.; Vianna, J.S.; Da Silva, L.V.; Ramis, I.B.; Da Silva, P.E.A. Mycobacterium avium: An overview. Tuberculosis 2019, 114, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Honda, J.R.; Virdi, R.; Chan, E.D. Global Environmental Nontuberculous Mycobacteria and Their Contemporaneous Man-Made and Natural Niches. Front. Microbiol. 2018, 9, 2029. [Google Scholar] [CrossRef]
- Park, H.-E.; Kim, K.-M.; Trinh, M.P.; Yoo, J.-W.; Shin, S.J.; Shin, M.-K. Bigger problems from smaller colonies: Emergence of antibiotic-tolerant small colony variants of Mycobacterium avium complex in MAC-pulmonary disease patients. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 25. [Google Scholar] [CrossRef]
- Cowman, S.; Van Ingen, J.; Griffith, D.E.; Loebinger, M.R. Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J. 2019, 54, 1900250. [Google Scholar] [CrossRef]
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Hasegawa, N.; Ato, M.; Mitarai, S. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan1. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Koh, W.-J.; Moon, S.M.; Kim, S.-Y.; Woo, M.-A.; Kim, S.; Jhun, B.W.; Park, H.Y.; Jeon, K.; Huh, H.J.; Ki, C.-S.; et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur. Respir. J. 2017, 50, 1602503. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Jeon, K.; Park, H.Y.; Kim, S.-Y.; Lee, K.S.; Huh, H.J.; Ki, C.-S.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Intermittent Antibiotic Therapy for Nodular Bronchiectatic Mycobacterium avium Complex Lung Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 96–103. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef] [PubMed]
- M24-A2; Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard—Second Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011.
- Van Ingen, J.; Wagner, D.; Gallagher, J.; Morimoto, K.; Lange, C.; Haworth, C.S.; Floto, R.A.; Adjemian, J.; Prevots, D.R.; Griffith, D.E. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur. Respir. J. 2017, 49, 1601855. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Spencer, A.C.; Panda, S.S. DNA Gyrase as a Target for Quinolones. Biomedicines 2023, 11, 371. [Google Scholar] [CrossRef]
- Wigley, D.B. Structure and Mechanism of DNA Gyrase. In Nucleic Acids and Molecular Biology; Eckstein, F., Lilley, D.M.J., Eds.; Nucleic Acids and Molecular Biology; Springer: Berlin/Heidelberg, Germany, 1995; Volume 9, pp. 165–176. ISBN 978-3-642-79490-2. [Google Scholar]
- Thapa, J.; Chizimu, J.Y.; Kitamura, S.; Akapelwa, M.L.; Suwanthada, P.; Miura, N.; Toyting, J.; Nishimura, T.; Hasegawa, N.; Nishiuchi, Y.; et al. Characterization of DNA Gyrase Activity and Elucidation of the Impact of Amino Acid Substitution in GyrA on Fluoroquinolone Resistance in Mycobacterium avium. Microbiol. Spectr. 2023, 11, e05088-22. [Google Scholar] [CrossRef]
- Cambau, E.; Sougakoff, W.; Jarlier, V. Amplification and nucleotide sequence of the quinolone resistance-determining region in the gyrA gene of mycobacteria. FEMS Microbiol. Lett. 1994, 116, 49–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Catalogue of Mutations in Mycobacterium Tuberculosis Complex and Their Association with Drug Resistance; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002817-3.
- Itoh, K.; Kuramoto, Y.; Amano, H.; Kazamori, D.; Yazaki, A. Discovery of WQ-3810: Design, synthesis, and evaluation of 7-(3-alkylaminoazetidin-1-yl)fluoro-quinolones as orally active antibacterial agents. Eur. J. Med. Chem. 2015, 103, 354–360. [Google Scholar] [CrossRef]
- Kazamori, D.; Aoi, H.; Sugimoto, K.; Ueshima, T.; Amano, H.; Itoh, K.; Kuramoto, Y.; Yazaki, A. In vitro activity of WQ-3810, a novel fluoroquinolone, against multidrug-resistant and fluoroquinolone-resistant pathogens. Int. J. Antimicrob. Agents 2014, 44, 443–449. [Google Scholar] [CrossRef]
- Park, J.-H.; Yamaguchi, T.; Ouchi, Y.; Koide, K.; Mori, S.; Kim, H.; Mukai, T.; Nakajima, C.; Suzuki, Y. WQ-3810 inhibits DNA gyrase activity in ofloxacin-resistant Mycobacterium leprae. J. Infect. Chemother. 2020, 26, 335–342. [Google Scholar] [CrossRef]
- Ouchi, Y.; Mukai, T.; Koide, K.; Yamaguchi, T.; Park, J.-H.; Kim, H.; Yokoyama, K.; Tamaru, A.; Gordon, S.V.; Nakajima, C.; et al. WQ-3810: A new fluoroquinolone with a high potential against fluoroquinolone-resistant Mycobacterium tuberculosis. Tuberculosis 2020, 120, 101891. [Google Scholar] [CrossRef]
- Koide, K.; Kim, H.; Whelan, M.V.X.; Belotindos, L.P.; Tanomsridachchai, W.; Changkwanyeun, R.; Usui, M.; Ó Cróinín, T.; Thapa, J.; Nakajima, C.; et al. WQ-3810, a fluoroquinolone with difluoropyridine derivative as the R1 group exerts high potency against quinolone-resistant Campylobacter jejuni. Microbiol. Spectr. 2024, 12, e04322-23. [Google Scholar] [CrossRef] [PubMed]
- Yamaba, Y.; Ito, Y.; Suzuki, K.; Kikuchi, T.; Ogawa, K.; Fujiuchi, S.; Hasegawa, N.; Kurashima, A.; Higuchi, T.; Uchiya, K.; et al. Moxifloxacin resistance and genotyping of Mycobacterium avium and Mycobacterium intracellulare isolates in Japan. J. Infect. Chemother. 2019, 25, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.E.; Wildman, B.; Schwanz, H.A.; Kerns, R.J.; Aldred, K.J. Role of the Water–Metal Ion Bridge in Quinolone Interactions with Escherichia coli Gyrase. Int. J. Mol. Sci. 2023, 24, 2879. [Google Scholar] [CrossRef]
- Blower, T.R.; Williamson, B.H.; Kerns, R.J.; Berger, J.M. Crystal structure and stability of gyrase-fluoroquinolone cleaved complexes from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, 1706–1713. [Google Scholar] [CrossRef]
- Koh, W.-J.; Hong, G.; Kim, S.-Y.; Jeong, B.-H.; Park, H.Y.; Jeon, K.; Kwon, O.J.; Lee, S.-H.; Kim, C.K.; Shin, S.J. Treatment of Refractory Mycobacterium avium Complex Lung Disease with a Moxifloxacin-Containing Regimen. Antimicrob. Agents Chemother. 2013, 57, 2281–2285. [Google Scholar] [CrossRef]
- Khadawardi, H.; Marras, T.K.; Mehrabi, M.; Brode, S.K. Clinical efficacy and safety of fluoroquinolone containing regimens in patients with Mycobacterium avium complex pulmonary disease. Eur. Respir. J. 2020, 55, 1901240. [Google Scholar] [CrossRef]
- Griffith, D.E. Macrolide-Resistant Mycobacterium avium Complex: I Feel Like I’ve Been Here Before. Ann. Am. Thorac. Soc. 2016, 13, 1881–1882. [Google Scholar] [CrossRef]
- Jacobo-Delgado, Y.M.; Rodríguez-Carlos, A.; Serrano, C.J.; Rivas-Santiago, B. Mycobacterium tuberculosis cell-wall and antimicrobial peptides: A mission impossible? Front. Immunol. 2023, 14, 1194923. [Google Scholar] [CrossRef]
- Emami, S.; Shafiee, A.; Foroumadi, A. Quinolones: Recent Structural and Clinical Developments. Iran. J. Pharm. Res. 2005, 3, 123–136. [Google Scholar]
- Rodrigues, L.; Sampaio, D.; Couto, I.; Machado, D.; Kern, W.V.; Amaral, L.; Viveiros, M. The role of efflux pumps in macrolide resistance in Mycobacterium avium complex. Int. J. Antimicrob. Agents 2009, 34, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Aínsa, J.A.; Riccardi, G. Role of mycobacterial efflux transporters in drug resistance: An unresolved question. FEMS Microbiol. Rev. 2006, 30, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Bannantine, J.P.; Etienne, G.; Laval, F.; Stabel, J.R.; Lemassu, A.; Daffé, M.; Bayles, D.O.; Ganneau, C.; Bonhomme, F.; Branger, M.; et al. Cell wall peptidolipids of Mycobacterium avium: From genetic prediction to exact structure of a nonribosomal peptide. Mol. Microbiol. 2017, 105, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Lee, J.S.; Kwak, N.; Cho, J.; Lee, C.-H.; Han, S.K.; Yim, J.-J. Role of ethambutol and rifampicin in the treatment of Mycobacterium avium complex pulmonary disease. BMC Pulm. Med. 2019, 19, 212. [Google Scholar] [CrossRef]
- Timmins, G.S.; Deretic, V. Mechanisms of action of isoniazid. Mol. Microbiol. 2006, 62, 1220–1227. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
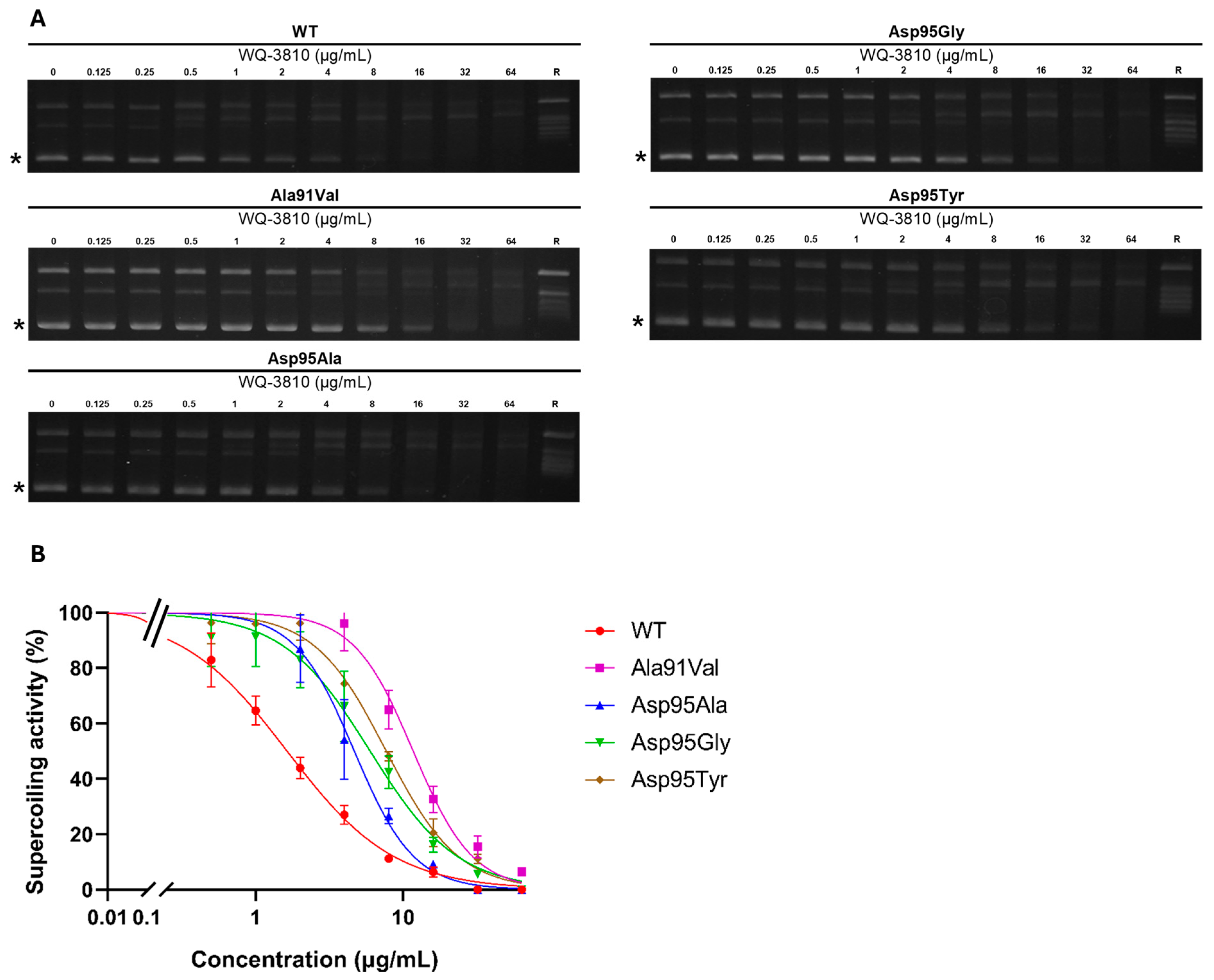
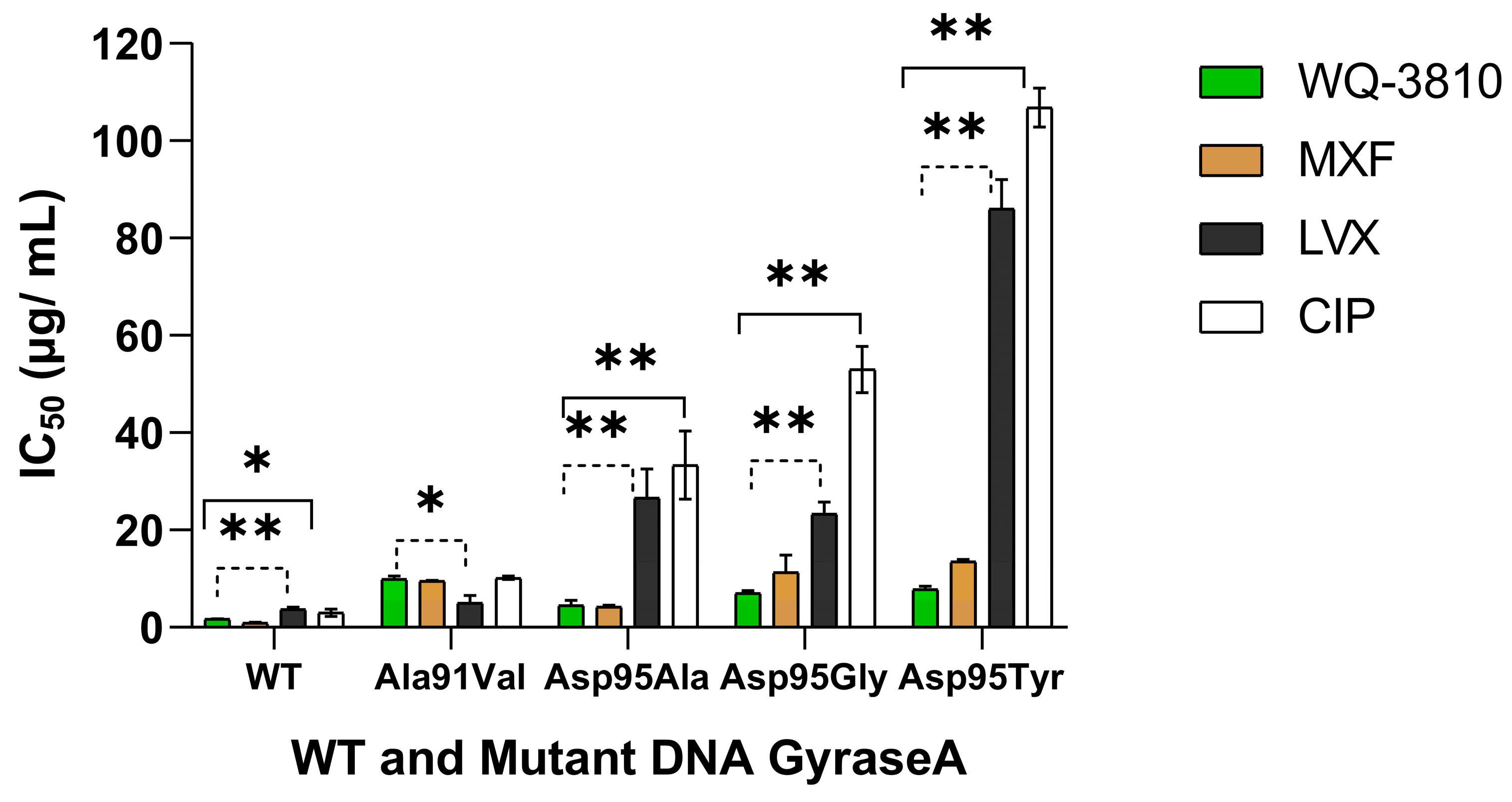
| WT | Ala91Val | Asp95Ala | Asp95Gly | Asp95Tyr | Reference | |
|---|---|---|---|---|---|---|
| WQ-3810 | 1.7 ± 0.1 | 9.9 ± 0.6 | 4.5 ± 1.1 | 7.0 ± 0.5 | 7.8 ± 0.6 | This study |
| CIP | 3 ± 0.8 | 10.2 ± 0.3 | 33.4 ± 7.0 | 53 ± 4.7 | 106.8 ± 4.0 | Previous study [17] |
| MXF | 0.9 ± 0.1 | 9.5 ± 0.2 | 4.2 ± 0.3 | 11.3 ± 3.5 | 13.5 ± 0.4 | Previous study [17] |
| LVX | 3.7 ± 0.4 | 5 ± 1.5 | 26.6 ± 5.9 | 23.3 ± 2.4 | 86.1 ± 6.0 | Previous study [17] |
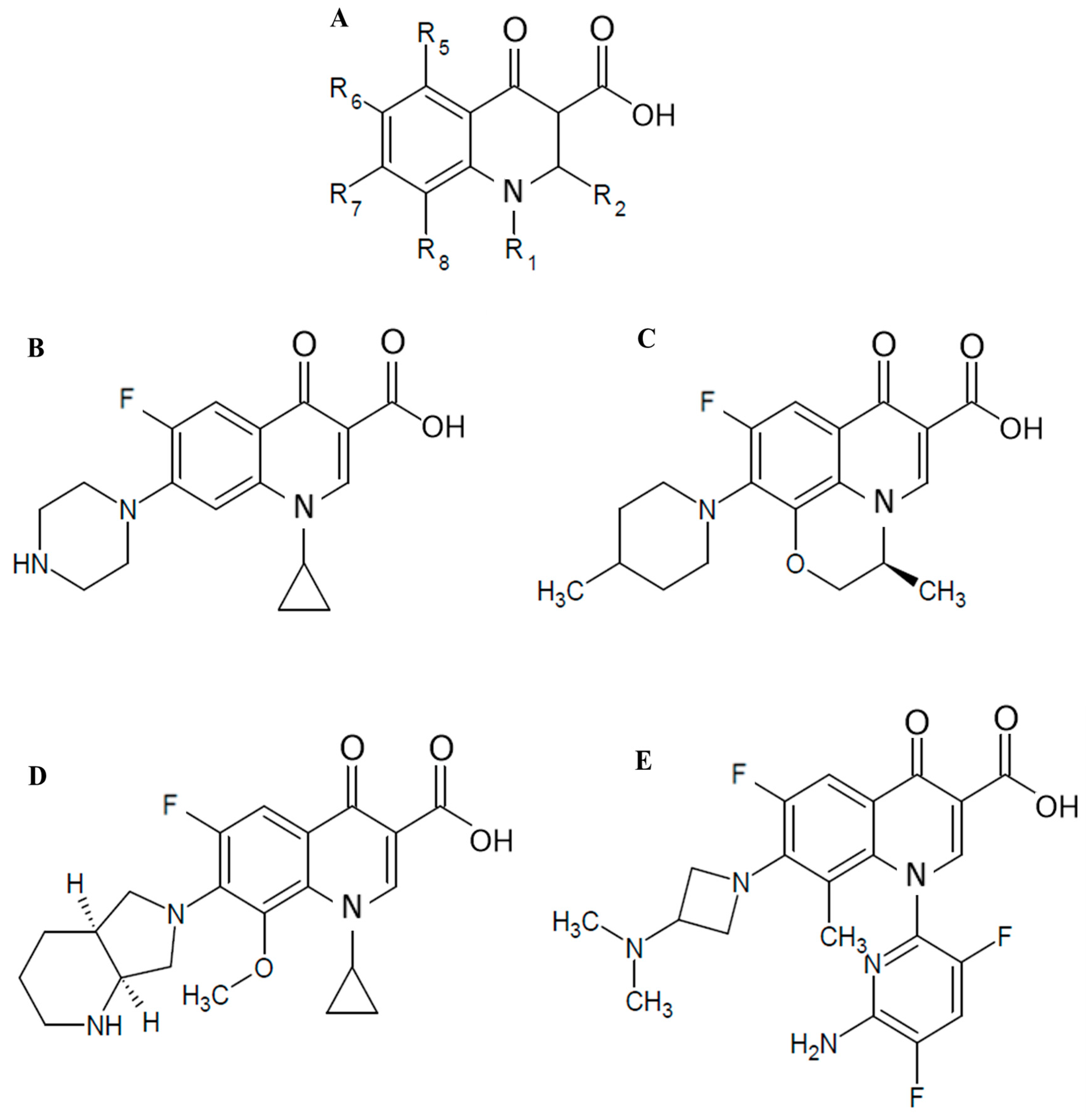
| Fluoroquinolones | N1 | R6 | R7 | R8 |
|---|---|---|---|---|
| CIP | Cyclopropyl | F | Piperazine | H |
| LVX | Bridge N1-C8 | F | 4-Methylpiperazinyl | Bridge N1-C8 |
| MXF | Cyclopropyl | F | Piperridino-pyrrolidinyl | CH3O |
| WQ-3810 | 5-Amino-2,4-difluoropyridine-2-yl | F | 3-Isopropylaminoazetizine-1-yl | CH3 |
| Sample ID | GyrA Mutation | MIC (µg/mL) of: | MIC (µg/mL) with 1 µg/mL EMB | MIC (µg/mL) with 1 µg/mL INH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LVX | CIP | MXF | WQ-3810 | EMB | INH | MXF | WQ-3810 | MXF | WQ-3810 | ||
| Koav 3 | Asp95Gly | 64 | 32 | 8 | 4 | 16 | 4 | 8 (×1) | 4 (×1) | 4 (×0.5) | 2 (×0.5) |
| Koav 11 | Asp95Tyr | 8 | 16 | 16 | 4 | 16 | 8 | 8 (×0.5) | 4 (×1) | 8 (×0.5) | 4 (×1) |
| Koav 13 | Asp95Gly | 128 | 64 | 16 | 8 | 32 | 32 | 16 (×1) | 4 (×0.5) | 8 (×0.5) | 4 (×0.5) |
| Cl-A-2 | Asp95Tyr | 64 | 32 | 8 | 4 | 16 | 4–8 | 4 (×0.5) | 1 (×0.25) | 8 (×1) | 1(×0.25) |
| Koav 1 | None (WT) | <0.5 | 0.5 | 0.25 | 0.125 | 2 | 8 | <0.01 (×0.04) | 0.01 (×0.08) | 0.03 (×0.12) | 0.03 (×0.24) |
| Koav 2 | None (WT) | 2 | 1 | 1 | 0.5 | 16 | 128 | ND | ND | ND | ND |
| Koav 15 | None (WT) | 4 | 2 | 1 | 1 | 16 | 32 | 1 (×1) | 1 (×1) | 1(×1) | 1 (×1) |
| Koav 19 | None (WT) | 2 | 2 | 0.5 | 0.5 | 16 | 8–16 | 0.5 (×1) | 0.25 (×0.5) | 0.13 (×0.25) | 0.13 (×0.5) |
| Koav 20 | None (WT) | <0.5 | 0.5 | 0.25 | 0.125 | 2 | 16 | 0.13–0.25 (×0.5–1) | 0.13 (×1) | 0.25 (×1) | 0.13 (×1) |
| Koav 21 | None (WT) | 4 | 1 | 1 | 1 | 32 | 16 | 1 (×1) | 1 (×1) | 0.5 (×0.5) | 1 (×1) |
| Koav 25 | None (WT) | 4 | 2 | 1 | 1 | 32 | 8 | 1 (×1) | 0.5 (×0.5) | 0.5 (×0.5) | 0.5 (×0.5) |
| Reference | Previous study [17] | This study | This study | This study | This study | This study | |||||
| Sample ID | Gyr A Mutation | Mean FICIm of WQ-3810 | Mean FICIm of MXF | ||
|---|---|---|---|---|---|
| with INH | with EMB | with INH | with EMB | ||
| Koav 3 | Asp95Gly | 0.71 | 0.75 | 0.66 | 0.52 |
| Koav 11 | Asp95Tyr | 0.46 | 0.83 | 0.41 | 0.52 |
| Koav 13 | Asp95Gly | 0.31 | 0.83 | 0.25 | 0.6 |
| Cl-A-2 | Asp95Tyr | 0.53 | 0.66 | 0.40 | 0.41 |
| Koav 1 | None (WT) | 0.45 | 0.62 | 0.27 | 0.5 |
| Koav 2 | None (WT) | 0.25 | 0.91 | 0.31 | 0.71 |
| Koav 15 | None (WT) | 0.33 | 0.71 | 0.38 | 0.66 |
| Koav 19 | None (WT) | 0.45 | 0.86 | 0.19 | 0.71 |
| Koav 20 | None (WT) | 0.25 | 0.66 | 0.41 | 0.81 |
| Koav 21 | None (WT) | 0.41 | 0.75 | 0.31 | 0.55 |
| Koav 25 | None (WT) | 0.39 | 0.75 | 0.49 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaweera, S.; Suwanthada, P.; Barnes, D.A.; Poussier, C.; Nishimura, T.; Hasegawa, N.; Nishiuchi, Y.; Thapa, J.; Gordon, S.V.; Kim, H.; et al. Investigation of WQ-3810, a Fluoroquinolone with a High Potential Against Fluoroquinolone-Resistant Mycobacterium avium. Antibiotics 2025, 14, 704. https://doi.org/10.3390/antibiotics14070704
Jayaweera S, Suwanthada P, Barnes DA, Poussier C, Nishimura T, Hasegawa N, Nishiuchi Y, Thapa J, Gordon SV, Kim H, et al. Investigation of WQ-3810, a Fluoroquinolone with a High Potential Against Fluoroquinolone-Resistant Mycobacterium avium. Antibiotics. 2025; 14(7):704. https://doi.org/10.3390/antibiotics14070704
Chicago/Turabian StyleJayaweera, Sasini, Pondpan Suwanthada, David Atomanyi Barnes, Charlotte Poussier, Tomoyasu Nishimura, Naoki Hasegawa, Yukiko Nishiuchi, Jeewan Thapa, Stephen V. Gordon, Hyun Kim, and et al. 2025. "Investigation of WQ-3810, a Fluoroquinolone with a High Potential Against Fluoroquinolone-Resistant Mycobacterium avium" Antibiotics 14, no. 7: 704. https://doi.org/10.3390/antibiotics14070704
APA StyleJayaweera, S., Suwanthada, P., Barnes, D. A., Poussier, C., Nishimura, T., Hasegawa, N., Nishiuchi, Y., Thapa, J., Gordon, S. V., Kim, H., Nakajima, C., & Suzuki, Y. (2025). Investigation of WQ-3810, a Fluoroquinolone with a High Potential Against Fluoroquinolone-Resistant Mycobacterium avium. Antibiotics, 14(7), 704. https://doi.org/10.3390/antibiotics14070704









